Background: The trafficking of the vasopressin-dependent water channel AQP2 is regulated by post-translational modifications as phosphorylations and ubiquitylation.
Results: AQP2 is subjected to S-glutathionylation, which is modulated by ROS production.
Conclusion: AQP2 is sensitive to oxidative stress.
Significance: Identifying this novel post-translational modification is crucial to understand renal diseases characterized by oxidative stress and AQP2-dependent water balance disturbance.
Keywords: Aquaporin, Glutathionylation, Kidney, Oxidative Stress, Post-translational Modification (PTM), AQP2, CaSR, Water Reabsorption
Abstract
Aquaporin-2 (AQP2) is the vasopressin-regulated water channel that controls renal water reabsorption and urine concentration. AQP2 undergoes different regulated post-translational modifications, including phosphorylation and ubiquitylation, which are fundamental for controlling AQP2 cellular localization, stability, and function. The relationship between AQP2 and S-glutathionylation is of potential interest because reactive oxygen species (ROS), produced under renal failure or nephrotoxic drugs, may influence renal function as well as the expression and the activity of different transporters and channels, including aquaporins. Here, we show for the first time that AQP2 is subjected to S-glutathionylation in kidney and in HEK-293 cells stably expressing AQP2. S-Glutathionylation is a redox-dependent post-translational modification controlling several signal transduction pathways and displaying an acute effect on free cytosolic calcium concentration. Interestingly, we found that in fresh kidney slices, the increased AQP2 S-glutathionylation correlated with tert-butyl hydroperoxide-induced ROS generation. Moreover, we also found that cells expressing wild-type human calcium-sensing receptor (hCaSR-wt) and its gain of function (hCaSR-R990G; hCaSR-N124K) had a significant decrease in AQP2 S-glutathionylation secondary to reduced ROS levels and reduced basal intracellular calcium concentration compared with mock cells. Together, these new findings provide fundamental insight into cell biological aspects of AQP2 function and may be relevant to better understand and explain pathological states characterized by an oxidative stress and AQP2-dependent water reabsorption disturbs.
Introduction
The aquaporins (AQPs)2 are a family of integral membrane proteins that mediate water transport across membranes in response to osmotic gradients. In the kidney, AQP2 is the most characterized aquaporin controlling renal collecting duct water balance, being a selective target of the hormone vasopressin. Vasopressin binds its cognate V2R receptor at the basolateral membrane of collecting duct principal cells activating the cAMP/PKA signal transduction cascade, resulting in multiple phosphorylating events in the C terminus of AQP2. Specifically, phosphorylation of AQP2 at Ser256 is required for the vasopressin-regulated translocation of the AQP2 bearing vesicles toward the apical plasma membrane, increasing water luminal permeability (1, 2). This process is of physiopathological relevance because the failure to insert functional AQP2 into the luminal side of renal collecting ducts can be the cause of different acute and/or chronic renal injury characterized by water balance disorders and often accompanied by inflammation, fibrosis, and reactive oxygen species (ROS) production.
A proper balance between oxidation and reduction reactions is fundamental to control numerous signaling cascades (3), and oxidative post-translational modifications of cysteines, like glutathionylation, can directly influence these processes. Interestingly, recent evidence demonstrated that vasopressin stimulation increased S-glutathionylation of different proteins in mpkccd cells, likely suggesting the involvement of ROS into vasopressin-activated signal transduction pathway (4). In addition, under physiological conditions, vasopressin causes a significant increase in intracellular calcium level that is reduced by inhibiting NADPH oxidases, which is the major source of ROS in the kidney (5). In this respect, a novel study indicates NADPH oxidases as new actor regulating AQP2 transcription because NADPH oxidase deficiency results in a relevant decrease in AQP2 mRNA synthesis (6). AQP2 is subjected to different protein post-translational modifications (PTMs) such as phosphorylations (7, 8), short chain ubiquitylation (9), and polyubiquitylation (10), which play key roles in controlling the cellular localization and stability of AQP2.
We show here that AQP2 is subjected to S-glutathionylation both in kidney and in renal cell culture. S-Glutathionylation is an important post-translational modification, which allows protection of cysteine residues against irreversible oxidation during redox imbalance. We provide evidence that AQP2 glutathionylation is tightly modulated by changes in cellular ROS content both in renal tissue and in HEK cells stably expressing AQP2. The relationship that binds AQP2, S-glutathionylation, and oxidative stress signaling can be of physiological interest and might be of clinical relevance in the field of renal water balance disorders associated with abnormal ROS production.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
All chemicals were purchased from Sigma-Aldrich. tert-Butyl hydroperoxide (tBHP) was kind gift from A. Signorile (University of Bari). To immunoprecipitate and detect the total amount of AQP2, we used antibodies (Pre-C-tail Ab) against the 20-amino acid residue segment just N-terminal from the polyphosphorylated region of AQP2 (CLKGLEPDTDWEEREVRRRQ) (11). Alternatively, AQP2 was detected using a specific antibody (C-tail Ab) raised against a synthetic peptide corresponding to the last 15 C-terminal amino acids of human AQP2 (12). Glutathione antibodies (D8), NADPH, and lucigenin were purchased from Santa Cruz Biotechnologies (DBA, Italy). BioGEE, DMEM, GlutaMAX, and fetal calf serum were purchased from Life Technologies (Monza, Italy).
Cell Culture and Transfection
Human embryonic kidney (HEK-293) cells stably expressing human AQP2 were grown in DMEM high glucose, GlutaMAX, pyruvate, supplemented with 10% (v/v) fetal bovine serum and 100 IU/ml penicillin, 100 μg/ml streptomycin at 37 °C in 5% CO2. HEK-293 cells were seeded on a poly-l-lysine hydrobromide substrate and grown for 24 h at 80% confluence.
Plasmids encoding for hCaSR-wt, hCaSR-R990G, and hCaSR-N124K were already described by Ranieri et al. (13). Cells were transiently transfected with plasmids (0.4 μg of DNA/cm2) encoding for EGFP (mock), hCaSR-wt, and its variants fused with the GFP, using Lipofectamine (1 μg/μl) according to the protocol provided by the manufacturer (Life Technologies). Experiments were performed 48–72 h post-transfection.
Studies in Kidney Slices
Studies in kidney slices were performed as reported (14). Briefly, kidneys were quickly removed and sections of 0.5 mm were made and divided in two groups. The sections were equilibrated for 10 min in a buffer containing 118 mm NaCl, 16 mm HEPES, 17 mm Na-HEPES, 14 mm glucose, 3.2 mm KCl, 2.5 mm CaCl2, 1.8 mm MgSO4, and 1.8 mm KH2PO4 (pH 7.4). S-Glutathionylation of AQP2 was evaluated under resting condition and after oxidant stimulation with tBHP (2 mm) for 30 min. The treated sections were subjected to immunoprecipitation studies or used for ROS detection. Animals were housed according to local and international requirements.
Immunoprecipitation
Immunoprecipitation experiments were performed as described (14). Briefly, mice kidney medulla, fresh kidney slices, or HEK-293 cells were lysed with 1% Triton X-100, 150 mm NaCl, 25 mm HEPES (pH 7.4). The obtained supernatants were precleared with 50 μl of immobilized protein-A for 1 h and incubated overnight with anti-AQP2 antibodies coupled to protein A-Sepharose. Immunocomplexes were washed three times, resuspended in 50 μl of Laemmli's buffer in not denaturating condition and subjected to immunoblotting using AQP2 (1:1000) and glutathione (1:100) antibodies.
S-Glutathionylation Assay
HEK-293 cells were seeded on Ø40-mm glass coverslips treated with poly-l-lysine hydrobromide and transfected as described above. 48 h after transfection, cells were incubated for 1 h with BioGEE reagent (250 μm) at 37 °C, 5%CO2. Cells were lysed in 1% Triton X-100, 150 mm NaCl, 25 mm HEPES (pH 7.4). Cellular debris were removed by centrifugation at 13,000 × g for 10 min at 4 °C. The clarified lysate supernatants were incubated with streptavidin-agarose-conjugated for 4h at 4 °C and then washed three times in the lysis buffer. Glutathionylated proteins were resuspended in 50 μl of Laemmli's buffer in nondenaturating conditions and subjected to immunoblotting using AQP2 (1:1000) and glutathione (1:100) antibodies.
ROS Detection
HEK-293 cells and kidney sections were incubated in serum- and antibiotic-free medium or in Ringer equilibration buffer for 1 h before assaying. Cells or kidney slices were then incubated with dihydrorhodamine-123 (10 μm) in PBS for 30 min at 37 °C with 5% CO2 and recovered in complete medium for 30 min. In the last 15 min of recovery, cells were treated with H2O2 (2 μm) and DTT (50 μm). Alternatively, kidney slices were treated with tBHP (2 mm) for 30 min. Cells and kidney slices were lysed in a buffer containing 1% Triton X-100 150 mm NaCl, 25 mm HEPES (pH 7.4). Lysates were analyzed by RF-5301PC fluorimeter (excitation wavelength, 512 nm; emission wavelength, 530 nm).
Evaluation of NADPH Oxidase
HEK-293 cells were grown and transfected as describe above. The cells were washed with ice-cold PBS, scraped, and centrifuged at 750 × g for 10 min at 4 °C. The obtained pellet was resuspended in 100 μl of homogenizing buffer (20 mm KH2PO4, 1 mm EGTA, pH 7.0). Cells suspension was homogenized by sonication, and protein content was measured. The lucigenin-enhanced chemiluminescence assay was used to evaluate NADPH oxidase activity (15). The reaction mixture solution (150 μl) contains 50 mm phosphate buffer, 1 mm EGTA (pH 7.0), and 5 μm lucigenin. The reaction was started by the addition of cell homogenate (40 μg), and the light emission was recorded. NADPH (1 mm) was added to the mixture, and light emission was measured for the all indicated time points.
RESULTS
AQP2 Is Glutathionylated in Kidney
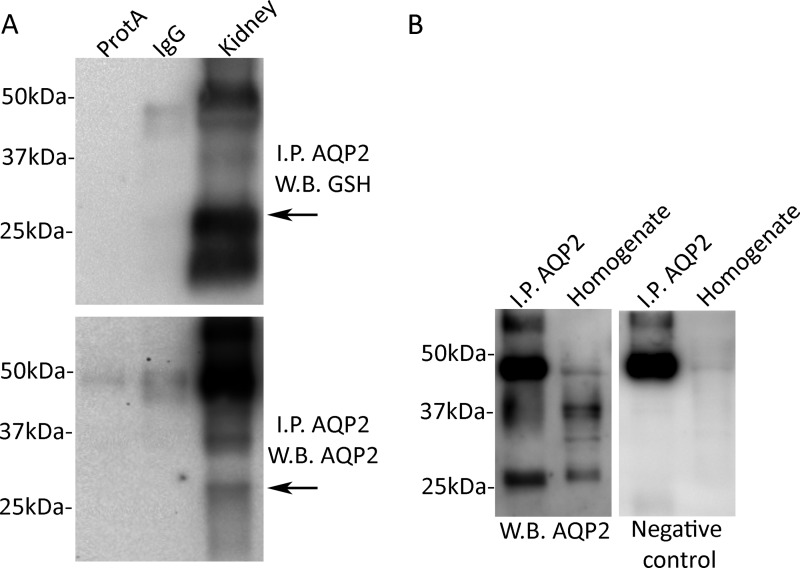
AQP2 glutathionylation was first analyzed from solubilized mice kidney medulla (Fig. 1A). AQP2 was immunoprecipitated and revealed using pre-C-tail antibodies. No specific bands were revealed after incubation of lysates isolated from native tissue, with unspecific IgG or with protein A beads only (Fig. 1A). Furthermore, immunoprecipitates were immunoblotted and revealed for glutathione. Obtained results indicate glutathionylated AQP2 band approximately at 29 kDa (Fig. 1A). As further control, AQP2 immunoprecipitated from renal tissue and kidney homogenate (15 μg) was immunoblotted with or without antibodies against the C-tail. The data in Fig. 1B showed the specificity of the immunoprecipitation because no specific bands were detected in the negative control. Together, these data indicate that AQP2 is modified by S-glutathionylation in native tissue.
FIGURE 1.
AQP2 is subjected to S-glutathionylation. A, AQP2 was immunoprecipitated (I.P. AQP2) using pre-C-tail antibodies from the renal medulla of five mice. Lysates were also subjected to precipitation with only protein A-coupled Sepharose (ProtA) or with nonspecific IgG. AQP2 was visualized using anti pre-C-tail antibodies (1:1000). B, AQP2 from immunoprecipitation and renal homogenate was detected with anti-C-terminal antibodies. Negative control is shown on the right panel. W.B., Western blot.
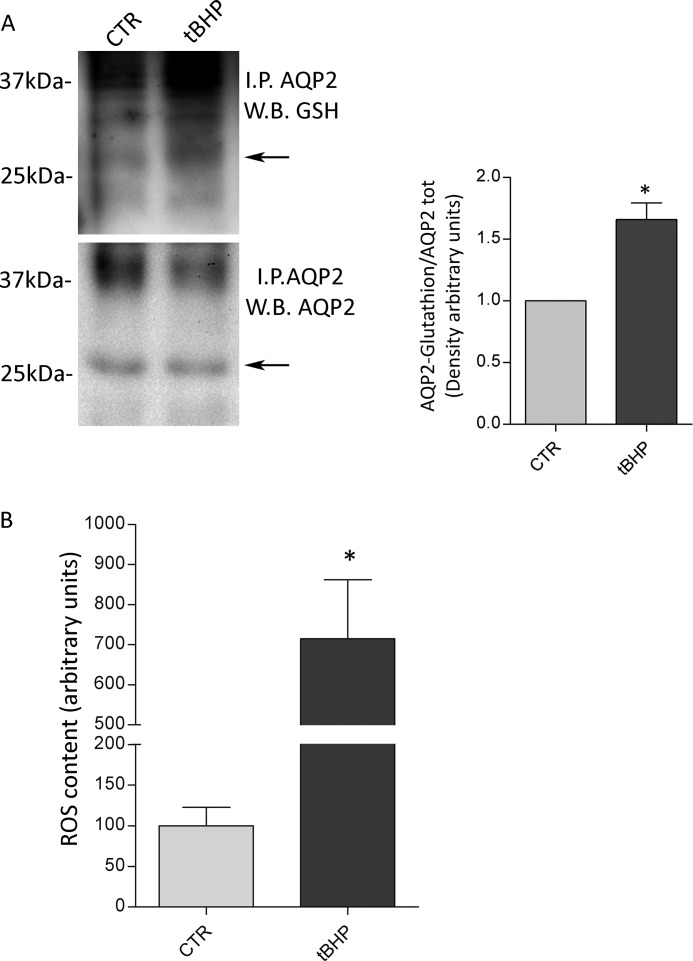
To evaluate whether AQP2 S-glutathionylation is modulated by oxidative changes, ex vivo experiments were performed. Renal kidney slices were prepared and treated as described under methods. Oxidative stimulation was obtained with tBHP (2 mm) for 30 min. Compared with the total amount of AQP2, the relative immunoreactive signal of AQP2 S-glutathionylated significantly increased after tBHP treatment (Fig. 2A, tBHP: 1.66 ± 0.1364 versus control: 1.00, n = 3; *, p < 0.01). The redox state of renal sections was studied using a ROS indicator named dihydrorhodamine-123, which is an uncharged and nonfluorescent probe that became highly fluorescent when oxidized by intracellular ROS and other peroxides. Relative to untreated kidney slices, incubation with tBHP resulted in a significant increase in ROS content (Fig. 2B; tBHP: 715.2 ± 147.0 versus control: 100 ± 23.0, n = 6; *, p < 0.01). Together, these findings likely indicate that changes of the redox state correlate with alterations of AQP2 S-glutathionylation in renal native tissue.
FIGURE 2.
A, AQP2 was immunoprecipitated from fresh kidney slices left untreated or stimulated with the oxidant tBHP (2 mm) for 30 min. Density on the right indicates that tBHP treatment significantly increases glutathionylation of AQP2. The data (means ± S.E.) were analyzed by Student's t test with p < 0.05 (*) considered to be statistically different. B, ROS content was measured using dihydrorhodamine-123 fluorescence in fresh renal sections left untreated or stimulated with the oxidant tBHP (2 mm) for 30 min. The data (means ± S.E.) were analyzed by Student's t test with p < 0.01 (*) considered to be statistically different. CTR, control; I.P., immunoprecipitated AQP2; W.B., Western blot.
AQP2 Is Glutathionylated in HEK-293 Cells
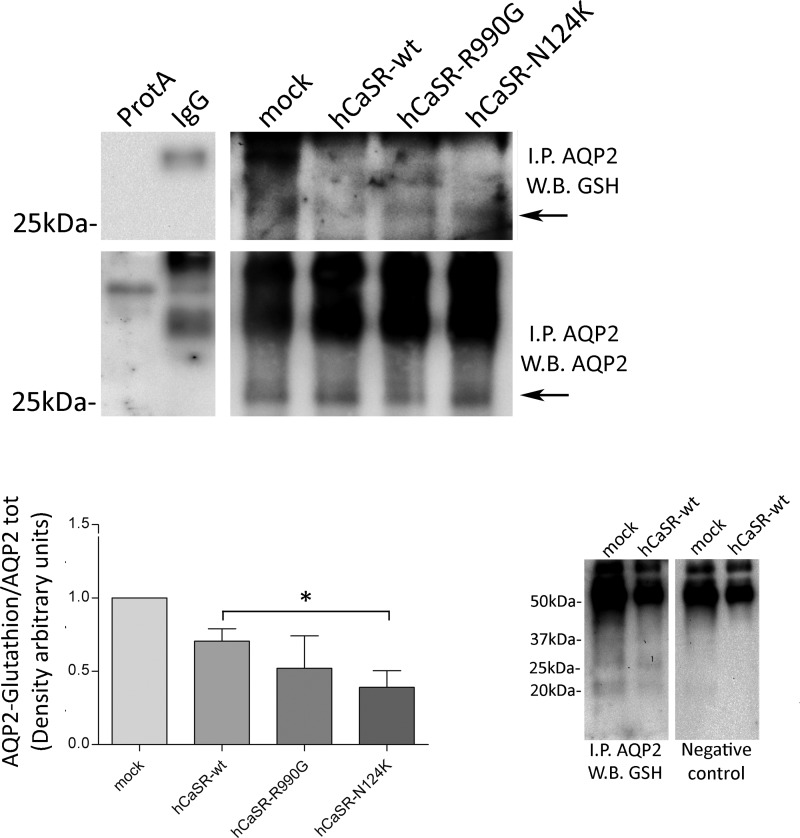
AQP2 S-glutathionylation was further investigated in HEK-293 cells stably expressing human AQP2. Interestingly, we found that the level of S-glutathionylated AQP2 was significantly reduced in cells expressing hCaSR-wt, hCaSR-R990G, and hCaSR-N124K, suggesting an interplay between CaSR signaling and this post-transcriptional modification of AQP2 (Fig. 3: hCaSR-wt: 0.663 ± 0.102; hCaSR-R990G: 0.453 ± 0.144; hCaSR-N124K: 0.408 ± 0.098 versus mock: 1.00). No specific bands were revealed after incubation of lysates isolated from mock cells, with unspecific IgG or with protein A beads only. As a control, AQP2 immunoprecipitated from mock and hCaSR-wt expressing cells immunoblotted with or without glutathione antibodies (Fig. 3, right panels) showed the specificity of GSH antibodies because no selective bands were detected at 29 kDa (negative control).
FIGURE 3.
AQP2 was immunoprecipitated (I.P. AQP2) from HEK-293 cells stably expressing hAQP2. Lysates from HEK-293 cells were also subjected to precipitation with only protein A-coupled Sepharose (ProtA) or with nonspecific IgG. Throughout this study, nonequivalents (10 and 90%) of the immunoprecipitates were immunoblotted for AQP2 or GSH, respectively. The data (means ± S.E.) were analyzed by one-way analysis of variance followed by Newman-Keuls multiple comparison test with p < 0.05 (*) considered to be statistically different. AQP2 was immunoprecipitated from mock and hCaSR-wt expressing cells and immunoblotted with or without glutathione antibodies. W.B., Western blot.
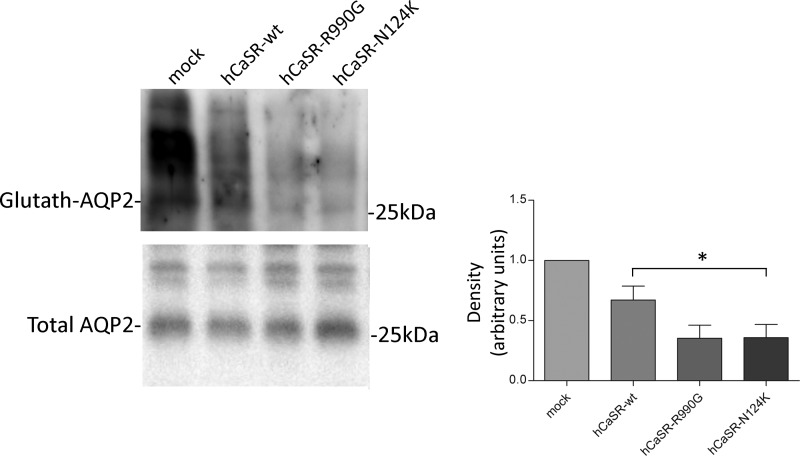
Alternatively, AQP2 S-glutathionylation was analyzed with a different methodological procedure using BioGEE reagent, a cell-permeant biotinylated glutathione ethyl ester raised for the detection of protein glutathionylation. After affinity precipitation of cellular glutathionylated proteins with streptavidin-agarose beads, immunoblotting analysis, with pre-C-tail antibodies, confirmed AQP2 S-glutathionylation, which was found decreased in HEK-293 cells expressing hCaSR-wt and even more in CaSR gain of function variants (hCaSR-wt: 0.670 ± 0.115; hCaSR-R990G: 0.353 ± 0.107; hCaSR-N124K: 0.358 ± 0.108 versus mock: 1.00) (Fig. 4), confirming our previous findings obtained with immunoprecipitation experiments (Figs. 1–3).
FIGURE 4.
S-Glutathionylation assay with BIOGEE reagent was performed in HEK-293 cells as described under “Experimental Procedures.” Glutathionylated proteins were separated on electrophoresis gel and immunoblotted for AQP2. Densitometry revealed that AQP2 S-glutathionylation decreased in HEK-293 cells expressing hCaSR-wt and its variants with respect to mock. The data (means ± S.E.) were analyzed by one-way analysis of variance followed by Newman-Keuls multiple comparison test with p < 0.05 (*) considered to be statistically different.
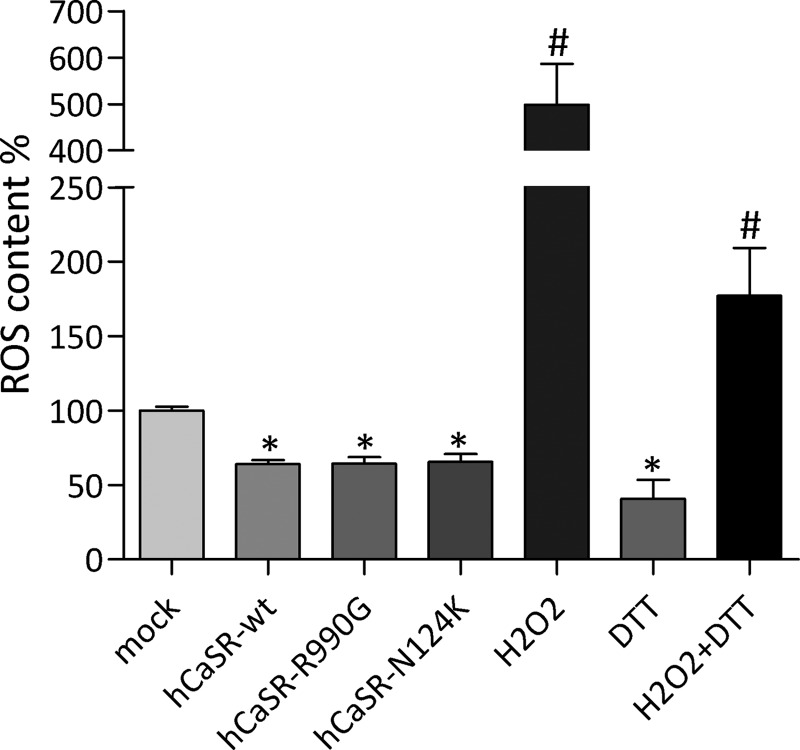
ROS content was measured to evaluate whether changes in AQP2 S-glutathionylation in CaSR-wt and its gain of function variants are associated with alterations in intracellular ROS level. To this end, cells were incubated with the ROS indicator dihydrorhodamine-123. Compared with cells transfected with the empty vector encoding for EGFP, HEK-293 cells expressing CaSR-wt and its gain of function variants (hCaSR-R990G and hCaSR-N124K) showed a significant decrease of intracellular ROS (Fig. 5; hCaSR-wt: 64.13 ± 2.5%; hCaSR-R990G: 64.32 ± 4.55%; CaSR-N124K: 65.46 ± 5.22% versus mock: 100 ± 2.24%). Additionally, as internal experimental control, HEK-293 cells were incubated with H2O2 (2 μm for 15 min) or with DTT (50 μm for 15 min) or co-treated with H2O2 and DTT. H2O2 incubation increases, whereas DTT reduces ROS content, respectively (H2O2: 499.00 ± 87.5; DTT: 40.68 ± 12.90 versus mock: 100 ± 2.24%). Treatment with DTT partially abolished H2O2-induced increase of ROS content (H2O2 + DTT: 177.0 ± 32.33 versus mock: 100 ± 2.24%).
FIGURE 5.
ROS content was measured using dihydrorhodamine-123 fluorescence in HEK-293 cells expressing empty vector encoding for EGFP (mock) or CaSR-wt and its gain of function variants. Alternatively mock cells were treated with H2O2 or DTT as positive and negative control, respectively. The data are shown as means ± S.E. and analyzed by one-way analysis of variance followed by Newman-Keuls multiple comparison test with p < 0.05 (*) considered to be statistically different.
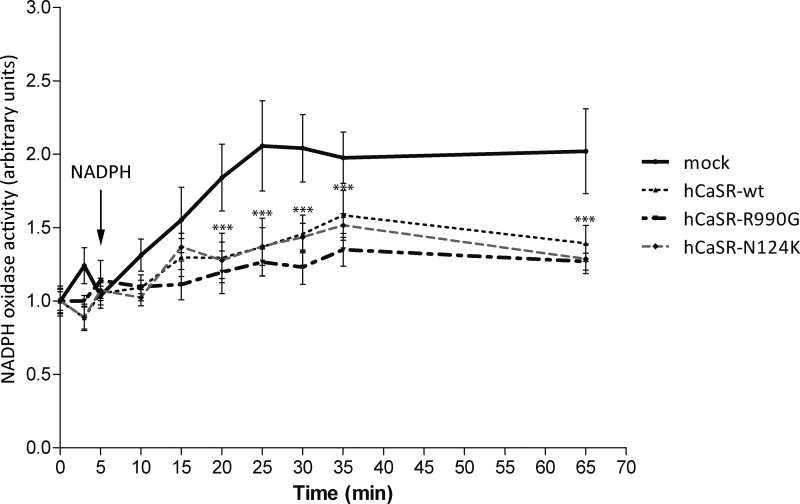
NADPH oxidase is known to be the major source for ROS production in several cell models including kidney (6, 16). Therefore, we next examined NADPH oxidase activity with a lucigenin-based assay. The time course of lucigenin chemiluminescence revealed that mock cells hold higher NADPH oxidase activity compared with cells expressing hCaSR-wt or its gain of function variants (hCaSR-R990G and hCaSR-N124K) (Fig. 6) as indicated by the linear regression analysis within 5 min after NADPH addition (for slope, hCaSR-wt: 0.01705 ± 0.005629; hCaSR-R990G: 0.009804 ± 0.005220; CaSR-N124K: 0.01706 ± 0.006094 versus mock: 0.05157 ± 0.02061). Statistical analysis revealed that mock cells showed a significantly higher NADPH oxidase activity already 15 min following NADPH addition. Remarkably, with respect to mock cells, in hCaSR-wt and its gain of function variants, the difference in NADPH oxidase activity remained significantly higher until the end of the assay (Fig. 6).
FIGURE 6.
Time course of NADPH oxidase activity in HEK-293 cells expressing empty vector encoding for EGFP (mock) or CaSR-wt and its gain of function variants.
DISCUSSION
We report here the first evidence that AQP2 is glutathionylated in native mammalian kidney and in renal cell culture and that this post-translational modification is modulated by the oxidative stress. Specifically, in ex vivo experiments, tBHP, a known oxidant inducer (17), causes a significant increase in AQP2 S-glutathionylation secondary to an increases in ROS content, indicating that this redox sensitive PTM is linked to the redox condition of the tissue. Moreover, functional expression of hCaSR and its gain of function variants (hCaSR-R990G and hCaSR-N124K) in renal HEK cells caused a reduction in ROS which in turn was accompanied by a reduction in AQP2 S-glutathionylation with respect to mock cells.
Protein PTMs play key roles in cellular signaling (18). Among them, AQP2 undergoes ubiquitylation (9, 19) and phosphorylation at different sites (8), two events known to regulate intracellular distribution and cellular stability of AQP2.
We show here that AQP2 is also glutathionylated, apparently as a consequence of modification of the redox cellular state, both in kidney and in renal cell culture. Redox PTMs have emerged as important molecular signals described in all organisms. Increasing evidence revealed that ROS and reactive nitrogen species function as signaling components transducing extracellular or intracellular information and elaborating highly selective responses by promoting PTMs of thiol residues on target proteins (3, 20). Glutathione can be considered one of the major cellular antioxidant molecules that are continuously converted into the reduced form of GSH. Glutathione can bind an accessible free thiol on a target protein through the thiol group of its cysteine promoting S-glutathionylation. Topological analysis of AQP2 suggests that Cys75 and Cys79, on cytosolic B-loop and highly conserved among species, might be target of S-glutathionylation. Analysis of the primary amino acid sequence, surrounding possible residues of S-glutathionylation, did not highlight any consensus site motif, suggesting that the nature of this PTM may be dependent on the microenvironment features of the cysteine within the three-dimensional structure of the protein (21). However, Chen et al. (22) have recently published a database of cysteine glutathionylation, suggesting that the GSH motifs contain a higher preference of negatively charged residues than non-GSH sites. S-Glutathionylation allows protection of specific cysteine residues from irreversible oxidation. In addition several data demonstrated that S-glutathionylation may also affect the activity and the cellular distribution of target proteins (23, 24). At steady state, whether or not S-glutathionylation affects the localization and the activity of AQP2 remains unclear. We found here, however, that the expression of hCaSR and its gain of function variants (hCaSR-R990G and hCaSR-N124K) reduced AQP2 S-glutathionylation. In vivo and in vitro studies demonstrated a clear interplay between the vasopressin-stimulated signal transduction cascade activating AQP2 trafficking and CaSR (25). In particular, under physiological conditions, vasopressin caused a significant increase in AQP2 excretion accompanied by an increase in urinary osmolality, as expected. Hypercalciuric patients showed higher excretion of AQP2, which did not increase after vasopressin treatment. Consistent with these findings, in vitro experiments revealed that the cell surface abundance of AQP2 in cells exposed to CaSR agonists was higher than in control cells and did not increase in response to short term exposure to the cAMP-elevating agent forskolin (25). It is still unclear whether CaSR agonists control the cellular distribution of AQP2 by affecting PTMs, which are known to regulate AQP2 trafficking. However, we found that expression of hCaSR and its gain of function variants reduced intracellular calcium level (13) and significantly altered AQP2 phosphorylation (unpublished data). The role of calcium on AQP2 localization and phosphorylation has been debated for long time. In this respect, we have recently demonstrated that low cytosolic calcium level down-regulates the phosphatase PP2A increasing phosphorylation of AQP2 at serine 256 (14). Therefore, to avoid transmission failure, cells tightly control cytosolic and ER calcium concentration by modulating the expression and the relative activity of different calcium signaling proteins. In fact, the decrease in cytosolic calcium observed in cells expressing hCaSR and its gain of function variants is paralleled by calcium accumulation in the endoplasmic reticulum (ER) because of increased sarcoplasmic/endoplasmic reticulum Ca2+-ATPase activity and expression and a reduced plasma membrane Ca2+ ATPase abundance (13). Mitochondria are also deeply involved in calcium homeostasis because they promote ER depletion by calcium buffering. Importantly, mitochondria calcium uptake stimulates ROS production, which plays a role in modulating ER and cytosolic calcium concentration (26) by affecting the activity of pivotal calcium signaling proteins via S-glutathionylation (20). We found that expression hCaSR-wt, hCaSR-R990G, and hCaSR-N124K not only reduced intracellular calcium level (13) but is also accompanied by a significant decrease of AQP2 S-glutathionylation secondary to a decrease of ROS content, which is associated to a relevant decrease of NADPH oxidase activity.
The finding that AQP2 is subjected to S-glutathionylation is itself important to better elucidate the involvement of AQP2 in a broad spectrum of acute and chronic renal diseases characterized by exacerbate oxidative stress. Together, these findings provide evidence that changes in oxidative cellular state correlate with glutathionylation of AQP2. On the other hand, these observations strengthen the importance of AQP2-CaSR signaling interplay involving, in addition to modulation of AQP2 phosphorylation, AQP2 alteration of its glutathionylation state, which emerges as a novel post-translational modification modulated by intracellular calcium and the oxidative stress.
This work was supported by a grant from University of Bari, Italy (Idea Giovani 2011), by Research Program of National Interest projects (to G. T.) (Tamma01373409Prin), and by Telethon Grant GGP13227 (to G. V.).
- AQP
- aquaporin
- ROS
- reactive oxygen species
- tBHP
- tert-butyl hydroperoxide
- hCaSR
- human calcium-sensing receptor
- PTM
- post-translational modification
- ER
- endoplasmic reticulum.
REFERENCES
- 1. Fenton R. A., Pedersen C. N., Moeller H. B. (2013) New insights into regulated aquaporin-2 function. Curr. Opin. Nephrol. Hypertens. 22, 551–558 [DOI] [PubMed] [Google Scholar]
- 2. Valenti G., Procino G., Tamma G., Carmosino M., Svelto M. (2005) Aquaporin 2 trafficking. Endocrinology 146, 5063–5070 [DOI] [PubMed] [Google Scholar]
- 3. Grek C. L., Zhang J., Manevich Y., Townsend D. M., Tew K. D. (2013) Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem. 288, 26497–26504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sandoval P. C., Slentz D. H., Pisitkun T., Saeed F., Hoffert J. D., Knepper M. A. (2013) Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J. Am. Soc. Nephrol. 24, 1793–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding Y., Winters A., Ding M., Graham S., Akopova I., Muallem S., Wang Y., Hong J. H., Gryczynski Z., Yang S. H., Birnbaumer L., Ma R. (2011) Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J. Biol. Chem. 286, 31799–31809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Féraille E., Dizin E., Roth I., Derouette J. P., Szanto I., Martin P. Y., de Seigneux S., Hasler U. (2014) NADPH oxidase 4 deficiency reduces aquaporin-2 mRNA expression in cultured renal collecting duct principal cells via increased PDE3 and PDE4 activity. PLoS One 9, e87239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown D., Hasler U., Nunes P., Bouley R., Lu H. A. (2008) Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr. Opin. Nephrol. Hypertens. 17, 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moeller H. B., Olesen E. T., Fenton R. A. (2011) Regulation of the water channel aquaporin-2 by posttranslational modification. Am. J. Physiol. Renal Physiol. 300, F1062–F1073 [DOI] [PubMed] [Google Scholar]
- 9. Kamsteeg E. J., Hendriks G., Boone M., Konings I. B., Oorschot V., van der Sluijs P., Klumperman J., Deen P. M. (2006) Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc. Natl. Acad. Sci. U.S.A. 103, 18344–18349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nedvetsky P. I., Tabor V., Tamma G., Beulshausen S., Skroblin P., Kirschner A., Mutig K., Boltzen M., Petrucci O., Vossenkämper A., Wiesner B., Bachmann S., Rosenthal W., Klussmann E. (2010) Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J. Am. Soc. Nephrol. 21, 1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamma G., Lasorsa D., Ranieri M., Mastrofrancesco L., Valenti G., Svelto M. (2011) Integrin signaling modulates AQP2 trafficking via Arg-Gly-Asp (RGD) motif. Cell. Physiol. Biochem. 27, 739–748 [DOI] [PubMed] [Google Scholar]
- 12. Tamma G., Procino G., Strafino A., Bononi E., Meyer G., Paulmichl M., Formoso V., Svelto M., Valenti G. (2007) Hypotonicity induces aquaporin-2 internalization and cytosol-to-membrane translocation of ICln in renal cells. Endocrinology 148, 1118–1130 [DOI] [PubMed] [Google Scholar]
- 13. Ranieri M., Tamma G., Di Mise A., Vezzoli G., Soldati L., Svelto M., Valenti G. (2013) Excessive signal transduction of gain-of-function variants of the calcium-sensing receptor (CaSR) are associated with increased ER to cytosol calcium gradient. PLoS One 8, e79113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamma G., Lasorsa D., Trimpert C., Ranieri M., Di Mise A., Mola M. G., Mastrofrancesco L., Devuyst O., Svelto M., Deen P. M., Valenti G. (2014) A protein kinase A-independent pathway controlling aquaporin 2 trafficking as a possible cause for the syndrome of inappropriate antidiuresis associated with polycystic kidney disease 1 haploinsufficiency. J. Am. Soc. Nephrol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ungvari Z., Csiszar A., Huang A., Kaminski P. M., Wolin M. S., Koller A. (2003) High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108, 1253–1258 [DOI] [PubMed] [Google Scholar]
- 16. Morigi M., Macconi D., Zoja C., Donadelli R., Buelli S., Zanchi C., Ghilardi M., Remuzzi G. (2002) Protein overload-induced NF-κB activation in proximal tubular cells requires H2O2 through a PKC-dependent pathway. J. Am. Soc. Nephrol. 13, 1179–1189 [PubMed] [Google Scholar]
- 17. Taylor E. R., Hurrell F., Shannon R. J., Lin T. K., Hirst J., Murphy M. P. (2003) Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 278, 19603–19610 [DOI] [PubMed] [Google Scholar]
- 18. Choudhary C., Mann M. (2010) Decoding signalling networks by mass spectrometry-based proteomics. Nat. Rev. Mol. Cell Biol. 11, 427–439 [DOI] [PubMed] [Google Scholar]
- 19. Tamma G., Robben J. H., Trimpert C., Boone M., Deen P. M. (2011) Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am. J. Physiol. Cell Physiol. 300, C636–C646 [DOI] [PubMed] [Google Scholar]
- 20. Pastore A., Piemonte F. (2012) S-Glutathionylation signaling in cell biology: progress and prospects. Eur. J. Pharm. Sci. 46, 279–292 [DOI] [PubMed] [Google Scholar]
- 21. Zaffagnini M., Bedhomme M., Groni H., Marchand C. H., Puppo C., Gontero B., Cassier-Chauvat C., Decottignies P., Lemaire S. D. (2012) Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: a proteomic survey. Mol. Cell. Proteomics 11, M111.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y. J., Lu C. T., Lee T. Y., Chen Y. J. (2014) dbGSH: a database of S-glutathionylation. Bioinformatics 30, 2386–2388 [DOI] [PubMed] [Google Scholar]
- 23. Hawkins B. J., Irrinki K. M., Mallilankaraman K., Lien Y. C., Wang Y., Bhanumathy C. D., Subbiah R., Ritchie M. F., Soboloff J., Baba Y., Kurosaki T., Joseph S. K., Gill D. L., Madesh M. (2010) S-Glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 190, 391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang W., Oliva C., Li G., Holmgren A., Lillig C. H., Kirk K. L. (2005) Reversible silencing of CFTR chloride channels by glutathionylation. J. Gen. Physiol. 125, 127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Procino G., Mastrofrancesco L., Tamma G., Lasorsa D. R., Ranieri M., Stringini G., Emma F., Svelto M., Valenti G. (2012) Calcium-sensing receptor and aquaporin 2 interplay in hypercalciuria-associated renal concentrating defect in humans: an in vivo and in vitro study. PLoS One 7, e33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huddleston A. T., Tang W., Takeshima H., Hamilton S. L., Klann E. (2008) Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J. Neurophysiol. 99, 1565–1571 [DOI] [PubMed] [Google Scholar]