Abstract
Background and Aims
NK cells are essential early after infection not only for viral containment but also for timely and efficient induction of adaptive responses. An inhibitory effect of HCV-E2 proteins on NK cells has been reported but the features of NK cell responses in the acute phase of hepatitis C are still largely undefined. Therefore, the aim of this study was to characterize function and phenotype of natural killer cells in the acute phase of infection and compare individuals with chronic and self-limited outcomes.
Methods
Twenty-two individuals with acute HCV infection, 14 with chronic evolution and 8 with self-limited infection, were studied using NK phenotypic and functional assays.
Results
An increased expression of NKG2D on both CD56bright and CD56dim NK cells was detected in acute HCV patients, irrespective of the outcome, as compared to healthy controls. Also IFN-γ production and cytotoxicity by NK cells were higher in individuals with acute HCV infection than in healthy controls. Subset analysis demonstrated an increased IFN-γ production in both NK cell subsets carrying group 1 and group 2 HLA-C specific KIRs. However, increased CD107a was noted only on NK cells expressing the group 1 HLA-C specific KIR and was maximal in self-limited infection.
Conclusions
Our data demonstrate that in the acute phase of HCV infection NK cells are activated regardless of outcome with no evidence of a suppressive effect of HCV on NK cell function.
Keywords: NK receptors, Cytotoxicity, Interferon-gamma
INTRODUCTION
Hepatitis C virus (HCV) can establish a chronic persistent infection in the vast majority of exposed individuals and the host immune response is believed to play an important role in determining the fate of infection. Chronic evolution of infection is associated with an impairment of the adaptive immunity1-3, which can be influenced by innate responses because the innate immune system, in addition to its direct anti-viral activity, can also direct downstream adaptive responses, through a number of different mechanisms2,4. Among them, the natural killer (NK) cell function is critical because NK cells are enriched in the human liver, have cytotoxic potential, secrete cytokines including TNF-α and IFN-γ and can cross-talk with dendritic cells (DCs)5-7. Therefore, they are both anti-viral effectors and also important regulators of adaptive responses through their interplay with dendritic cells8.
NK cells are controlled by combinations of activating and inhibitory receptors9 and the integration of signals derived from these receptors can determine whether the NK cell will become activated. These receptors include the inhibitory killer cell immunoglobulin-like receptors (KIR), as well as the activating natural cytotoxicity receptors and NKG2D10. KIR bind polymorphic MHC class I molecules; in particular, KIR2DL2 and KIR2DL3 bind group 1 HLA-C alleles and the inhibitory receptor KIR2DL1 bind the group 2 HLA-C alleles11. Of relevance to HCV infection is that a specific combination of inhibitory KIR and its MHC class I ligand (KIR2DL3 and HLA-C group 1) is protective against chronic HCV infection12,13. In addition, HLA-C can impact on the maturation of NK cells, because during development NK cells are required to interact with a cognate MHC class I ligand in order to become fully functional14. Thus, the presence or absence of a cognate HLA-C ligand can influence the cytokine response of a KIR-expressing NK cells, and hence the anti-viral immune response.
Cross sectional studies have indicated that NK cells are perturbed in chronic HCV infection. There may be a decrease in the number of circulating NK cells and skewing of NK cell subset distribution towards increased numbers of the cytokine producing CD56bright population, relative to the cytotoxic CD56dim sub-population15,16. This may be due to a defect in IL-15 production17, but may also be consistent with chronic stimulation by IFN-α18. Furthermore, natural cytotoxicity receptor and NKG2D expression may be abnormal and cytokine production by NK cells in chronic HCV is skewed towards production of the Th2 type cytokine IL-10, with impairment of IFN-γ production18-21. The function of NK cells in chronic HCV infection may be directly impaired by the binding of the E2 protein of HCV to CD81, which has an inhibitory function on NK cells22,23. During therapy with IFN-α there is a decrease in CD81 expression on NK cells and there may also be restoration of suppressed NK cell activity24,25.
NK/DC cross-talk is abnormal in chronic HCV infection and this may be due to an abnormality of the NKG2A-expressing sub-population26,27. Furthermore, NK cells are activated by IFN-α and production of this cytokine by plasmacytoid DCs is abnormal in chronic HCV infection28. CD56bright NK cells are increased in chronically infected individuals as compared to those spontaneously clearing infection29. These individuals also have a higher frequency of NKG2A/C expression and CD56bright NK cells produce more IFN-γ. Additionally, in chronic infection NKG2A+ NK cells have been correlated with disease activity30.
Given the lack of information about NK cell responses in acute HCV infection, the aim of the present study was to analyze frequency and functional features of NK cells in the acute phase of HCV infection in order to determine whether NK cells become activated acutely and whether NK cell receptor expression might influence the outcome of acute HCV infection.
MATERIALS AND METHODS
Patients and Virological Assessment
Twenty-two patients with acute HCV infection and seventeen seronegative healthy subjects were enrolled at the Unit of Infectious Diseases and Hepatology of the Azienda Ospedaliero-Universitaria of Parma, Italy. The study was approved by the ethical committee of the Azienda Ospedaliero-Universitaria of Parma. All subjects gave written informed consent. Blood samples were drawn at the time of clinical presentation or 1-2 weeks later for the study of the acute phase of infection, or up to 12 months after clinical presentation (follow-up). Serum HCV-RNA was analysed by qualitative PCR (Cobas Amplicor, Roche Molecular Systems Inc., Branchburg, NJ) and quantified by branched DNA assay (Bayer System 340bDNA Analyzer, Bayer Corporation, Tarrytown, NY). HLA typing was performed as previously described12.
Immunostaining of NK cells
All phenotypic and functional NK cell analyses were performed on frozen and subsequently thawed PBMC. PBMC were incubated with the following Abs: CD56-FITC (BD Biosciences-Pharmingen, San Jose, CA); NKG2D-PE (R&D Systems, Inc., Minneapolis, MN), and CD3-PerCP, CD158b-FITC (KIR2DL2/2DL3/2DS2), CD56-PE-Cy7, CD3-APC-Cy7 (BD Biosciences-Pharmingen), 3DL1-biotin and Streptavidin-PerCP (Abcam, Cambridge, UK), CD158a-APC (KIR2DL1/2DS1; Beckman Coulter, High Wycombe, UK) and analysed on a BD Biosciences flow cytometer (FACSCalibur or FACSCanto).
IFN-γ assays
PBMCs were incubated for 18 hours at 37°C with or without rhIL-12 (Sigma-Aldrich, St.Louis, MO); brefeldin A (Sigma) (10 μg/mL) was added for the last 3 h of incubation. After surface staining with NK cell surface markers and CD3-PerCP mAb, cells were fixed and permeabilised. The cells were then stained with PE-conjugated anti-IFN-γ mAb (Sigma) and analysed on a FACSCalibur. The proportion of IFN-γ producing cells was determined by subtracting the percentage of IFN-γ+ cells in unstimulated samples from IL-12 stimulated samples.
Degranulation assay
PBMC were cultured in the presence or absence of 1 ng/mL rhIL-15 (R&D Systems, Abingdon, UK). After 16 hours, PE-conjugated CD107a or isotype control Ab (BD Biosciences) was added to the PBMC cultures together with K562 target cells at an E:T ratio of 5:1. Following 1 h incubation, GolgiStop was added and 3 hours later cells were harvested and stained for NK cell surface markers. The percentage of degranulating NK cells was obtained by subtracting the isotype control value from the CD107a value.
Chromium release assay
PBMC were incubated overnight with or without 1ng/ml of rhIL-15 (Sigma). K562 target cells (1×106) were incubated with 100 μCi of Na251CrO4 at 37°C for 1 h, washed and cocultured for 4 hrs in triplicate at 3×103 with PBMCs at E:T ratios ranging from 20:1 to 5:1. Killing efficiency was given by dividing the percent lysis values by the percentage of total NK cells, obtained by flow cytometry analysis.
Statistical analysis
The expression of NK cell markers were compared by Student’s T test, Mann-Whitney test, or Wilcoxon matched pairs test, as appropriate. Correlations were evaluated by linear regression analysis and Spearman’s correlation coefficient. Serial analyses of variations in MK cell parameters throughout the follow-up were performed by the repeated measurement ANOVA test.
RESULTS
NK cell proportions are abnormal in patients with acute HCV infection
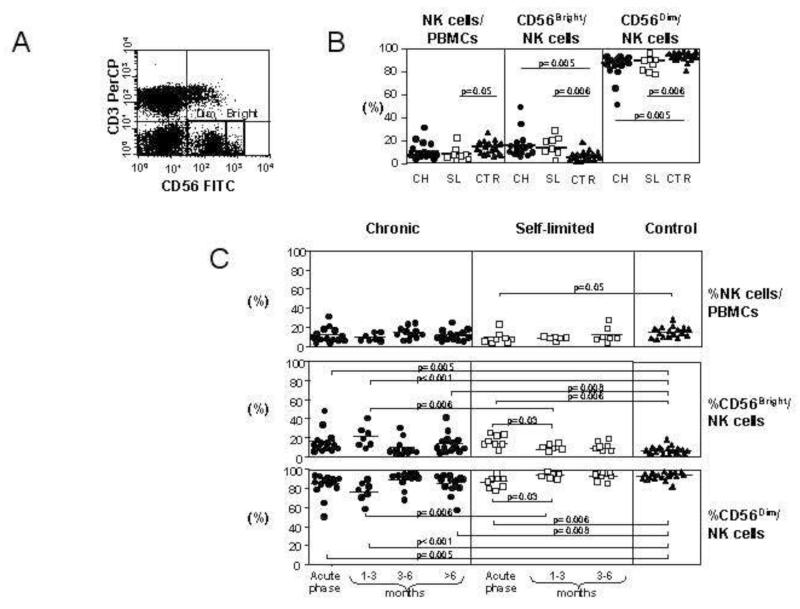
The frequency of NK cells was analyzed in PBMC of 22 Caucasian individuals with acute HCV infection and different outcomes of the disease (Table 1) and in 17 seronegative healthy control subjects. Patients were enrolled and tested at the time of clinical presentation, which coincided with the detection of elevated ALT levels, or 1-2 weeks later when ALT were already declining (Table 1). NK cells were identified by flow cytometry as CD56+CD3− and were further divided into CD56bright and CD56dim sub-populations (Figure 1A). Individuals with acute hepatitis had a lower percentage of NK cells as compared to healthy controls but the difference was statistically significant only between controls and self-limited infections (9.2%±6.1% vs 14.5%±5.8%, p=0.05) (Figure 1B). No significant difference in the percentage of NK cells was detectable between patients with different outcomes of acute HCV infection. Within the NK cell pool, the proportion of CD56bright NK cells was higher in patients with both self-limited (13.1%±6.5%) and chronic disease (16.1%±12.0%) than in control subjects (6.7%±4.1%; p=0.006 and p=0.005, respectively). CD56dim NK cells, were similarly reduced in both groups of patients with acute HCV infection as compared to controls (Figure 1B). Thus, in acute HCV infection the relative proportions of CD56bright and CD56dim NK cells are altered, regardless of outcome. Analysis of serial samples during the convalescent phase of infection showed a decline in CD56bright NK cells in patients with self-limited infection which was already evident 1 to 3 months after the acute phase (from 13.1±6.5 to 6.0±3.2; p=0.03 by Student t test and p=0.0062 by repeated measures ANOVA). This early decline was not detectable in patients with chronic evolution who showed a transient drop of CD56bright NK cells at the 3-6 months time point which was not maintained later (figure 1C). Changes in the opposite direction were observed among CD56dim NK cells that progressively increased in self-limited infection reaching values comparable to those of healthy controls 1-3 months after the acute phase. This behavior was not seen in patients with chronic evolution because the frequency of CD56dim NK cells was still significantly lower than in healthy controls at both 1-3 and >6 months time points (figure 1C).
Table 1. Clinical characteristics of the individuals in the study.
| Patient outcome | SEX | AGE | ALT (at time of lymphocyte collection) | HCV-RNA Copies/mL | Genotype | HLA-C group |
|---|---|---|---|---|---|---|
| Chronic 1 | F | 64 | 417 | 3,100 | 2 | 1,2 |
| Chronic 2 | M | 29 | 875 | 12,897,741 | 2 | 2,2 |
| Chronic 3 | M | 29 | 245 | 16,242 | 3 | 2,2 |
| Chronic 4 | M | 44 | 852 | 451,916 | 1A | 1,2 |
| Chronic 5 | M | 18 | 1114 | 6,775,737 | 3 | 1,1 |
| Chronic 6 | M | 67 | 490 | 10,170 | 2 | 1,1 |
| Chronic 7 | F | 33 | 34 | <3200 | 1 | ND |
| Chronic 8 | M | 57 | 1027 | 1,344,724 | 2 | 1,2 |
| Chronic 9 | M | 25 | 146 | 528,241 | 1A/1B | 1,2 |
| Chronic 10 | M | 22 | 358 | ND | 3 | 2,2 |
| Chronic 11 | M | 22 | 459 | 3,807 | 3 | 1,1 |
| Chronic 12 | F | 41 | 225 | 1,361,225 | 1A | ND |
| Chronic 13 | MM | 39 | 210 | 3,1 998 | 1B | ND |
| Chronic 14 | F | 50 | 78 | ND | 2C | ND |
| Self-limited 1 | F | 50 | 567 | 5,517 | 1 | ND |
| Self-limited 2 | F | 52 | 67 | 1,030,000 | 1 | 1,2 |
| Self-limited 3 | M | 20 | 1299 | <3200 | n/a | 1,2 |
| Self-limited 4 | M | 28 | 152 | 4,957 | n/a | 1,2 |
| Self-limited 5 | M | 18 | 54 | 12,991 | 4 | 1,2 |
| Self-limited 6 | M | 27 | 54 | ND | 1 | 2,2 |
| Self-limited 7 | M | 32 | 940 | 7,355 | 2 | 1,1 |
| Self-limited 8 | F | 25 | 1966 | 240,807 | n/a | 1,2 |
Figure 1. Changes in NK cell subsets during acute HCV infection.
Panel A: representative flow cytometry dot plot of the CD56bright CD3− and CD56dim CD3− populations in acute HCV infection. Panel B: frequency of NK cells as a percentage of the lymphocyte population and the frequencies of CD56bright and CD56dim NK cells as a percentage of the total NK population in 14 chronically evolving patients (CH, circles), 8 self-limited patients (SL, squares) and 17 healthy controls (CTR, triangles). Panel C: changes in NK cells in the follow-up phase of infection. The upper panel shows NK cells as a percentage of the live lymphocyte gate, and the middle and lower panels show the relative proportions of CD56bright (middle) and CD56dim (lower) NK cells.
NKG2D, but not KIR, expression is altered in acute HCV infection
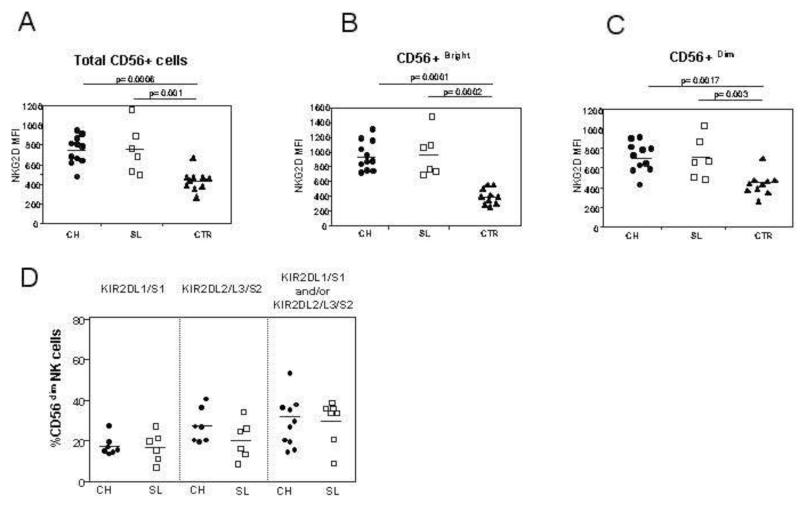
The expression of the activating receptor NKG2D was first analyzed on the surface of NK cells (Figure 2A). It was more highly expressed on total NK cells as well as on CD56bright and CD56dim subsets in patients with acute HCV infection irrespective of the subsequent evolution compared to controls (Figure 2B and C).
Figure 2. NK cell receptor expression in the three patient populations.
Panels A-C: NKG2D expression on total NK cells (A), on CD56bright NK cells (B) and on CD56dim NK cells (C). The expression of these receptors in the acute phase are compared between 11 patients with acute HCV infection and chronic evolution (CH, circles), 6 with self-limited infection (SL, squares) and 10 healthy controls (CTR, triangles). Panel D: percentage of NK cells expressing specific KIR molecules in the acute phase of infection, as determined by GL183 (anti-KIR2DL2/3/S2) and EB6 (anti-KIR2DL1/S1) in individuals with acute HCV infection. The left panel shows the frequency of KIR2DL1/S1 NK cells in individuals with group 2 HLA-C allotypes; the middle panel shows the frequency of KIR2DL2/3/S2 in individuals with group 1 HLA-C alleles and the right panel shows the total number of NK cells expressing a KIR cognate for the HLA-C alleles of the individual (either group1 HLA-C, group 2 HLA-C or both group 1 and group 2 HLA-C).
The level of expression of inhibitory KIR receptors was then analyzed. Current antibodies do not distinguish the inhibitory receptors KIR2DL1 or KIR2DL2 and KIR2DL3 from their activating counterparts KIR2DS1 and KIR2DS2, respectively; so, the combined expression of KIR2DL1 and KIR2DS1, and of KIR2DL2, KIR2DL3 and KIR2DS2, was determined. This was performed on CD56dim NK cells only, as CD56bright NK cells do not express KIR. Furthermore, as the education of NK cells is dependent on the presence of a cognate HLA ligand for the inhibitory KIR, we also determined the HLA-C type of the HCV infected individuals (Table 1). No significant differences in KIR expression were found between individuals with self-limited infection and with chronic evolution, even when corrected for the presence of a cognate HLA-C ligand (Figures 2D). Furthermore, the expression of these receptors on the surface of NK cells did not change after stimulation with IL-12 or IL-15 (data not shown).
IFN-γ production by NK cells is increased in acute hepatitis C virus infection
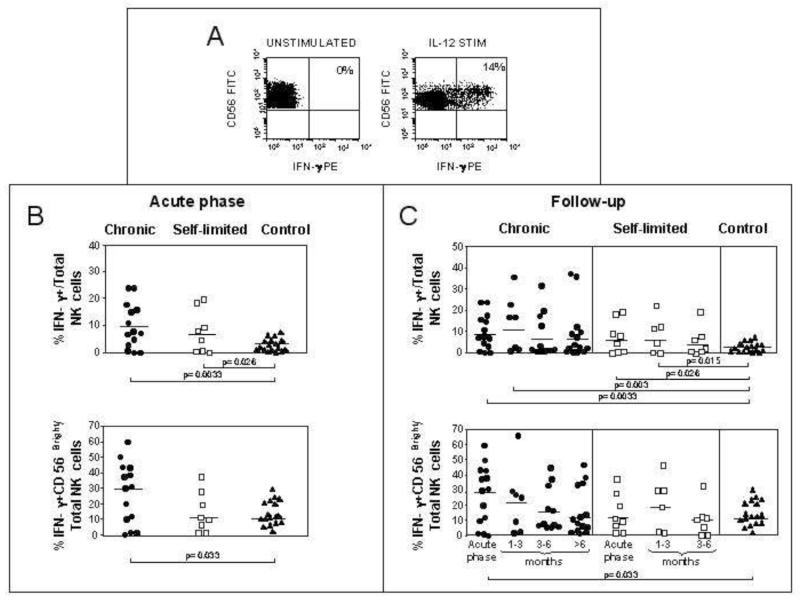
To investigate further the behavior of NK cells during acute HCV infection, we assessed the ability of total and CD56bright NK cells to produce IFN-γ following IL-12 stimulation (Figure 3A). A higher proportion of NK cells produced IFN-γ in patients with both self-limited (8.1%±7.8) and chronically evolving (9.98%±8.3) acute HCV infection as compared to controls (3.28%±2.29; p=0.026 and p=0.0033, respectively) (Figure 3B). The difference with controls was greater in patients with chronically evolving infections, although IFN-γ production in chronic and self-limited evolutions was not statistically different. In line with this trend, the proportion of CD56bright NK cells producing IFN-γ was significantly higher in chronically evolving (27.0%±19.6%) but not in self-limited (14.9±12.7%) infections compared to controls (15.4%±8.0%; p=0.033) (Figure 3B and C). In patients with a chronic course there was a trend towards a decline in IFN-γ secretion by the CD56bright NK cells during the follow-up phase of infection, but this did not reach statistical significance by repeated measures ANOVA (Figure 3C).
Figure 3. Increased production of IFN-γ by NK cells.
PBMCs were incubated overnight with or without IL-12. The cells were then stained with mAbs to detect IFN-γ and identify NK cells (CD56+ CD3−). Panel A: representative flow cytometry dot plots of IFN-γ producing cells following overnight culture with or without IL-12. Panel B: percentage of IFN-γ+ total NK (upper plot) and IFN-γ+ CD56bright NK cells (lower plot) in IL-12 stimulated PBMC in the various patient populations in the acute phase of infection (8 self-limited, 14 chronically evolving, 17 controls). Panel C: IFN-γ production during the follow-up phase of HCV infection in individuals with self-limited and chronic hepatitis on total NK cells and on CD56bright NK cells.
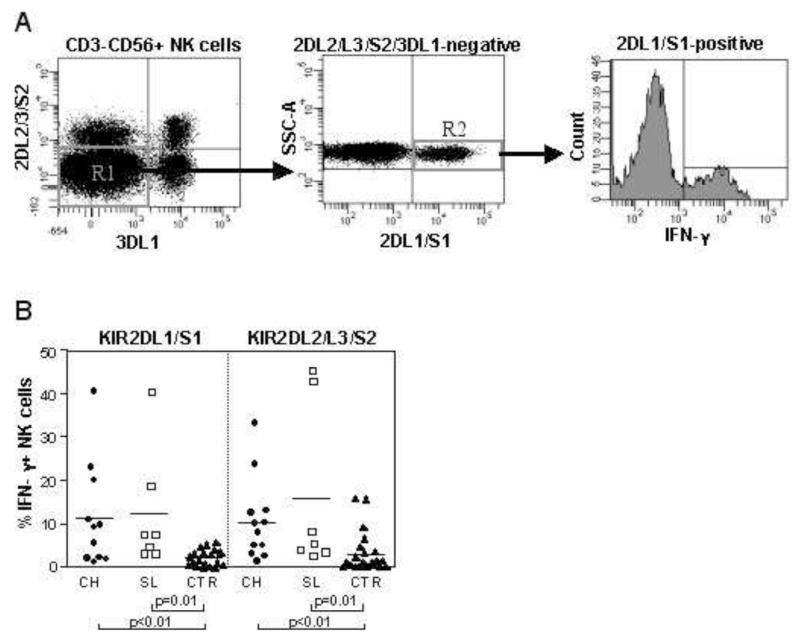
As KIR2DL3 is associated with spontaneous resolution of HCV infection we determined whether this sub-population of NK cells was preferentially activated in acute HCV infection by analysis of IFN-γ production (Figure 4). The inhibitory KIR, KIR2DL1, KIR2DL2, KIR2DL3, and KIR3DL1, are important for determining the ability of NK cells to mature and produce IFN-γ14. Thus, in order to avoid the confounding variable of co-expressing KIR we gated on NK cells that only expressed KIR2DL1/S1 or KIR2DL2/L3/S2 (figure 4A). Both NK cell subsets expressing the HLA-C group 2-specific KIR2DL1/S1 and the HLA-C group 1 specific KIR2DL2/3/S2 showed an increased proportion of IFN-γ secreting cells in both acutely infected patients with a self-limited and a chronic evolution compared to controls (IFN-γ secreting KIR2DL1/S1 NK cells: self-limited, 12.2%±13.63; chronic, 11.32%±12.11 versus controls: 2.1±1.83, p=0.01 and p<0.01, respectively; IFN-γ secreting KIR2DL2/3/S2 NK cells: self-limited, 15.6%±19.3; chronic, 10.36%±9.45 versus controls, 3±4.7, p=0.01 and p<0.01, respectively) (Figure 4B). No significant differences were observed between IFN-γ production by NK cells in individuals with chronic versus self-limited hepatitis.
Figure 4. Activation of KIR+ NK cells during acute HCV infection.
Panel A: representative flow cytometry dot plot to illustrate the gating strategy used to obtain NK cells single positive (SP) for one specific KIR group, in this case KIR2DL1/S1. In this plot CD3− CD56dim NK cells were gated and NK cells negative for both KIR2DL2/L3/S2 and KIR3DL1 were analysed for expression of KIR2DL1/S1. The histogram shows IFN-γ production by these KIR2DL1/S1 positive NK cells. Panel B: percentage of IFN-γ producing NK cells single positive for KIR2DL1/S1 or KIR2DL2/3/S2 in the acute phase of infection in patients with acute hepatitis and healthy controls. Circles depict patients with chronically evolving infection (n=12), squares individuals with self-limited infection (n=7) and triangles represent the controls (n=17).
Increased cytotoxicity of NK cells in acute HCV infection
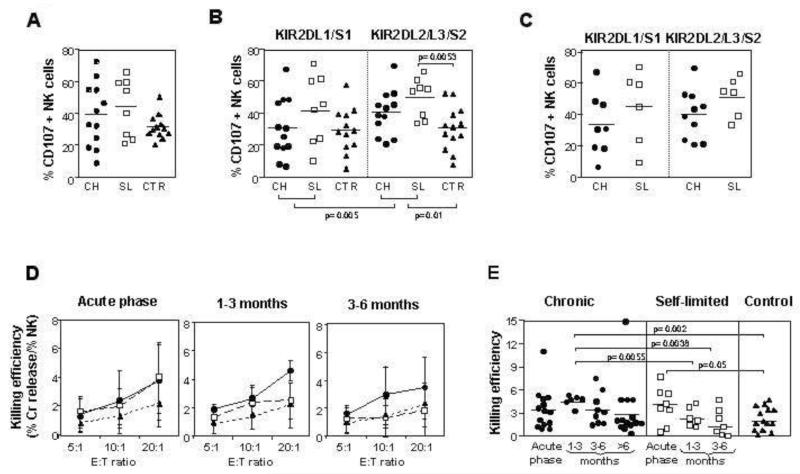
NK cell degranulation was investigated by following the emergence of the lysosomal associated membrane protein CD107a at the surface of NK cells. A trend towards increased activation of NK cells was observed during acute HCV infection as compared to healthy controls (Figure 5A). The difference was significant for NK cells expressing group 1 HLA-C specific KIR2DL2/3 comparing acute HCV patients with controls (44.2%±13.8% versus 29.9%±14.4%, p=0.01) (Figure 5B). In acute patients there was also greater activation of KIR2DL2/3 NK cells as compared to KIR2DL1-positive NK cells (44.2%±13.8% versus 35.0%±20.9%; p=0.005, Wilcoxon matched pairs test) (Figure 5B). Degranulation of KIR2DL2/3 NK cells appeared to be greater in self-limited than in chronically evolving infections. Although this difference was not significant, only KIR2DL2/3 NK cells from self-limited (49.45%±12.25) but not from chronically evolving (40.69±14.06) infections were significantly more active than those from controls (30.96+13.5; p=0.0053, figure 5B).
Figure 5. Up-regulation of NK cell degranulation and cytotoxicity during acute HCV infection.
Degranulation, assessed by surface expression of CD107a, was measured in IL-15 stimulated NK cells following co-culture with K562 target cells. Panel A: the percentage of degranulating CD56dim NK cells, analyzed in the acute phase, is compared between 12 patients with acute HCV infection and chronic evolution, 8 patients with self-limited infection and 11 healthy controls. No statistically significant differences were found between the groups. Panel B: percentage of NK cells single positive for KIR2DL1/S1 or KIR2DL2/3/S2 that express CD107a in the acute phase in individuals with acute hepatitis and in the healthy controls. Circles depict individuals with chronic infection, squares those with self-limited infection and triangles represent the controls. Comparisons by Mann-Whitney U test revealed significant differences in CD107 upregulation between NK cells single positive for KIR2DL2/L3/S2 in the total patient population, as well as in the self-limited group compared with healthy controls. A statistical difference was also detected between NK cells single positive for KIR2DL1/S1 and those single positive for KIR2DL2/L3/S2 in the whole patient population. Panel C: comparison of CD107a expression on KIR2DL1/S1 or KIR2DL2/3/S2 NK cells in the acute phase in HCV infected individuals who have the cognate HLA-C ligand (group 2 and group 1 respectively). Individuals with chronic evolution are depicted with closed circles and those with self-limited hepatitis with open squares. No statistically significant differences were found between the different types of infection. Panel D: cytotoxic activity in the acute and follow-up phases of infection normalised for NK cell number, as tested by 51Cr-release assay against K562 cells. Results are shown for individuals with chronic evolution of acute infection (circles and full line), self-limited infection (squares and dashed line) and 14 healthy controls (triangles and dotted line) at varying effector to target (E:T) ratios. Fourteen individuals with chronic evolution and 8 with self-limited infection were evaluated in the acute phase; 6 in each group at one to three months, and 9 with chronic evolution and 7 with self-limited at three to six months. The mean and standard deviations are shown. Panel E: cytotoxicity against K562 targets normalized for NK cell number in the acute phase and early follow-up phase of infection at the E:T ratio of 20:1. The numbers of patients and controls are the same as those illustrated in panel D, with 14 additional chronic patients analyzed at later follow-up time points.
Cytolytic function of NK cells is determined by the presence or absence of a cognate ligand for inhibitory KIR, a process termed “licensing”14, and KIR2DL3 in combination with its cognate group 1 HLA-C ligand has been shown to be associated with self-limited hepatitis C infection12,13. We therefore analyzed the activation of KIR-positive NK cells by grouping patients based upon expression of the appropriate cognate HLA-C ligand. Although there was a trend towards greater CD107a expression in KIR-positive NK cells in patients with self-limited infection, this was not statistically significant (Figure 5C). We also observed no significant differences in IFN-γ expression on KIR-positive NK cells between patients with self-limited and chronic courses of infection when controlled for the presence of a cognate HLA-C ligand (data not shown).
Cytotoxicity of total NK cells was also tested by the chromium release assay at different E:T ratios. Although NK cells from patients with acute HCV infection generally showed a killing efficiency greater than NK cells from controls, a statistically significant difference was detected only between controls and acute phase of self-limited infections (p=0.05) and between controls and 1-3 months follow-up in chronically evolving patients (p=0.002) (figure 5D and E). Cytotoxicity declined after the acute phase more rapidly in self-limited than in chronic infections (Figure 5D), so that killing efficiency was greater in individuals with a chronic course as compared to those with self-limited infection during follow-up, with a statistically significant difference at early time points (1-3 months follow-up; 4.6±0.7 vs 2.5±1.3, p=0.0055 at an E:T ratio of 20:1) (figure 5E). No significant differences were detected throughout the various time points of the follow-up by the repeated measurement ANOVA in both groups of HCV infected patients.
To test whether HCV-RNA levels could influence NK cell function during acute HCV infection we assessed possible correlations between viremia and killing efficiency (at an E:T ratio of 20:1) or between viremia and the percentage of IFN-γ producing NK cells (data not shown). However, no correlations were observed, suggesting that the level of viremia does not influence the level of activation of the innate immune responses sustained by NK cells.
DISCUSSION
Different mechanisms have been proposed to explain the high rate of chronic HCV persistence. Among them, an insufficient promotion of the adaptive immunity by defective downstream innate responses has been suggested by a number of in vitro studies, showing interference of HCV gene products with the anti-viral function of the innate immunity at different levels1,2, including the inhibition of the NK cell activity by E2 proteins22,23,31. This putative impairment of NK responses may not only affect the initial control of infection directly but may also influence T cell priming by precluding a productive cross-talk between NK and dendritic cells26,27. On the other hand, the mechanisms favoring virus control in HCV infection are still largely undefined. In this context, preferential expression of the inhibitory receptor KIR 2DL3 on NK cells in patients with a self-limited outcome of infection may play a role12,13. Since KIR 2DL3 has a lower affinity for its HLA-C ligand than other KIRs, KIR 2DL3-mediated inhibition of NK cells is inherently weak; this may predispose NK cells from these individuals to be more easily activated by viral infection, thereby protecting them from virus persistence12,13. The final impact of these mechanisms on NK cell function in vivo was to date unknown because studies in acute HCV infection have not been performed so far.
To characterize the behavior of NK cells in acute HCV infection and their contribution to HCV pathogenesis we analyzed frequency, phenotype and functional properties of CD56+ CD3− NK cells and their CD56dim and CD56bright subsets longitudinally in patients with self-limited and chronically evolving acute hepatitis C. The most evident finding is that NK cells in acute HCV infection are functionally more active than NK cells from uninfected healthy controls. This is principally indicated by a more efficient production of IFN-γ following in vitro stimulation with IL12 and by a stronger cytotoxicity in the acute phase (significant in self-limited infections) and in the first 3 months of follow-up (significant in chronically evolving infections). Also the degranulation activity, tended to be stronger in acute patients than in controls, but a significant difference was only observed for KIR2DL2/3. Enhanced NK cell function was detectable in both groups of acute patients with a self-limited and a chronic evolution of infection and was more evident at the early stages of infection (acute phase and 1 to 3 months of follow-up). It was not associated with a parallel increase in the overall NK cell number. However, when we analyzed the expression of the CD56dim and CD56bright subsets, CD56bright cells appeared to be significantly increased and CD56dim significantly reduced in acute patients compared to controls. Thus, the relative representation of NK cell subsets rather than the absolute NK cell number is altered in the acute stage of HCV infection. Moreover, NK cells tended in general to be more active and to remain activated for longer time in chronically evolving than in self-limiting infections, with a slower kinetics of functional decline which was more evident for cytolytic activity. In cross-sectional studies changes in the CD56dim and CD56bright subsets in chronic HCV infection have been previously reported, and our data are consistent with the alterations observed in the acute phase of infection persisting into the chronic phase in patients who do not clear infection16,21,29.
NK cell activity is regulated by a complex interplay between activating and inhibitory cell surface receptors and an altered balance between positive and negative signals released by these receptors is likely to result in NK cell functional changes9. To address this possibility, we looked at the expression of the NKG2D receptor, which is known to mediate NK cell activation by binding stress-inducible class I like molecules (MICA/B) and ULBPs on target cells, and at the expression of the KIR2DL1 and KIR2DL2/3 receptors, which can mediate NK cell inhibition by interacting with HLA-C ligands32. In line with functional data, the activating NKG2D receptor was expressed at higher levels in acute HCV patients than in controls on both CD56dim and CD56bright NK subsets.
In contrast, the expression of inhibitory KIR receptors was not significantly different in the different patient groups. However, a preferential CD107a expression was observed on KIR2DL2/L3/S2-positive, as compared to KIR2DL1/S1-positive NK cells in the acute phase of infection. KIR2DL3 and group 1 HLA-C is protective against chronic HCV infection12,13 as a likely result of stronger inhibitory signals generated by KIR2DL1 than KIR2DL3 with a subsequent slower rate of activation of NK cells expressing KIR2DL1 compared to those expressing KIR2DL333. In our study, however, we have only detected a trend towards a higher degranulation activity in KIR2DL3 positive NK cells from self-limited as compared to chronically evolving infections. The lack of a stronger association between KIR2DL3 and resolution of infection may be due to the limited number of patients that can be recruited for functional studies given the asymptomatic nature of acute HCV infections. Similarly, we had limited power to detect differences between the groups in the cytotoxicity assays during the follow-up phases, due to the difficulty in obtaining samples at defined timepoints. This was in part due to the behavior of the IVDU population, and is a further hindrance to the accurate study of acute HCV infection.
In summary, our study suggests a model of increased NK cell activation in the acute phase of infection irrespective of the subsequent outcome, with a prolonged duration in individuals with chronic evolution of infection. Although HCV has the potential to inhibit the NK cell function by cross-linking the tetraspannin CD81 molecule at the surface of NK cells with its E2 protein22,23, NK cells seem to be efficiently induced during acute HCV infection, because IFN-γ production and cytotoxicity are stronger in acute patients than in controls. This is consistent with the observations of Yoon et al showing that infectious HCV particles generated in cell culture did not impair NK cell function in vitro even when used at concentrations higher than those found in infected individuals34.
Thus, in acute infection HCV does not appear to down-modulate NK cell responses as may be predicted if the HCV-E2 protein had in vivo NK cell inhibitory activity. However, the evidence of a slow but progressive decline of NK cell activity in patients who subsequently progress to chronicity does not exclude the possibility that long-term persistence of HCV may eventually lead to NK cell inhibition resulting from a balance between inhibitory and activating NK cell receptors in favor of inhibition, as reported by previous studies29. NK cells have rarely been studied in acute human viral infections, and so we are unable to determine if our observations are specific for HCV. However, the activation of NK cells during acute HCV infection indicates that they play a role in HCV immunopathogenesis, and that further studies are needed to define their specific contribution to the outcome of infection.
Grant support
This work was supported by the DANA foundation, a Wellcome Trust Senior Clinical Fellowship.
Abbreviations
- DC
dendritic cell
- IL
interleukin
- RIBA
recombinant immunoblot assay
- PCR
polymerase chain reaction
- PBMC
peripheral blood mononuclear cells
- rh
recombinant human
- E:T
effector:target
- ALT
alanine transaminase
Footnotes
Potential conflict of interest: Nothing to report
REFERENCES
- 1.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 2.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen D, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–52. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. Nk cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15:226–33. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 5.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- 6.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moretta A. The dialogue between human natural killer cells and dendritic cells. Curr Opin Immunol. 2005;17:306–11. doi: 10.1016/j.coi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. NK Cell Recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 10.Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol. 2002;3:807–13. doi: 10.1038/ni0902-807. [DOI] [PubMed] [Google Scholar]
- 11.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 12.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O’Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 13.Romero V, Azocar J, Zuniga J, Clavijo OP, Terreros D, Gu X, Husain Z, Chung RT, Amos C, Yunis EJ. Interaction of NK inhibitory receptor genes with HLA-C and MHC class II alleles in Hepatitis C virus infection outcome. Mol Immunol. 2008;45:2429–36. doi: 10.1016/j.molimm.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, Emerson SS, Shuhart MC, Gretch DR. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–80. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 16.Lin AW, Gonzalez SA, Cunningham-Rundles S, Dorante G, Marshall S, Tignor A, Ha C, Jacobson IM, Talal AH. CD56(+dim) and CD56(+bright) cell activation and apoptosis in hepatitis C virus infection. Clin Exp Immunol. 2004;137:408–16. doi: 10.1111/j.1365-2249.2004.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I, Klenerman P, Borrow P. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. Natural Killer Cells are Polarized towards Cytotoxicity in Chronic Hepatitis C in an Interferon-alpha-Dependent Manner. Gastroenterology. 2010;138:325–335. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–55. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 20.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–77. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural Killer Cell Functional Dichotomy in Chronic Hepatitis B and Chronic Hepatitis C Virus Infections. Gastroenterology. 2009;137:1151–60. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Crotta S, Stilla A, Wack A, D’Andrea A, Nuti S, D’Oro U, Mosca M, Filliponi F, Brunetto RM, Bonino F, Abrignani S, Valiante NM. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronenberger B, Herrmann E, Hofmann WP, Wedemeyer H, Sester M, Mihm U, Ghaliai T, Zeuzem S, Sarrazin C. Dynamics of CD81 expression on lymphocyte subsets during interferon-alpha-based antiviral treatment of patients with chronic hepatitis C. J Leukoc Biol. 2006;80:298–308. doi: 10.1189/jlb.0106047. [DOI] [PubMed] [Google Scholar]
- 25.Bonavita MS, Franco A, Paroli M, Santilio I, Benvenuto R, De Petrillo G, Levrero M, Perrone A, Balsano C, Barnaba V. Normalization of depressed natural killer activity after interferon-alpha therapy is associated with a low frequency of relapse in patients with chronic hepatitis C. Int J Tissue React. 1993;15:11–6. [PubMed] [Google Scholar]
- 26.Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative Regulation of NK Cell Activities by Inhibitory Receptor CD94/NKG2A Leads to Altered NK Cell-Induced Modulation of Dendritic Cell Functions in Chronic Hepatitis C Virus Infection. J Immunol. 2004;173:6072–81. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 27.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol. 2005;43:1013–20. doi: 10.1016/j.jhep.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–68. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 29.Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, O’Farrelly C, Doherty DG. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–8. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 30.Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N, Dariz A, Guillermet C, Pernollet M, Zarski JP, Marche PN, Leroy V, Jouvin-Marche E. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol. 2009;51:458–67. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Sklan EH, Charuworn P, Pang PS, Glenn JS. Mechanisms of HCV survival in the host. Nat Rev Gastroenterol Hepatol. 2009;6:217–27. doi: 10.1038/nrgastro.2009.32. [DOI] [PubMed] [Google Scholar]
- 32.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahlenstiel G, Martin MP, Gao X, Carrington M, Rehermann B. Distinct KIR/HLA compound genotypes affect the kinetics of human antiviral natural killer cell responses. J Clin Invest. 2008;118:1017–26. doi: 10.1172/JCI32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]