Abstract
Background:
Over the past few years, there has been growing interest in assessing the relationship between exposure to radon at home and the risk of childhood cancer. Previous studies have produced conflicting results, probably because of limitations assessing radon exposure, too few cancer cases and poorly documented health statistics.
Methods:
We used a cohort approach of 0–15-year-old children to examine whether residential radon exposure was associated with childhood leukaemia and cancer in the central nervous system in the Oslo region. The study was based on Norwegian population registers and identified cancer cases from The Cancer Registry of Norway. The residence of every child was geo-coded and assigned a radon exposure.
Results:
In all, 712 674 children were followed from 1967 to 2009 from birth to date of cancer diagnosis, death, emigration or 15 years of age. A total of 864 cancer cases were identified, 437 children got leukaemia and 427 got cancer in the central nervous system.
Conclusions or interpretation:
No association was found for childhood leukaemia. An elevated nonsignificant risk for cancer in the central nervous system was observed. This association should be interpreted with caution owing to the crude exposure assessment and possibilities of confounding.
Keywords: leukaemia, CNS cancer, radon, childhood, population-based study
An association between leukaemia and medical radiation exposure was identified more than 60 years ago (Cour-Brown and Doll, 1957). Since the 1990s it has been hypothesised that radon inhalation may deliver a small amount of irradiation to the red bone marrow, and consequently may increase the risk of leukaemia among children. The causes of childhood leukaemia and cancer in the central nervous system (CNS) are poorly understood (Idowu and Idowu, 2008; Eden, 2010). Moderate to high doses of ionising radiation are an established environmental risk factor for cancer in the CNS, but exposures to low-level radiation are not well characterised (Braganza et al, 2012).
A recent meta-analysis summarised data from ecological and case–control studies on radon exposure and childhood leukaemia, showing an increase in leukaemia with increasing radon exposure (Tong et al, 2012). Since 1987 eight case–control studies and a cohort study have been reported. Studies from the United States/Canada (Lubin et al, 1998; Steinbuch et al, 1999), Germany (Kaletsch et al, 1999), Japan (Yoshinaga et al, 2005) and the United Kingdom (UK Childhood Cancer Study Investigators, 2002a; Kendall et al, 2013) and an even more recent cohort study did not find any association between radon exposure and childhood cancer including leukaemia and CNS cancers (Hauri et al, 2013) Contrary to this, studies from Egypt (Maged et al, 2000) and Denmark (Raaschou-Nielsen et al, 2008) found an association with leukaemia. Authors of two British studies showed the problems associated with assessing radon exposure (UK Childhood Cancer Study Investigators, 2002a, 2002b). Some of the limitations in many of the previous studies include too few cancer cases and limited documented health statistics, especially organ-specific cancer rates (Brenner et al, 2003).
Norway has one of the highest incidences of childhood cancer in the world (Stiller et al, 2006; Schmidt et al, 2011), and one of the highest indoor radon concentrations (Stranden, 1986; Stigum et al, 2003) with an average level of radon in Norwegian dwellings around 90 Bq m−3 (Swedish Radiation Safety Authority et al, 2009). Because of its geologic conditions, the Oslo area has a wide variation in indoor radon exposure. In addition, the cold climate leads to limited ventilation in the winter season. This in combination with good cancer and population records makes Norway a suitable ground for studies of this type.
The purpose of the present paper was to test the hypothesis that children who grow up in homes with elevated radon concentrations have increased risk of developing leukaemia or cancer in the CNS.
Materials and methods
Description of the cohort
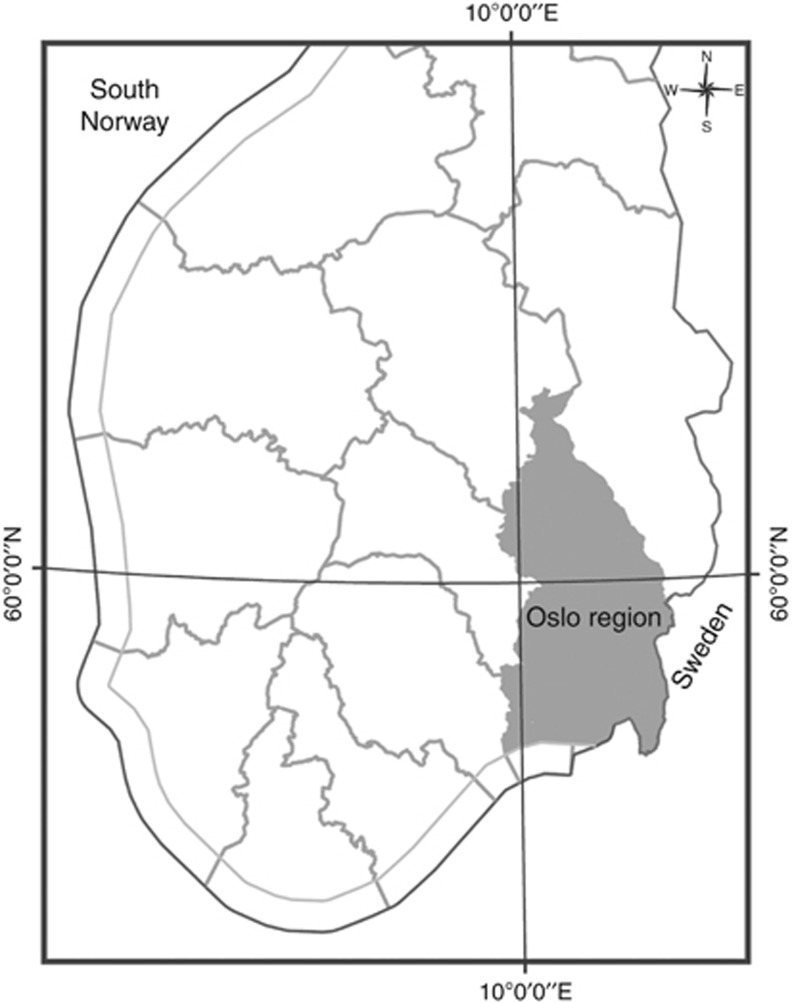
This study includes all children born alive in the counties of Oslo, Akershus, Vestfold and Østfold, together with the municipalities of Gran, Jevnaker, Lunner, Lillehammer, Gjøvik, Vestre Toten, Østre Toten, Søndre Land, Ringerike, Hole, Lier, Nedre Eiker, Røyken, Drammen and Hurum, which are situated in the geologic Oslo area (Figure 1), from 1 January 1967 to December 2009.
Figure 1.
The study region in south central Norway contains 4 counties, 15 municipalities and is referred to as the Oslo region in this study.
The sample was identified from The Norwegian Population Register. Everyone registered as a resident in Norway is assigned a unique Norwegian personal ID number that allows linkage to several nationwide population-based registers.
From The Medical Birth Register of Norway, we obtained date of birth, sex, birth weight, gestational age, congenital malformations and date of death. We defined preterm birth as being born before gestational week 37. For the mother, we obtained year of birth, parity and complications during pregnancy, including haemorrhage and hypertensive condition. Parent's highest educational level and annual income data were obtained from other registers (Statistics Norway, 2006).
Cases
Cases were children diagnosed with leukaemia or cancer in the CNS, before 15 years of age between 1 January 1967 and 31 December 2009, classified according to the International Classification of Diseases of Oncology (April Fritz et al, 2000). Information on cases was obtained from The Cancer Registry of Norway, which has been considered to be very close to complete (Larsen et al, 2009).
Residential history
We had accurate information on addresses at 1 January 1980 and each year from 1987 to 2009. In addition, we had addresses from a population census in 1970. Addresses at the 1970 census were based on residential area. All other addresses were based on exact numeric addresses. Moving history between municipalities was available from 1 January 1981. Between 1984 and 1986, all children in the cohort were regarded as living in the same place as in January 1987 and between 1975 and 1983 at the same address as in January 1980. For children in the cohort between 1967 and 1974 addresses from the census in 1970 were used. Children born before 1987 had at least information on two addresses in the follow-up period. For children with missing information on radon exposure in some of the years of the follow-up period and in the absence of moving to another municipality the time-weighted average concentration for the part of the childhood period with know radon concentration was used as a best estimate. If the child moved out of the study area, it was given the known radon concentration of the years the child lived in the study area.
Exposure assessment
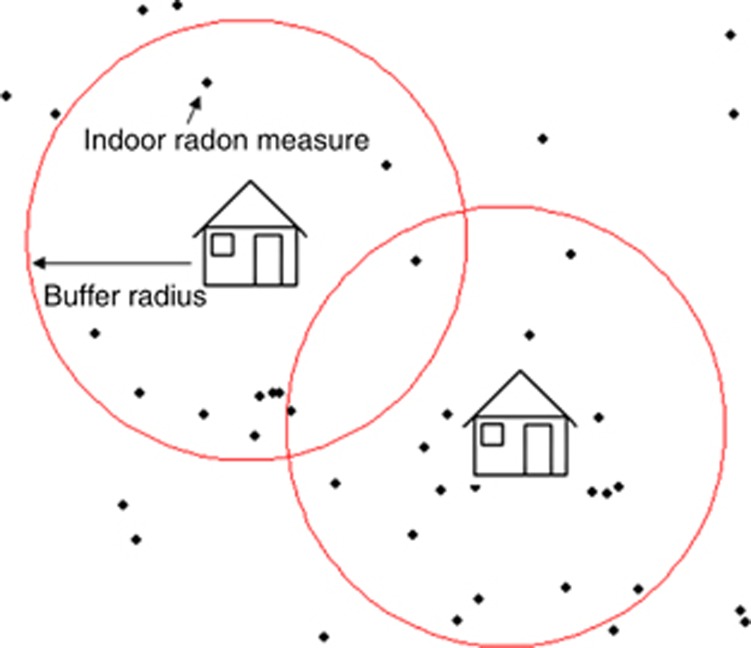
We had on-site indoor radon measurements only for 6% of the residences in the study region. Therefore, we used a buffer model with different radius size to estimate radon exposure to the rest of the cohort (Figure 2). The model was based on 41 515 indoor radon measurements. When this was missing, indoor radon measurements from at least five dwellings found around each non-measured unit were used to estimate a radon value (geometric mean). In all, 53.7% of these dwellings were assigned a radon mean from five or more measured dwellings found in a radius of 300 m. For 17.9% we had to increase the radius to 500 m to find at least five measured dwellings, for 15.5% the radius was 1000 m and for 13.0% 2000 m. If we could not find five dwellings inside a radius of 2000 m around an unmeasured dwelling, the house got the same value as the closest measured dwelling. This method is explained in more detail elsewhere (Kollerud et al, 2014). Residential areas identified from the population census in 1970 were assigned radon mean concentrations found in their actual residential area. In addition, 45.5% of the houses occupied by children born after 1986 had information on level of radon hazard: low, medium or high. The radon hazard categories were based on distribution of uranium-rich rock types, uranium concentrations in the ground and permeable drift deposits found at each coordinate point. Rocks like alum shale and granite are associated with elevated levels of radon in indoor air, and sand and gravel can permit the transport of radon from large ground volumes into dwellings. The presence of these kinds of deposits in the substrates of dwellings is generally regarded as a high radon hazard factor (Smethurst et al, 2008).
Figure 2.
The house in the centre represents a dwelling without radon value, and the circle is the associated buffer. Black points represent dwellings with indoor radon measurements.
Radon exposure was divided into tertiles (1–56.8, 56.9–93.2 and 93.3–6315 Bq m−3), which were rounded to <50, 50–100 and >100 Bq m−3. We also grouped exposure into <100, 100–199 and ⩾200 Bq m−3, according to national and international recommendations (Norwegian Radiation Protection Authority, 2009; Zeeb and Shannoun, 2009). To explore a possible exposure–response hypothesis, we analysed radon exposure as a continuous variable grouped into five groups by increments of 100 Bq m−3.
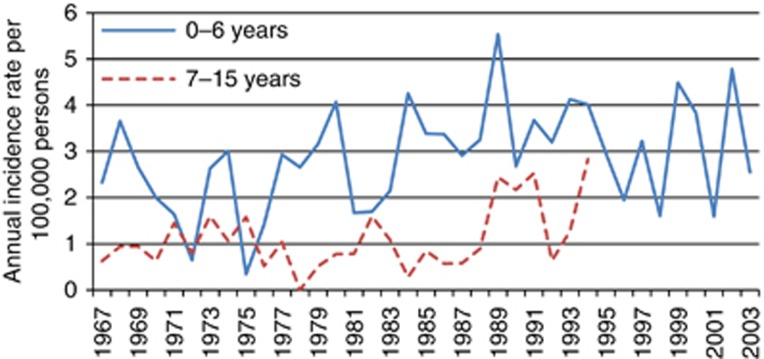
Exposure was estimated each year from birth to whatever came first: the age of 15 years, the date of diagnosis of leukaemia or cancer in the CNS, emigration, death or the end of the follow-up period. More specific information on exposure windows may improve the risk assessment by identifying the most sensitive windows for evaluation of radon exposure in children (Selevan et al, 2000). We analysed radon mean concentration in three exposure periods: at birth, in the first 4 years of life and in the whole follow-up period. Because of high incidence of especially leukaemia among the youngest children (Figure 3), we did not find use of cumulative exposure suitable to use in our material.
Figure 3.
Annual incidence of leukaemia across age groups in 1967–2003.
Statistics
Logistic regression was used to analyse the cancer types according to exposure during the first year after birth, whereas Cox proportional hazard models were used to analyse time to event of the two cancer types after exposure for 0–4 years and during whole study period (0–15 years). We used logistic regression to analyses cancers in the first year of life as there was not time aspect involved and Cox regression later to take into account the time to diagnosis. Radon exposure and cancer are independent observations, and the incidence of cancer in children was relatively constant across time. Figures 3 and 4 show full age ranges plotted against year of diagnosis. We used two models for adjusting, first, adjusted for paternal and maternal education and annual family income (Model I; Table 2). Then, we added the child's sex, birth weight, congenital malformations and parity to the model (Model II). Preterm birth, mother's age and complications during pregnancy had no significant effect on the estimates and were excluded in the regressions. Analyses were run in SPSS version 20 (IBM, Armonk, NY, USA).
Figure 4.
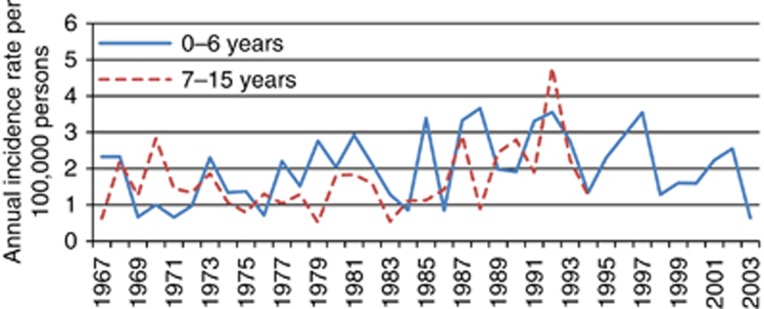
Annual incidence of cancer in the CNS across age groups in 1967–2003.
Table 2. Crude and adjusted OR and HR (95% confidence interval) for all leukaemia, acute lymphoblastic leukaemia, CNS cancers and both types across radon exposure level and age in two models across age groups.
| |
|
<1 year |
0–4 years |
0–15 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | Radon (Bq m−3) | No of cases | Crude OR | Model I OR | Model II OR | No. of cases | Crude HR | Model I HR | Model II HR | No of cases | Crude HR | Model I HR | Model II HR |
| All lleukaemia |
<50 |
99 |
Ref. |
Ref. |
Ref. |
99 |
Ref. |
Ref. |
Ref. |
95 |
Ref. |
Ref. |
Ref. |
| |
50–100 |
205 |
1.21 (0.95–1.54) |
1.15 (0.90–1.48) |
1.12 (0.88–1.44) |
207 |
1.12 (0.88–1.42) |
1.07 (0.84–1.37) |
1.04 (0.81–1.34) |
209 |
1.05 (0.82–1.33) |
1.00 (0.78–1.29) |
0.97 (0.76–1.25) |
| |
>100 |
127 |
1.18 (0.91–1.55) |
1.13 (0.87–1.48) |
1.11 (0.85–1.46) |
125 |
1.10 (0.84–1.43) |
1.07 (0.82–1.40) |
1.05 (0.800–1.37) |
127 |
1.06 (0.82–1.39 |
1.03 (0.79–1.36) |
1.02 (0.78–1.34) |
| |
Per 100 Bq m−3a |
|
1.00 (0.87–1.14) |
0.99 (0.87–1.14) |
1.00 (0.87–1.14) |
|
0.99 (0.86–1.13) |
0.99 (0.86–1.13) |
0.99 (0.86–1.14) |
|
0.99 (0.86–1.13) |
0.99 (0.86–1.14) |
1.00 (0.87–1.15) |
| Acute lymphoblastic leukaemia |
<50 |
79 |
Ref. |
Ref. |
Ref. |
78 |
Ref. |
Ref. |
Ref. |
74 |
Ref. |
Ref. |
Ref. |
| |
50–100 |
153 |
1.13 (0.86–1.49) |
1.08 (0.82–1.43) |
1.03 (0.78–1.36) |
155 |
1.06 (0.81–1.39) |
1.02 (0.77–1.35) |
0.97 (0.74–1.29) |
157 |
1.01 (0.76–1.33) |
0.97 (0.73–1.28) |
0.93 (0.70–1.23) |
| |
100 |
92 |
1.07 (0.80–1.46) |
1.04 (0.77–1.41) |
1.02 (0.75–1.39) |
91 |
1.02 (0.75–1.37) |
0.98 (0.72–1.34) |
1.00 (0.70–1.31) |
93 |
1.00 (0.74–1.36) |
0.70 (0.71–1.32) |
0.95 (0.69–1.29) |
| |
Per 100 Bq m−3a |
|
0.97 (0.83–1.14) |
0.97 (0.83–1.14) |
0.98 (0.83–1.15) |
|
0.96 (0.82–1.13) |
0.96 (0.23–1.13) |
0.97 (0.82–1.14) |
|
0.97 (0.82–1.14) |
0.97 (0.82–1.14) |
0.97 (0.83–1.15) |
| CNS cancer |
<50 |
100 |
Ref. |
Ref. |
Ref. |
98 |
Ref. |
Ref. |
Ref. |
92 |
Ref. |
Ref. |
Ref. |
| |
50–100 |
172 |
1.00 (0.79–1.29) |
0.95 (0.74–1.23) |
0.96 (0.74–1.24) |
174 |
0.95 (0.74–1.22) |
0.90 (0.70–1.16) |
0.90 (0.69–1.16) |
182 |
0.94 (0.73–1.21) |
0.88 (0.68–1.14) |
0.88 (0.68–1.14) |
| |
>100 |
145 |
1.34 (1.04–1.73) |
1.25 (0.96–1.62) |
1.27 (0.97–1.65) |
145 |
1.29 (1.00–1.66) |
1.22 (0.94–1.59) |
1.21 (0.93–1.58) |
143 |
1.23 (0.95–1.60) |
1.16 (0.89–1.51) |
1.15 (0.87–1.50) |
| |
Per 100 Bq m−3a |
|
1.17 (1.04–1.32) |
1.14 (1.00–1.29) |
1.13 (1.00–1.29) |
|
1.17 (1.04–1.32) |
1.14 (1.00–1.29) |
1.14 (1.00–1.29) |
|
1.15 (1.02–1.30) |
1.12 (0.99–1.27) |
1.13 (0.99–1.28) |
| Leukaemia and CNS cancer |
<50 |
199 |
Ref. |
Ref. |
Ref. |
197 |
Ref. |
Ref. |
Ref. |
187 |
Ref. |
Ref. |
Ref. |
| |
50–100 |
377 |
1.10 (0.93–1.32) |
1.05 (0.88–1.26) |
1.04 (0.87–1.25) |
381 |
1.03 (0.87–1.23) |
0.99 (0.83–1.18) |
0.97 (0.81–1.16) |
391 |
0.99 (0.83–1.18) |
0.94 (0.79–1.13) |
0.93 (0.78–1.11) |
| |
>100 |
272 |
1.26 (1.05–1.52) |
1.19 (0.99–1.44) |
1.19 (0.98–1.44) |
270 |
1.19 (0.99–1.43) |
1.15 (0.95–1.38) |
1.13 (0.93–1.36) |
270 |
1.15 (0.95–1.38) |
1.10 (0.91–1.32) |
1.08 (0.89–1.31) |
| Per 100 Bq m−3a | 1.09 (0.99–1.19) | 1.07 (0.98–1.17) | 1.07 (0.97–1.17) | 1.08 (0.99–1.18) | 1.08 (0.81–1.15) | 1.07 (0.97–1.17) | 1.07 (0.98–1.17) | 1.06 (0.96–1.16) | 1.06 (0.97–1.17) | ||||
Abbreviations: HR=hazard ratio; OR=odds ratio.
Model I is adjusted for socioeconomic status (family income, mother and father's level of education).
Model II is adjusted for all variables (parity, birth weight, sex, congenital malformations, family income, mother and father's level of education).
Grouped into five groups by increments of 100 Bq m−3.
We had more detailed residential information for the time period from 1987 including type of dwelling and radon risk category. However, we lacked information on some factors that might influence the radon concentration in the dwellings such as floor number and building materials. Radon concentrations may be lower on high level floors compared with low-level floors. Building materials may be a less important factor (Zeeb and Shannoun, 2009). Thus, we performed separate subanalyses for children born in 1987 or later (Table 3). First, we included only dwellings with information of numeric address, excluding children living in high apartment buildings. Second, we analysed children living in detached and semidetached dwellings. Third, we only included children living in houses with on-site indoor radon measurements. Finally, we performed an analysis of radon exposure adjusted by the level of radon hazard.
Table 3. Subanalyses of the associations between household radon concentration and childhood cancer by diagnostic group (adjusted HRs and 95% confidence interval) for children of age 0–15 years and born 1987 or later.
| |
Leukaemia |
CNS |
||
|---|---|---|---|---|
| Radon (Bq m−3) | No. of cases | Adjusted HR | No. of cases | Adjusted HR |
|
All children excluding children living in high apartment buildings | ||||
| <50 | 30 | Ref. | 30 | Ref. |
| 50–100 | 77 | 0.89 (0.58–1.36) | 62 | 0.77 (0.49–1.21) |
| >100 | 55 | 1.00 (0.64–1.56) | 55 | 0.94 (0.58–1.51) |
| Per 100 Bq m−3a |
162 |
0.97 (0.78–1.20) |
147 |
1.04 (0.84–1.30) |
|
Children who lived in detached and semidetached houses | ||||
| <50 | 30 | Ref. | 30 | Ref. |
| 50–100 | 77 | 0.90 (0.58–1.40) | 62 | 0.75 (0.47–1.19) |
| >100 | 55 | 1.04 (0.66–1.65) | 55 | 0.92 (0.57–1.48) |
| Per 100 Bq m−3a |
162 |
0.98 (0.79–1.22) |
147 |
1.04 (0.83–1.30) |
|
Children who lived in houses with on-site indoor radon measurements | ||||
| ⩽100 | 10 | Ref. | 7 | Ref. |
| >100 | 5 | 0.90 (0.31–2.66) | 6 | 2.33 (0.70–7.69) |
|
aPer 100 Bq m−3 |
15 |
0.91 (0.54–1.55) |
13 |
1.36 (0.87–2.11) |
|
Total children born 1987 or later | ||||
| <50 | 49 | Ref. | 50 | Ref. |
| 50–100 | 118 | 0.99 (0.70–1.40) | 104 | 0.86 (0.60–1.22) |
| >100 | 69 | 0.99 (0.67–1.44) | 81 | 1.07 (0.74–1.55) |
| Per 100 Bq m−3a | 236 | 0.93 (0.76–1.13) | 235 | 1.09 (0.92–1.29) |
Abbreviations: CNS, central nervous system; HR, hazard ratio.
Grouped into five groups by increments of 100 Bq m−3.
Adjusted for parity, birth weight, sex, congenital malformations, family income, mother and father's level of education.
The project was approved by the Norwegian Data Protection Authority and the Regional Committees for Medical and Health Research Ethics.
Results
The cohort consisted of 712 674 children 0–15 years of age born between 1967 and 2009 (Table 1). A total of 864 cancer cases were identified, 437 children with leukaemia and 427 children with cancer in the CNS. Highest incidence rates were found among the youngest children, especially for leukaemia. The study had relatively many cases for each value of the independent variables.
Table 1. Distribution of 864 children with leukaemia or cancer in the CNS and corresponding person-years at risk across age in a population-based cohort of 712 674 children born in the Oslo region, Norway, in 1967–2009.
| |
Number of cases leukaemia (n=437) |
Number of cases CNS (n=427) |
Person-years at risk |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 0–4 | 5–15 | Total | 0–4 | 5–15 | Total | 0–4 | 5–15 | Total |
|
Sex | |||||||||
| Male | 142 | 99 | 241 | 97 | 128 | 225 | 162 973 | 4 370 205 | 4 533 178 |
| Female |
123 |
73 |
196 |
95 |
107 |
202 |
151 905 |
4 171 404 |
4 323 309 |
|
Mother's age (years) | |||||||||
| <35 | 215 | 153 | 368 | 166 | 194 | 360 | 234 614 | 5 900 368 | 7 547 476 |
| ⩾35 |
44 |
14 |
58 |
22 |
26 |
48 |
71 094 |
556 160 |
940 820 |
|
Parity | |||||||||
| No child | 109 | 65 | 174 | 88 | 87 | 175 | 37 695 | 3 564 454 | 3 670 691 |
| 1 Child | 86 | 58 | 144 | 63 | 82 | 145 | 44 070 | 2 940 098 | 3 057 787 |
| ⩾2 Children |
59 |
42 |
101 |
34 |
50 |
84 |
29 564 |
1 629 533 |
1 699 742 |
|
Birth weight (g) | |||||||||
| <4000 | 203 | 127 | 330 | 151 | 177 | 328 | 249 615 | 6 659 616 | 6 909 231 |
| ⩾4000 |
56 |
39 |
95 |
37 |
43 |
80 |
55 668 |
1 512 785 |
1 568 453 |
|
Congenital malformations | |||||||||
| Yes | 23 | 7 | 30 | 23 | 13 | 36 | 16 999 | 278 497 | 295 496 |
| No |
242 |
165 |
407 |
169 |
222 |
391 |
297 879 |
8 263 112 |
8 560 991 |
|
Complications during pregnancy | |||||||||
|
Haemorrhage | |||||||||
| Yes | 9 | 7 | 16 | 2 | 8 | 10 | 5905 | 14 962 | 20 867 |
| No |
256 |
165 |
421 |
190 |
227 |
417 |
112 773 |
579 034 |
691 807 |
|
Hypertension | |||||||||
| Yes | 10 | 8 | 18 | 10 | 7 | 17 | 5441 | 23 879 | 29 320 |
| No |
255 |
164 |
419 |
182 |
228 |
410 |
113 237 |
570 117 |
683 354 |
|
Maternal education (years) | |||||||||
| <10 | 62 | 44 | 106 | 34 | 58 | 92 | 53 826 | 2 007 801 | 2 061 627 |
| 10–12 | 99 | 68 | 167 | 72 | 91 | 163 | 82 480 | 3 274 549 | 3 357 029 |
| >12 |
97 |
58 |
155 |
80 |
84 |
164 |
160 468 |
3 058 493 |
3 218 961 |
|
Paternal education (years) | |||||||||
| <10 | 56 | 36 | 92 | 41 | 46 | 87 | 53 780 | 1 828 921 | 1 882 701 |
| 10–12 | 102 | 79 | 181 | 71 | 95 | 166 | 97 632 | 3 411 785 | 3 509 417 |
| >12 |
96 |
54 |
150 |
72 |
90 |
162 |
142 101 |
3 022 737 |
3 164 838 |
|
Annual family income | |||||||||
| Low | 102 | 64 | 166 | 59 | 75 | 134 | 90 388 | 2 846 697 | 2 937 085 |
| Medium | 85 | 56 | 141 | 65 | 75 | 140 | 94 804 | 2 869 245 | 2 964 049 |
| High | 77 | 51 | 128 | 68 | 82 | 150 | 127 869 | 2 718 325 | 2 846 194 |
Abbreviations: CNS=central nervous system; HR, hazard ratio.
Radon exposure assessment was available for 98.7% of cases of leukaemia and for 97.7% of CNS cancer cases. In all, 5.7% of the children, for whom a radon exposure was not obtained and could not be reconstructed, were excluded.
Geometric mean radon concentration for leukaemia was 89.1 Bq m−3 with a median of 75.6 Bq m−3. For cancers in the CNS, the radon mean was 100.2 Bq m−3 and the median was 77.9 Bq m−3. The mean radon concentration for the whole cohort was 91 Bq m−3 and the median was 74 Bq m−3. These differences were not statistically significant. Thus, mean radon concentration was similar across cases and in the whole cohort.
Table 2 shows crude and adjusted odds and hazard ratios for leukaemia and cancer in the CNS in the different radon exposure categories. There were only a few marginal changes in the effect estimates across the various models. We observed a small increased risk of both cancers among children under 1 year of age in the highest radon exposure group compared with the controls (crude OR=1.26; 95% CI: 1.05–1.52), and similar results for cancer in the CNS (OR=1.34; 95% CI: 1.04–1.73). We also observed a borderline increased risk among children when only taking into account the exposure during the first 0–4 years of life in the highest radon exposure group (crude HR=1.29; 95% CI: 1.00–1.66). When adjusted for socioeconomic and birth variables (Model I or II), the observed increased risk was no longer statistically significant at the 5% level. Hazard ratios were also estimated with radon exposure as a continuous variable to explore a possible linear exposure response. We observed an increase of 17% for CNS cancers for each 100 Bq m−3 increase in exposure when only taking into account the exposure during the first 0–4 years of life. It was only borderline significant after adjustments (Table 2). No association was observed when radon exposure was classified in just three categories <100, 100–199 and ⩾200 Bq m−3.
Table 3 shows the analyses regarding type of house for children 0–15 years old born after 1987 and living in homes we believed had the most accurate exposure assessment. These subanalyses showed the same results as the main analyses in Table 2 for leukaemia. For CNS cancer, however, among children living in houses with on-site measurements, we observed a nonsignificant 36% increased risk for every 100 Bq m−3 increase in exposure and an increased risk of 2.33 (0.70–7.69) in houses with radon concentrations above 100 Bq m−3. A total of 27 186 children (cases=28) with available data on results from radon measurements taken in living rooms were also analysed separately with similar results, that is, no association was observed.
We performed a subanalysis taking into account mean radon exposure in the follow-up period and level of radon hazard of the houses occupied by the children in the first year of life. No association was observed (leukaemia–n=118, HR=0.99, 95% CI=0.76–1–30; and CNS cancer–n=104, HR=1.08, 95%CI=0.83–1.40).
Discussion
This study offers no support for the hypothesis that elevated indoor radon concentration is associated with childhood leukaemia. The observed association between childhood radon exposure and cancer in the CNS was no longer statistically significant after adjustment for socioeconomic and birth variables. Results from four subanalyses were consistent with the main results. However, we observed a nonsignificant increased risk of CNS cancer in homes with on-site radon measurements and during time periods with more accurate address information. We conducted several analyses concerning age of the child and radon exposure as certain types of cancer such as leukaemia may have peaks between 2 and 5 years. No association was found in the different time periods.
Strength and limitations
A major strength of this study is the prospective cohort approach and the quality of data on cancer diagnoses and multiple risk factors, which allowed us to adjust for several conditions that may confound the association between childhood cancer and radon exposure. To our knowledge, this study is the second cohort study reported in the literature, and it is the first cohort study following radon exposure for all children from birth.
Additionally, there was available residential history for children in the majority of the years of follow-up and a detailed residential history for children born after 1987. This allows us to have a more accurate estimate of the exposure. Exposure during pregnancy is important because foetuses and young children may be more sensitive to radiation owing to immature biologic response (Kim et al, 2006), and residential address at birth is the best available proxy for the place of residence during pregnancy. In this way, we may have a surrogate estimate of radon exposure for mothers during pregnancy.
Another advantage of this study is the variation of radon exposure in the study area (Kollerud et al, 2014). Mean radon concentration is the highest reported in analytical studies so far. It is important to note that if exposures tend to be homogeneous or have low variability their effects might be underestimated or even completely obscured (Rothman et al, 2008). The high heterogeneity of radon exposure may improve the statistical power of the present study.
We used a model to estimate radon exposure in as short distances as possible around each unmeasured building. Only two other studies used such radon density measurements, to characterise individual radon exposure within small geographical areas (Hauri et al, 2013; Kendall et al, 2013). When estimating radon concentrations with radon measurements taken in the same area as the unmeasured buildings, it is more likely that the dwellings share important factors associated with radon concentration, such as geology and ground permeability. It is also likely that radon estimates from this method might express similarities in other factors influencing indoor radon concentrations such as building styles and living habits.
An important limitation is the potential for exposure misclassification which in this cohort study may lead to a reduction in the estimated ORs. The modelling of indoor radon estimates used in this study is based on mean radon values found within circles around each building. The uncertainty of the radon value increases with the increasing radius. Radon values from the buffers were validated by comparing with indoor radon measurements and with radon values from a regression model constructed with important radon predictors as radiometric data, equivalent concentrations of thorium, uranium and bedrock geology. It was good agreement with both methods (Kollerud et al, 2014). Nevertheless, the risk of exposure misclassification must be taken into account when interpreting the results of this study.
Another limitation is the lack of control over the habits of dwelling inhabitants, such as whether windows are opened, the average indoor temperature and other occupancy patterns.
A further limitation of the exposure assessment method is that we assessed only indoor concentrations at home and lacked information on radon exposure outside homes such as care institutions and school.
Discussion of results
Earlier ecologic studies have observed a positive association between radon concentration and the risk of leukaemia, and this is the ‘common knowledge' today (Evrard et al, 2005).
Since 1987, nine good analytical studies have been reported of which a recent cohort study (Hauri et al, 2013) and six case–control studies, in accordance with our study, found no association for leukaemia (Lubin et al, 1998; Kaletsch et al, 1999; Steinbuch et al, 1999; UK Childhood Cancer Study Investigators, 2002a; Yoshinaga et al, 2005; Kendall et al, 2013). Two of these studies, on the contrary to our study, found a positive association with leukaemia, but not with CNS cancer (Maged et al, 2000; Raaschou-Nielsen et al, 2008).
Maged et al (2000) reported a significant association for leukaemia. This case–control study only had access to 50 cases and 110 controls. Radon concentrations were measured in bedroom and living room. The other case–control study with a positive association by Raaschou-Nielsen et al (2008) had a large number of cancer cases, 1153 leukaemia and 922 cases of cancer in the CNS. The difference from our study may be due the use of different study design and the use of different models to assess indoor radon concentration. The Danish study used 3120 indoor radon measurements geologic maps and information on buildings for the whole of Denmark. Although the model has a R2 of 40% (Andersen et al, 2007), this approach might lead to a risk of exposure misclassification. Several authors point out geology as a useful, but not sufficient indicator for estimating radon in buildings (Gundersen and Schumann, 1996; Hulka et al, 1997; Miles, 1998). Indoor radon concentrations are extremely variable, even for houses on identical geology. There might also be some uncertainty arising from the use of geologic maps regarding the coordinates of geologic boundaries (Hunter et al, 2009). Geologic conditions such as rocks containing high levels of uranium and high permeability of soils and rocks for gases are some of the main parameters for final radon risk classification of buildings. Even areas with low radon concentration in the soil can cause significant indoor radon concentrations in cases of high permeability in the contact area between the building and the soil environment (Neznal and Neznal, 2005). More accurate radon exposure may be obtained from direct indoor measurements combined with geologic maps (Miles and Appleton, 2005). Our model was based on high-density indoor radon measurements in the whole study area. We had also information on important predictors for indoor radon such as radiometric data, permeability in the ground and bedrock geology.
We observed a nonsignificant increased risk of CNS cancer in this study based on 13 CNS cancers. In Germany, a case–control (Kaletsch et al, 1999) report a nonsignificant elevated risk (OR=2.61; 95% CI=0.96–7.13) based on six CNS tumours. More recent larger studies report no associations between elevated radon exposure and cancer in CNS (UK Childhood Cancer Study Investigators, 2002a; Raaschou-Nielsen et al, 2008; Hauri et al, 2013; Kendall et al, 2013).
Childhood leukaemia and cancers in the CNS are diseases with low incidence rates. Only few analytical studies had access to more cases than our study (UK Childhood Cancer Study Investigators, 2002a; Raaschou-Nielsen et al, 2008; Kendall et al, 2013). Two of them (UK Childhood Cancer Study Investigators, 2002a; Kendall et al, 2013) did not have complete residential histories.
Conclusion
We did not find any association between radon exposure at home and the risk for developing leukaemia or cancer in the CNS among children under 15 years of age living in the Oslo area. The present study gives an indication of an association between CNS cancer and radon exposure in homes with on-site radon measurements and during time periods with more accurate address information. However, the association is not observed in the full material after adjustments for potential confounders. The results regarding CNS cancer should be interpreted with caution owing to the crude exposure assessment and possibilities of residual confounding.
Acknowledgments
This study was supported by the Agency for Health of the City of Oslo. The study used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors and no endorsement by the Cancer Registry of Norway is intended nor should it be inferred. We are grateful to Professor Hein Stigum for statistics comments and to Professor Erling Stranden for helping in understanding the effect of low radiation in children.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Andersen CE, Raaschou-Nielsen O, Andersen HP, Lind M, Gravesen P, Thomsen BL, Ulbak K. Prediction of 222Rn in Danish dwellings using geology and house construction information from central databases. Radiat Prot Dosimetry. 2007;123:83–94. doi: 10.1093/rpd/ncl082. [DOI] [PubMed] [Google Scholar]
- April Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S.2000International Classification of Diseases for Oncology3rd edn67.WHO: Geneva, Switzerland [Google Scholar]
- Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14:1316–1324. doi: 10.1093/neuonc/nos208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Kubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaiser M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;25:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cour-Brown WM, Doll R. Leukaemia and aplastic anaemia in patients irradiated for ankylosing spondylitis. Spec Rep Ser Med Res Counc. 1957;295:1–135. [PubMed] [Google Scholar]
- Eden T. Aetiology of childhood leukaemia. Cancer Treat Rev. 2010;36:286–297. doi: 10.1016/j.ctrv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Evrard AS, Hemon D, Billon S, Laurier D, Jougla E, Tirmarche M, Clavel J. Ecological association between indoor radon concentration and childhood leukaemia incidence in France, 1990–1998. Eur J Cancer Prev. 2005;14:147–157. doi: 10.1097/00008469-200504000-00011. [DOI] [PubMed] [Google Scholar]
- Gundersen LC, Schumann RR. Mapping the radon potential of the United States: examples from the Appalachians. Environ Int. 1996;22:829–837. [Google Scholar]
- Hauri D, Spycher B, Huss A, Zimmerman F, Grotzer M, von der Weid N, Weber D, Sporerri A, Kuehni CE, Röösli M, Swiss National Cohort, Swiss Paediatric Oncology Group (SPOG) Domestic radon exposure and risk of childhood cancer: a prospective census-based cohort study. Environ Health Perspect. 2013;121:1239–1244. doi: 10.1289/ehp.1306500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka J, Fojtikova I, Borecky Z, Tomasek L, Burian I, Thomas J. Presented at the European Conference on Protection Against Radon at Home and at Work. Praha 10. National Radiation Protection Institute: Czech Republic; 1997. Indoor radon risk mapping in the Czech Republic. [Google Scholar]
- Hunter N, Muirhead CR, Miles JC, Appleton JD. Uncertainties in radon related to house-specific factors and proximity to geological boundaries in England. Radiat Prot Dosimetry. 2009;136:17–22. doi: 10.1093/rpd/ncp148. [DOI] [PubMed] [Google Scholar]
- Idowu OE, Idowu MA. Environmental causes of childhood brain tumours. Afr Health Sci. 2008;8:1–4. [PMC free article] [PubMed] [Google Scholar]
- Kaletsch U, Kaatsch P, Meinert R, Schüz J, Czarwinski R, Michaelis J. Childhood cancer and residential radon exposure – results of a population-based case–control study in Lower Saxony (Germany) Radiat Environ Biophys. 1999;38:211–215. doi: 10.1007/s004110050158. [DOI] [PubMed] [Google Scholar]
- Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JC, Vincent TJ, Meara JR, Murphy MF. A record-based case–control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia. 2013;27:3–9. doi: 10.1038/leu.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AS, Eastmond DA, Preston RJ. Childhood acute lymphocytic leukemia and perspectives on risk assessment of early-life stage exposures. Mutat Res. 2006;613:138–160. doi: 10.1016/j.mrrev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Kollerud R, Blaasaas K, Ganerød G, Daviknes HK, Aune E, Claussen B. Using geographic information systems for radon exposure assessment in dwellings in the Oslo region, Norway. Nat Hazards Earth Syst Sci. 2014;14:739–749. [Google Scholar]
- Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, Møller B. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Linet MS, Boice JD, Buckley J, Conrath SM, Hatch EE, Kleinerman RA, Tarone RE, Wacholder S, Robison LL. Case–control study of childhood acute lymphoblastic leukemia and residential radon exposure. J Natl Cancer Inst. 1998;18:294–300. doi: 10.1093/jnci/90.4.294. [DOI] [PubMed] [Google Scholar]
- Maged AF, Mokhtarb GM, El-Tobguic MM, Gabbrc AA, Attiad NI, Abu Shadyc MM. Domestic radon concentration and childhood cancer study in Cairo, Egypt. J Environ Sci Health C. 2000;18:153–170. [Google Scholar]
- Miles J. Development of maps of radon-prone areas using radon measurements in houses. J Hazard Mater. 1998;61:53–58. [Google Scholar]
- Miles JC, Appleton JD. Mapping variation in radon potential both between and within geological units. J Radiol Prot. 2005;25:257–276. doi: 10.1088/0952-4746/25/3/003. [DOI] [PubMed] [Google Scholar]
- Neznal M, Neznal M. Permeability as an important parameter for radon risk classification of foundation soils. Ann Geophys. 2005;48:175–180. [Google Scholar]
- Norwegian Radiation Protection Authority NRPA's new recommendations for radon in Norway. StrålevernInfo. 2009;25:9. [Google Scholar]
- Raaschou-Nielsen O, Andersen CE, Andersen HE, Gravesen P, Schüz J. Domestic radon and childhood cancer in Denmark. Epidemiology. 2008;19:536–543. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- Rothman K, Greenland S, Lash T.2008Modern Epidemiology3th edn.603.Lippincott Williams Wilkins: USA [Google Scholar]
- Schmidt LS, Schmiegelow K, Lahteenmaki P, Trager C, Stokland T, Grell K, Gustafson G, Sehested A, Raashou-Nielsen O, Johansen C, Schuz J. Incidence of childhood central nervous system tumors in the Nordic countries. Pediatr Blood Cancer. 2011;56:65–69. doi: 10.1002/pbc.22585. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000;108:451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethurst MA, Strand T, Sundal AV, Rudjord AL. Large-scale radon hazard evaluation in the Oslofjord region of Norway utilizing indoor radon concentrations, airborne gamma ray spectrometry and geological mapping. Sci Total Environ. 2008;15:379–393. doi: 10.1016/j.scitotenv.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Statistics Norway . Individually Based Education Statistics, Documentation 2005. Statistics Norway: Norway; 2006. [Google Scholar]
- Steinbuch M, Weinberg CR, Buckley JD, Robison LL, Sandler DP. Indoor residential radon exposure and risk of childhood acute myeloid leukaemia. Br J Cancer. 1999;81:900–906. doi: 10.1038/sj.bjc.6690784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigum H, Strand T, Magnus P. Should radon be reduced in homes? A cost-effect analysis. Health Phys. 2003;84:227–235. doi: 10.1097/00004032-200302000-00011. [DOI] [PubMed] [Google Scholar]
- Stiller CA, Marcos-Gragera R, Ardanaz E, Pannelli F, Almar Marques E, Cañada Martinez A, Steliarova-Foucher E. Geographical patterns of childhood cancer incidence in Europe, 1988–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:1952–1960. doi: 10.1016/j.ejca.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Stranden E. Radon in Norwegian dwellings and the feasibility of epidemiological studies. Radiat Environ Biophys. 1986;25:37–42. doi: 10.1007/BF01209683. [DOI] [PubMed] [Google Scholar]
- Swedish Radiation Safety Authority. Radiation and Nuclear Safety Authority, Finland. Norwegian Radiation Protection Authority, University of Oslo. Norway and National Institute of Radiation Protection, Denmark . Recommendations for Radon in Dwellings in the Nordic Countries. Vol. 15. University of Oslo: Oslo, Norway; 2009. p. 9. [Google Scholar]
- Tong J, Qin L, Cao Y, Li J, Zhang J, Nie J, An Y. Environmental radon exposure and childhood leukemia. J Toxicol Environ Health B. 2012;15:332–347. doi: 10.1080/10937404.2012.689555. [DOI] [PubMed] [Google Scholar]
- UK Childhood Cancer Study Investigators The United Kingdom Childhood Cancer Study of exposure to domestic sources of ionising radiation: 1: radon gas. Br J Cancer. 2002;86:1721–1726. doi: 10.1038/sj.bjc.6600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Childhood Cancer Study Investigators The United Kingdom Childhood Cancer Study of exposure to domestic sources of ionising radiation: 2: gamma radiation. Br J Cancer. 2002;86:1727–1730. doi: 10.1038/sj.bjc.6600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S, Tokonami S, Akiba S, Nitta H, Kabuto M. Case–control study of residential radon and childhood leukemia in Japan: results from preliminary analyses. Int Congr Ser. 2005;1276:233–235. [Google Scholar]
- Zeeb H, Shannoun F. WHO Handbook on Indoor Radon: A Public Health Perspective. WHO: France; 2009. pp. 1–11. [PubMed] [Google Scholar]