Abstract
Background
Recent studies have demonstrated safety, feasibility, and decreased hospital length of stay for patients with weakness acquired in the intensive care unit (ICU) who receive early physical rehabilitation. The scored Physical Function in Intensive Care Test (PFIT-s) was specifically designed for this population and demonstrated excellent psychometrics in an Australian ICU population.
Objective
The purpose of this study was to determine the responsiveness and predictive capabilities of the PFIT-s in patients in the United States admitted to the ICU who required mechanical ventilation (MV) for 4 days or longer.
Methods
This nested study within a randomized trial administered the PFIT-s, Medical Research Council (MRC) sum score, and grip strength test at ICU recruitment and then weekly until hospital discharge, including at ICU discharge. Spearman rho was used to determine validity. The effect size index was used to calculate measurement responsiveness for the PFIT-s. The receiver operating characteristic curve was used in predicting participants' ability to perform functional components of the PFIT-s.
Results
From August 2009 to July 2012, 51 patients were recruited from 4 ICUs in the Denver, Colorado, metro area. At ICU discharge, PFIT-s scores were highly correlated to MRC sum scores (rho=.923) and grip strength (rho=.763) (P<.0005). Using baseline test with ICU discharge (26 pairs), test responsiveness was large (1.14). At ICU discharge, an MRC sum score cut-point of 41.5 predicted participants' ability to perform the standing components of the PFIT-s.
Limitations
The small sample size was a limitation. However, the findings are consistent with those in a larger sample from Australia.
Conclusions
The PFIT-s is a feasible and valid measure of function for individuals who require MV for 4 days or longer and who are alert, able to follow commands, and have sufficient strength to participate.
A growing body of evidence supports the use of early mobilization and physical rehabilitation for patients while in the intensive care unit (ICU). Recent studies have demonstrated safety, feasibility, and decreased ICU and hospital length of stays for patients with ICU-acquired weakness (ICU-AW) who receive early physical rehabilitation.1–4 It is important to be able to objectively document patient functional status, assist with prognostication, and measure the impact of rehabilitation strategies on patient outcomes.
Recently, 3 tests specific to the ICU population were developed: the Functional Status Score for the ICU,5 the Chelsea Critical Care Physical Assessment Tool,6 and the Physical Function in Intensive Care Test (PFIT).4,6,7 These tests have documented safety, feasibility, and responsiveness in pilot studies and provide important information about patients' functional abilities. However, only the PFIT has undergone additional psychometric testing for validation and scale analysis with patients admitted to an Australian ICU for at least 5 days.4,7
The PFIT was specifically developed for use with patients who are critically ill who may never reach the ability to perform the Six-Minute Walk Test (6MWT) or other submaximal exercise tests.7 It includes 5 items: amount of assistance for sit-to-stand transfers, strength for shoulder flexion, strength for knee extension, marching in place, and an upper extremity (UE) endurance task of arm elevation to 90 degrees of shoulder flexion.7 Denehy et al4 used principal component analysis to further refine the test components, which resulted in the removal of the UE endurance item. An ordinal score was obtained for the new 4-item PFIT, and Rasch analysis was used to convert this score to an interval score. This summary score is referred to as the PFIT-s.4 The PFIT-s scores range from 0 (able to perform strength testing only with a maximal score of 2/5 for shoulder and knee) to 10 (performance without any difficulty).4 Hence, it is possible to test patients who are able to perform only certain parts of the test. The PFIT-s provides an objective measure of endurance (marching in place) for task performance of patients in the ICU, which in addition to typical strength and functional assessments may be important to examine and track over the course of a patient's hospital stay. In addition, the objective data from the PFIT-s can be used to guide exercise prescription. By recording rate of perceived exertion during marching in place, the PFIT-s provides an objective way to prescribe and evaluate exercise at an appropriate dose to achieve a training effect for patients at the bedside.4 In addition, the PFIT-s provides assessment of heart rate, blood pressure, respiratory rate, and oxygen saturation during a more strenuous activity.
Because patients who experience critical illness are a heterogeneous population in regard to severity and recovery, it is important to try to delineate patient characteristics to better develop physical therapist examination and intervention and to understand recovery trajectories. Comorbid conditions (such as sepsis), ICU length of stay, and age are factors that help to identify distinct phenotypes of patients with ICU-AW.8 Individuals who require mechanical ventilation (MV) have greater risk of developing ICU-AW.9 Therefore, patients who need MV may represent a distinct subset of patients in the ICU.
The original validation of the PFIT-s included patients admitted to an Australian ICU for 5 days; however, a specific MV duration was not required. Hence, it is important to establish the utility of the PFIT-s for patients in the United States who require MV. The overall purpose of this investigation was to validate findings of Denehy and colleagues4 to patients in a US medical ICU who required MV for 4 days or longer. Specifically, we report on the psychometric properties of the PFIT-s with respect to: (1) convergent validity of the PFIT-s with the Medical Research Council (MRC) sum score and grip strength measured using hand-held dynamometry; (2) change in PFIT-s over time, including responsiveness of the test; and (3) ability of the PFIT-s to predict hospital discharge disposition.
Method
Participants
This was a longitudinal observational study. All of our patients were also participants in an ongoing trial of intensive physical therapy for patients who are critically ill (ClinicalTrials.gov: NCT01058421). Participants in the parent study were all admitted to an ICU and required MV through an endotracheal tube for 4 days or longer. All patients were English speaking and lived within a 161-km (100-mile) radius from the study site. Exclusion criteria included: (1) age <18 years; (2) diagnosis of pre-existing disease of the peripheral motor nervous system (eg, Guillain-Barré syndrome, myasthenia gravis, polymyositis, amyotrophic lateral sclerosis); (3) any central nervous system disorder that would compromise participation (eg, cerebrovascular accident, anoxic encephalitis, meningitis following head trauma, spinal cord injury); (4) fewer than 2 limbs in which muscle strength could be tested; (5) referred from another hospital for patients who required MV for more than 24 hours; (6) pregnancy; (7) myocardial infarction within the last 3 weeks, unstable angina, or history of unstable arrhythmias, including ventricular tachycardia and atrial fibrillation (heart rate greater than 100 bpm); (8) pulmonary embolism within the last 6 weeks; (9) history of severe aortic stenosis; (10) presence of a dissecting aortic aneurysm; (11) disorder that made it unlikely that the patient will survive 6 months, which was determined through consultation with the medical team and based on patient acuity; (12) severe physical or cognitive impairment that would impair ability to exercise; and (13) burn greater than 30% of body surface area.
Consent was obtained from either a proxy or the patient prior to enrollment in the study. Once the participant was able, continuation of consent was sought from the participant.
Procedure
After patients were enrolled in the parent study, a physical therapist conducted daily assessments to determine medical stability and ability to consistently follow commands. Once patients were able to follow at least 2 of the commands from De Jonghe and colleagues' awakening criteria (Fig. 1),9 a physical therapist examination (baseline testing) was initiated. If a patient demonstrated delirium during the ICU stay, medical management was instituted and patients were screened daily by a physical therapist to determine ability to follow commands. Measurements were repeated every 7 days thereafter, up to 28 days or hospital discharge. In addition, the ICU discharge time point was defined as within ±3 days of discharge from the ICU. For the validation aspect of this study, outcomes measured included PFIT-s, MRC sum score, and grip strength. If participants were capable, additional measures of function were administered, including the Timed “Up & Go” Test (TUG), Berg Balance Scale (BBS), and Six-Minute Walk Test (6MWT). These measurements were obtained by physical therapists blinded to parent study group assignment. Demographic information included age, sex, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, MV days, ICU and hospital length of stay, ICU diagnosis, significant medical history, and discharge disposition.
Figure 1.
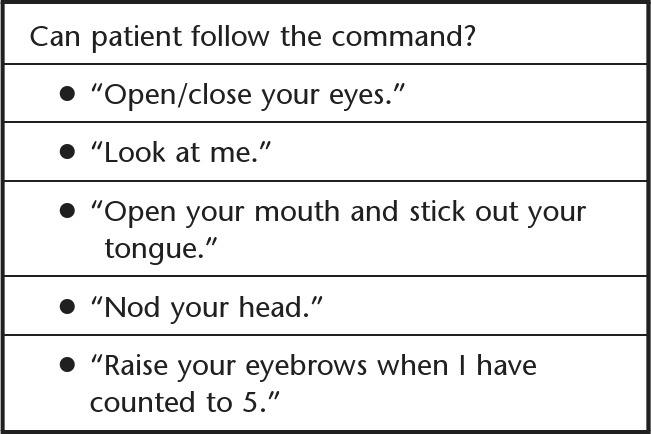
Awakening criteria.8
Outcome Measures
The original PFIT was used for testing of all participants because validation of the PFIT-s had not yet been reported at the time of initiation of the parent study. The original PFIT has excellent interrater reliability for the marching-in-place component (intraclass correlation coefficient [ICC]=.999 to 1.00).7 In recent work, Denehy et al4 reported convergent validity of the revised PFIT-s with the TUG (r=−.60, 95% confidence interval [95% CI]=−.70 to −.46), 6MWT (r=.41, 95% CI=.24 to .55) and MRC sum score (rho=.49; 95% CI=.33 to .62). In addition, the effect size index (ESI) revealed a large responsiveness to change (ESI=0.82, 95% CI=0.66 to 0.99) from the ICU baseline testing time point to ICU discharge. The only difference between the original 5-item PFIT and the revised 4-item PFIT-s version was the UE endurance component. Therefore, for quantifying baseline and outcome data, we excluded the UE endurance component scores when scoring the PFIT-s.4
The MRC sum score includes strength testing of 3 upper extremity and 3 lower extremity muscle groups and results in a score, which ranges from 0 to 60. The MRC sum score was first used by Kleyweg et al10 to quantify strength for patients with Guillain-Barré syndrome. De Jonghe et al9 reported that the MRC sum score can be used to diagnose ICU-acquired paresis if the sum score is less than 48/60. In a cohort of patients requiring MV for 5 days or more, Ali et al11 reported high interrater reliability and correlation with the diagnosis of ICU-acquired paresis (Pearson correlation coefficient=.96; P<.001 for both). Recent studies examining MRC sum scores in patients who are critically ill have shown interrater reliability ICC values of .95 for sum score and .83 for individual muscle group scores.12,13 Hermans et al13 reported disagreement in interrater reliability for muscle scores of 4 (good) and 5 (normal), which were attributed to the person-to-person variability in applied resistance.
Handgrip was first assessed by Ali et al11 in a population of patients who were critically ill with cutoff scores of less than 11 kg (men) and less than 7 kg (women), providing the best sensitivity (80.6%), specificity (83.2%), positive predictive value (63.0%), and negative predictive value (92.3%) for diagnosis of ICU-acquired paresis. For patients who are critically ill and able to follow commands, the interrater reliability is high (ICC=.93–.98).12–14 Recently, Baldwin et al14 reported minimal detectable differences for handgrip strength of 7.1 kg on the left and 5.7 kg on the right and recommended a minimum of 5 seconds of holding the contraction to achieve most accurate results. For this study, grip strength values were obtained by using the maximum value of the left and right scores.
There was no imputation of missing data. Participants who were unable to perform grip strength testing or all components of muscle strength testing were excluded from analyses. For the PFIT, some patients were unable to perform the sit-to-stand or marching-in-place component but could complete shoulder flexion and knee extension components. Therefore, patients were scored on these components performed and included in the analyses. If unable to perform, a patient received a zero for a specific component of the test.
Protocol
Once patients were medically stable and sufficiently alert, based on De Jonghe and colleagues' awakening criteria,9 the baseline physical therapist examination was performed. All components of the PFIT were attempted first, followed by muscle strength testing for MRC sum score and finally grip strength. The PFIT was performed based on descriptions by Skinner and colleagues.7 Sit-to-stand with assistance was recorded. Marching in place was performed next, and number of steps taken and time were recorded. Finally, shoulder flexion and knee extension strength in a sitting position were recorded. Before and after the PFIT, blood pressure, oxygen saturation, and Borg Rating of Perceived Exertion (RPE) on the 0 to 10 category ratio (CR10) scale were recorded.15 Patients were instructed to maintain between a 3 to 6 on the RPE scale during the marching and UE endurance tasks, representing moderate to somewhat hard exertion. Because this study began prior to the PFIT-s development, the UE endurance portion was recorded but excluded from scoring.
Next, manual muscle strength of 6 bilateral muscle groups was rated from 0 (no palpable contraction) to 5 (full force production) using the MRC sum scoring system.9,10 The muscle groups tested were shoulder and elbow flexors, wrist extensors, hip flexors, knee extensors, and ankle dorsiflexors; the total possible score was 60. Once strength testing was completed, participants were asked to perform grip strength 3 times with each hand and hold for 5 seconds on each hold using the Jamar handgrip dynamometer (Sammons Preston Rolyan, Bollingbrook, Illinois). Participants were placed in 90 degrees of upright sitting with the elbow at 90 degrees,11,16 and the average of the 3 trials was recorded in kilograms.
Based on a participant's abilities and physical limitations, the initial assessment required between 15 to 45 minutes to complete. If the participant was able to perform only muscle strength testing, grip strength, and bed mobility, the assessment required 15 minutes; if the participant was able to complete all components (ie, muscle and grip strength, mobility, PFIT, TUG, BBS, and 6MWT), the assessment required 30 to 45 minutes to complete.
Data Analysis
Demographic information, clinical acuity, and discharge disposition are presented as mean (SD) for normally distributed data and median (interquartile range [IQR]) for non-normally distributed data. The PFIT-s score was computed using the system outlined by Denehy et al4 and included the 4 items of the revised test. The floor and ceiling effects of the PFIT-s were calculated by computing the number of participants who scored either 0 (floor) on all components or the maximum score of 10 and expressed as a percentage of total N. Hand-grip dynamometry scores of the right and left hands were averaged, and the best of these scores was used for all analyses.
The Spearman rank correlation coefficient was used to determine correlations between PFIT-s, MRC sum score, and hand-grip dynamometry at ICU discharge. Correlations were categorized as follows: .25 to .5 as fair, .5 to .75 as moderate to good, and greater than .75 as excellent.17
The ESI was used to calculate PFIT-s responsiveness. An ESI of 0.2 corresponds to small responsiveness, 0.5 to moderate responsiveness, and 0.8 or higher to large responsiveness.18,19 Responsiveness was assessed using paired data at baseline, at ICU discharge, and post-ICU discharge. Separate logistic regression models were used to determine the predictive ability of the PFIT-s at baseline and ICU discharge in regard to discharge disposition. Three dichotomous discharge disposition variables were the outcome of interest (“home or other,” “acute rehabilitation or other,” and “long-term acute care [LTAC] or other”). Age, sex, APACHE II score, MRC sum score, ICU length of stay, ventilator days, and past history of chronic disease (defined as past history of diabetes, cirrhosis, cancer, or renal failure due to human immunodeficiency virus) were considered as potential covariates in the models. These covariates were assessed for collinearity. If 2 covariates had a bivariate correlation of .7 or higher, the covariate with the least significant association with the outcome of interest was omitted from the model. Odds ratios (ORs) and 95% CIs are reported for significant models. The level of significance was set at .05. Patients who had died prior to hospital discharge were not included in these analyses. The ability of ICU discharge MRC sum score to predict participants' ability to perform the sit-to-stand and marching-in-place components of the PFIT-s at ICU discharge was examined using the receiver operating characteristic (ROC) curve. The area under the curve (95% CI), sensitivity, and specificity are reported.
Role of the Funding Source
This work was made possible by support of the National Institutes of Health (R01 NR-11051).
Results
From August 2009 to July 2012, a total of 51 patients were recruited from 4 ICUs in the Denver, Colorado, metropolitan area; demographics are given in Table 1. The mean age of the participants was 50.5 years (SD=16.3). Sixty-three percent were men. The median ICU length of stay was 20 days (IQR=12–26), and median number of days on ventilator was 12 (IQR=8–24). Baseline testing occurred a mean of 15 days (SD=11) post-ICU admission.
Table 1.
Participant Demographics (N=51)a
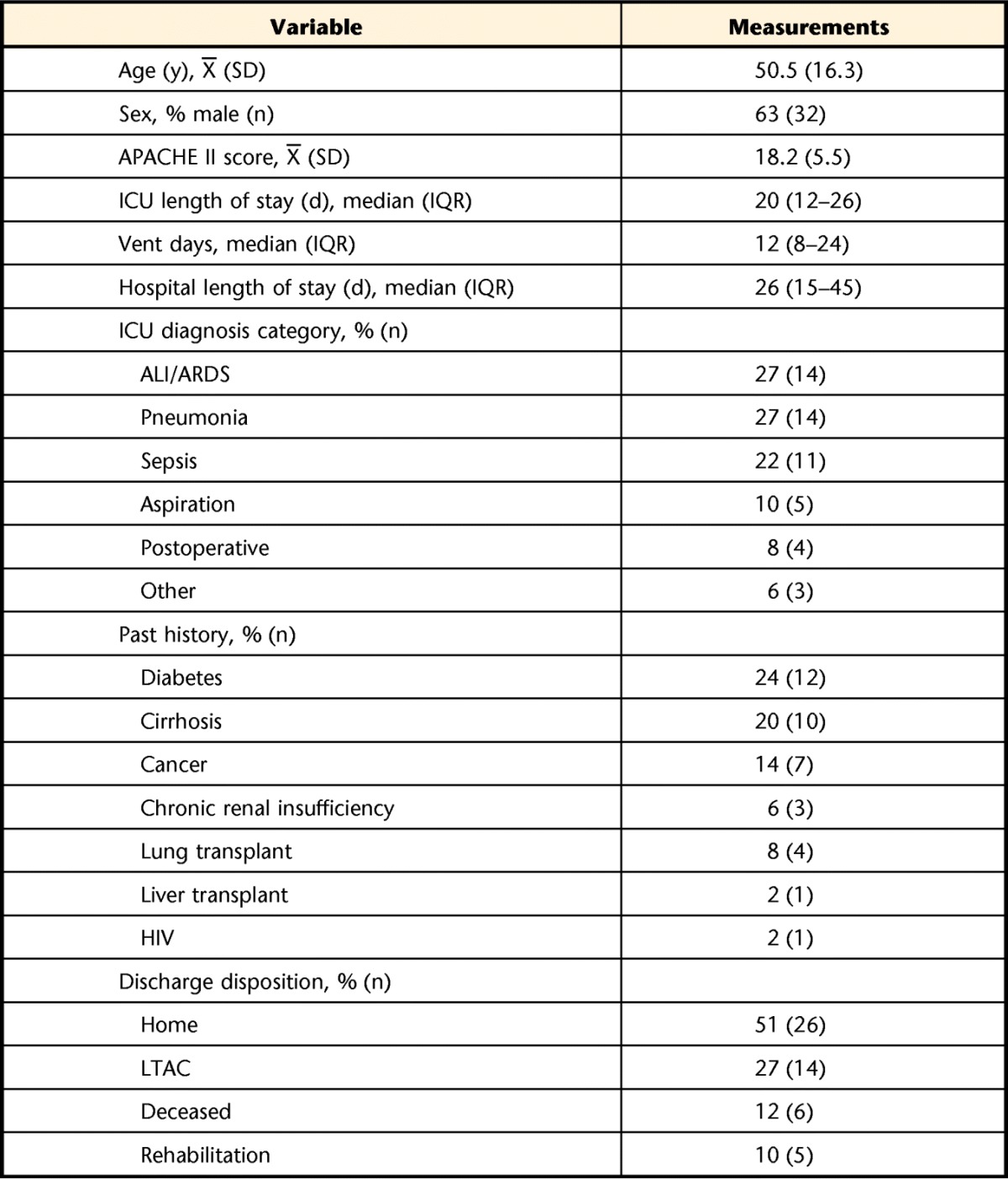
APACHE II=Acute Physiology and Chronic Health Evaluation II, ICU=intensive care unit, vent days=days of mechanical ventilation, ALI/ARDS=acute lung injury/acute respiratory distress syndrome, HIV=human immunodeficiency virus, LTAC=long-term acute care.
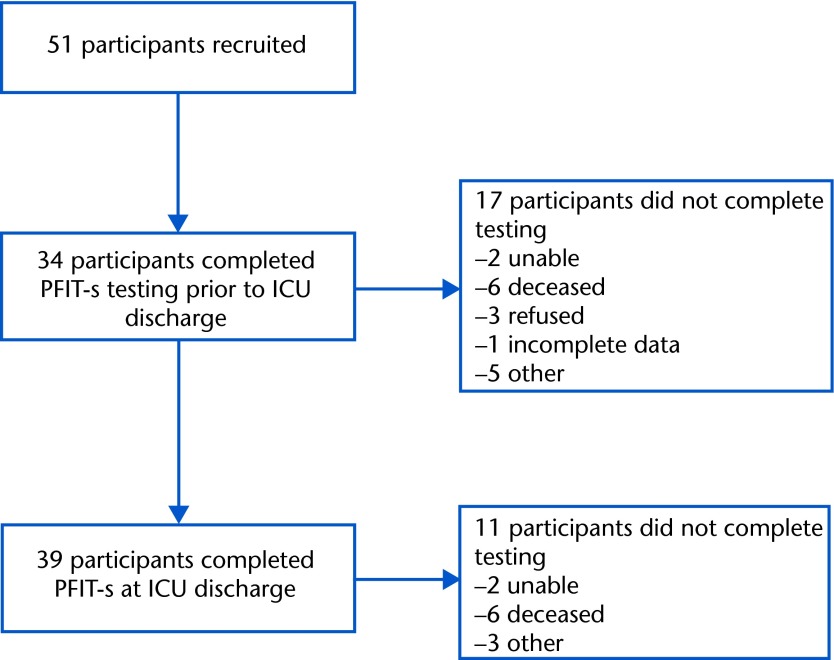
Due to medical acuity and inability to follow at least 2 commands, many participants were not able to perform the PFIT-s (15/51, 29%), muscle strength testing (15/51, 29%), or grip strength (19/51, 37%) at study enrollment (Fig. 2). However, 34 participants completed baseline PFIT-s testing prior to the ICU discharge time point (within 3 days of ICU discharge) (Tab. 2). Of the 34 baseline tests, 32% (11/34) of the participants scored the lowest possible score of 0, indicating a floor effect of the test. These individuals were characterized by a median MRC sum score of 30/60 and a median grip strength of 2.5 kg, which is 6% to 10% of the expected value for healthy individuals20 and indicates ICU-AW. The grip strength median (IQR) scores reported in Table 2 and data used in the correlations between grip strength, PFIT-s score, and MRC sum score at ICU discharge were obtained by using the maximum value of the left and right scores. These participants were all able to complete the shoulder and knee strength testing components of the PFIT-s, but as they were graded as 2 or less for strength, their overall PFIT-s remained 0.
Figure 2.
Participant flow. PFIT-s=scored Physical Function in Intensive Care Test, ICU=intensive care unit.
Table 2.
PFIT-s, MRC Sum Score, and Grip Strength Scores at Each Time Pointa
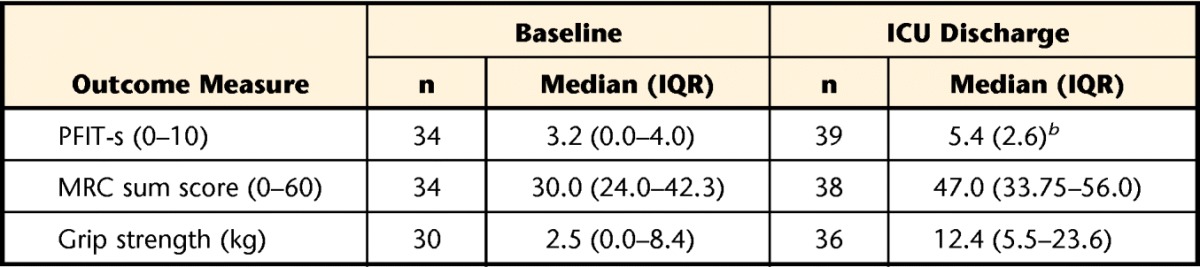
PFIT-s=scored Physical Function in Intensive Care Test, MRC=Medical Research Council, ICU=intensive care unit, IQR=interquartile range.
b Normally distributed data presented as mean (SD).
At ICU discharge, 39 patients completed testing, 2 remained unable, and 6 died prior to testing. Four discharge tests were not recorded due to patient refusals (n=3) or the therapist was unable to obtain data (n=1). Regarding floor effect at ICU discharge, only 5% (2/39) scored 0. The ceiling effect at ICU discharge was also 5%, with 2 of 39 participants scoring 10. Intensive care unit discharge PFIT-s testing occurred a mean of 8 days (SD=4) from baseline PFIT-s testing. The PFIT-s, MRC sum score, and grip strength scores are presented in Table 2. This study demonstrated no adverse events during the PFIT-s testing, which is similar to findings of other studies using this measure in the ICU.4,7
Because the PFIT-s contains shoulder and knee strength assessment, we hypothesized that MRC sum score and grip strength would be correlated with the composite score. At ICU discharge, PFIT-s was highly correlated to MRC sum score (rho=.923) and grip strength (rho=.763) (P<.0005).
In addition to convergent validity, this study also investigated responsiveness of the PFIT-s. Comparisons were made at 2 sets of time points, baseline with ICU discharge and ICU discharge, with the next test point (follow-up) after ICU discharge. Using baseline test with ICU discharge (26 pairs), test responsiveness was large (1.14). At follow-up PFIT testing (n=21 pairs), an average of 5.67 days (SD=2.01) post-ICU discharge, responsiveness was moderate (0.59).
Higher PFIT-s scores at baseline (OR=0.63, 95% CI=0.40 to 0.99, P=.043) and ICU discharge (OR=0.70, 95% CI=0.51 to 0.98, P=.038) were significantly associated with a reduced likelihood of discharge to LTAC. However, LTAC group numbers were small, and the sensitivity of these models was only 44% and 30%, respectively. Men were 3.7 times (95% CI=1.02 to 13.35) more likely be discharged home than women (P=.045). In this analysis, 3 discharge outcomes were evaluated: home versus other, LTAC versus other, and rehabilitation versus other. Long-term acute care was the only discharge disposition significantly associated with PFIT-s scores. Numbers in the group that was discharged to inpatient rehabilitation were low (n=5). At baseline and ICU discharge, PFIT-s scores did not predict discharge home (P=.087). Higher numbers of days of MV predicted discharge to LTAC (OR=1.17, 95% CI=1.05 to 1.30, P=.004). One outlier who was ventilated for 62 days and discharged home was excluded from this model. The MRC sum score is a diagnostic marker of ICU-AW, but we do not know how changes of MRC sum score over time relate to function. Therefore, we examined the ability of the MRC sum score at ICU discharge to predict whether the patient could perform the sit-to-stand component (and, therefore, also the marching-in-place component) of the PFIT-s. Five participants were able to perform the sit-to-stand component but not the marching-in-place component. In the ROC analysis, 14 participants were unable to perform the sit-to-stand and marching-in-place components (scored as 0), and 24 participants were able to perform the sit-to-stand component (scored as 1). The AUC was 0.93 (95% CI=0.85 to 1.00). An MRC sum score cut-point of 41.5 had a sensitivity of 85.7% and a specificity of 83.3% in predicting whether an individual would be unable to perform the sit-to-stand and marching-in-place components of the PFIT-s at ICU discharge.
Discussion
This investigation extends the initial validation of the PFIT-s reported by Denehy and colleagues.4 The US sample differed from the Australian sample in 3 important ways: First, all of the individuals in the US sample required MV for greater than 4 days compared with 55% for the Australian sample. Possibly, those in Australia had less severe primary disorders, necessitating reduced days on the ventilator, or this finding simply reflects a difference in medical practice between the 2 countries. Second, the US cohort had ICU length of stays that were almost 3 times longer than those of the Australian group (20 days versus 7 days, respectively). Third, a greater percentage of patients in the US cohort developed sepsis (US cohort=22%, Australian cohort=6.9%). Sepsis and ICU length of stay have been linked to differences in patient disability and recovery.2,8 These factors point to the US population being a different patient population in regard to disability and recovery trajectory.
Although patients in the US sample differed substantially from those in Australia, patient demographics were similar to the Australian cohort in regard to mean age (US cohort=51 years, Australian cohort=61 years), ICU admission diagnoses, and medical acuity, as reflected by mean APACHE II score (US cohort=18, Australian cohort=20).4 It is important to note that the APACHE II measures mortality, not morbidity. In addition, Schweickert et al2 reported that initial APACHE II scores were not associated with return to functional independence by hospital discharge for patients who were critically ill.
In recent work, Denehy et al4 reported convergent validity of the revised PFIT-s with the TUG (r=−.60, 95% CI=−.70 to −.46), 6MWT (r=.41, 95% CI=.24 to .55), and MRC sum score (rho=.49, 95% CI=.33 to .62). In addition, the ESI revealed a large responsiveness to change (ESI=0.82, 95% CI=0.66 to 0.99) from the ICU baseline testing time point to ICU discharge. We extended the findings in several ways. The PFIT-s was highly correlated with grip strength (rho=.763). Baseline PFIT-s scores predicted discharge to LTACs. Also, an MRC sum score cut-point of 41.5 had a sensitivity of 85.7% and a specificity of 83.3% in predicting whether the participant would be unable to perform the sit-to-stand and marching-in-place components of the PFIT-s at ICU discharge, which has not been reported previously. Finally, Denehy et al4 reported moderate correlations of the PFIT-s with MRC sum score (rho=.49) for patients admitted to ICUs in Australia. However, we found a strong correlation (rho=.92). This finding was possibly because all of the participants in our sample required MV for at least 4 days, increasing the likelihood of ICU-AW.
It is important to note that the Australian and US health care systems differ in regard to discharge disposition. Both the study by Denehy et al4 and the current study demonstrated similar percentages of patients being discharged to home (51% and 58%, respectively) and for those who expired (12% and 8%, respectively). However, 27% of the US sample were admitted to LTAC facilities, whereas 23% of the Australian cohort were admitted to acute rehabilitation facilities. This difference reflects the fact that LTACs are not part of the Australian health care system.
The PFIT-s is particularly well suited for use in the ICU. Because the PFIT-s contains a combination of body system impairments as well as functional components, physical therapists can begin to objectively categorize strength and functional deficits early in a patient's hospital stay. Thus, the test can be administered very early in recovery, while the patient is substantially limited in functional abilities, providing objective data about underlying impairments and function for early commencement of rehabilitation strategies. In contrast to MRC sum score and grip strength, the PFIT-s allows the physical therapist to assess vital sign response (heart rate, respiratory rate, blood pressure, and oxygen saturation) and RPE during a strenuous activity. By incorporating vital sign response and RPE, the PFIT-s can inform exercise intensity for patients in the ICU who are typically unable to perform traditional submaximal exercise tests such as the 6MWT or step tests. Exercise can be prescribed from the different components. For example, marching in place can be given as an exercise at 80% of the measured test cadence or duration, providing a means for systematic progression of exercise intensity. Future research regarding use of the PFIT-s to guide exercise prescription and progression is an important next step.
Furthermore, by using the PFIT-s at initial physical therapist examination and at ICU discharge, an objective assessment of change in overall functional ability can easily be captured, which may assist with discharge planning. In addition, this measure allows for assessment of functional limitations (such as transfers, standing, and challenges to dynamic balance), which can directly influence a patient's ability to perform activities such as gait and stair negotiation.
The PFIT-s has limitations that should be considered. This measure is not appropriate for patients who are unable to follow commands. In this respect, it is similar to all volitional tests for patients in the unique environment of the ICU. These limitations were reflected in floor effects for both the US and Australian cohorts. At baseline testing, 32% of our cohort scored the lowest possible score for the PFIT-s versus 21.5% reported by Denehy et al.4 One explanation for the higher floor effect with our population is sedation issues surrounding MV. Furthermore, at ICU discharge, there continued to be a floor effect (5%) for the US population but not for the Australian cohort, again possibly reflecting differences in health care practices in the 2 countries. Because of the heterogeneity of patient abilities and deficits, no single measure is appropriate for all patents with ICU-AW, and the PFIT-s appears best suited for patients early in the critical care stay.21
Before attempting to administer the PFIT-s in the ICU, we recommend testing grip strength and MRC sum score to ensure that the patient has sufficient ability to follow commands. Because portions of the MRC sum score are part of the PFIT-s, this testing will reduce overall time of administration. Although grip strength and MRC sum score are important markers of body system impairments, these measures provide limited information regarding the patient's ability to carry out basic functional activities. In contrast, the PFIT-s provides important information about abilities that are important to function, such as sit-to-stand performance and maintaining balance and postural control while performing an endurance test.
As patients become medically stable and transition to the medical floor, the PFIT-s remains appropriate in a high proportion of patients. In addition, tests of additional activities of daily living and walking may be indicated. Several investigations of patients with ICU-AW used the Barthel Index as both a self-report and performance measure to categorize patients' ability to perform activities of daily living.2,22–24 In addition, the TUG4,25,26 and 6MWT4,20,27–29 have been reported for use after ICU discharge. Depending on patient recovery, these measures also may be appropriate for the subacute or outpatient setting. A combination of additional tests and measures may need to be identified to address the long-term deficits in strength and function reported in this population28,30 and more generally in measuring the functional and performance impairments of survivors of the ICU with post–intensive care syndrome.4,30–32
We acknowledge that the sample size was small, which is a limitation to this study. However, the findings in this sample are consistent with those in a similar sample in Australia, lending support to their validity.4
In conclusion, the PFIT-s is a feasible and valid measure of function for individuals who require MV in the ICU and who are alert, able to follow commands, and have sufficient strength to participate. This measure can be applied in combination with other existing measures of body system impairments to fully assess the impact of activity limitations of people diagnosed with ICU-AW in the ICU setting. In addition to being a useful examination strategy, the PFIT-s can help direct physical therapy intervention. Finally, findings in this sample are consistent with those of Denehy et al4 while extending their initial validation.
Footnotes
Dr Nordon-Craft, Dr Schenkman, Dr Malone, Dr Moss, and Dr Denehy provided concept/idea/research. All authors provided writing. Dr Nordon-Craft, Dr Malone, and Dr Moss provided data collection. Dr Nordon-Craft, Ms Edbrooke, Dr Moss, and Dr Denehy provided data analysis. Dr Moss provided project management, fund procurement, study participants, and facilities/equipment. Ms Edbrooke, Dr Moss, and Dr Denehy provided consultation (including review of manuscript before submission).
Study approval was obtained from the Colorado Multiple Institutional Review Board and the institutional review boards of the local participating hospitals.
The manuscript was presented as a poster at the American Thoracic Society International Conference; May 17–22, 2013; Philadelphia, Pennsylvania.
This work was made possible by support of the National Institutes of Health (R01 NR-11051).
References
- 1. Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145 [DOI] [PubMed] [Google Scholar]
- 2. Schweickert W, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243 [DOI] [PubMed] [Google Scholar]
- 4. Denehy L, de Morton NA, Skinner EH, et al. A physical function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the Physical Function ICU Test (scored). Phys Ther. 2013;93:1636–1645 [DOI] [PubMed] [Google Scholar]
- 5. Zanni J, Korupolu R, Fan E, et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25:254–262 [DOI] [PubMed] [Google Scholar]
- 6. Corner EJ, Wood H, Englebretsen C, et al. The Chelsea Critical Care Physical Assessment Tool (CPAx): validation of an innovative new tool to measure physical morbidity in the general adult critical care population; an observational proof-of-concept pilot study. Physiotherapy. 2013;99:33–41 [DOI] [PubMed] [Google Scholar]
- 7. Skinner EH, Berney S, Warrillow S, Denehy L. Development of a physical function outcome measure (PFIT) and a pilot exercise training protocol for use in intensive care. Crit Care Resusc. 2009;11:110–115 [PubMed] [Google Scholar]
- 8. Batt J, dos Santos CC, Cameron JI, Herridge MS. Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Resp Crit Care Med. 2013;187:238–246 [DOI] [PubMed] [Google Scholar]
- 9. De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867 [DOI] [PubMed] [Google Scholar]
- 10. Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–1109 [DOI] [PubMed] [Google Scholar]
- 11. Ali NA, O'Brien JM, Jr, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268 [DOI] [PubMed] [Google Scholar]
- 12. Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hermans G, Clerckx B, Vanhullenbusch T, et al. Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 2012;45:18–25 [DOI] [PubMed] [Google Scholar]
- 14. Baldwin CE, Paratz JD, Bersten AD. Muscle strength assessment in critically ill patients with handheld dynamometry: an investigation of reliability, minimal detectable change, and time to peak force generation. J Crit Care. 2013:28:77–86 [DOI] [PubMed] [Google Scholar]
- 15. Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998 [Google Scholar]
- 16. Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2006;31:3–10 [DOI] [PubMed] [Google Scholar]
- 17. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Norwalk, CT: Appleton & Lange; 1993 [Google Scholar]
- 18. Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–S189 [DOI] [PubMed] [Google Scholar]
- 19. Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53:459–468 [DOI] [PubMed] [Google Scholar]
- 20. Klum M, Wolf MB, Hahn P, et al. Normative data on wrist function. J Hand Surg Am. 2012;37:2050–2060 [DOI] [PubMed] [Google Scholar]
- 21. Elliott D, Denehy L, Berney S, Alison JA. Assessing physical function and activity for survivors of a critical illness: a review of instruments. Aust Crit Care. 2011;24:155–166 [DOI] [PubMed] [Google Scholar]
- 22. Pohlman MC, Schweickert WD, Pohlman AS, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38:2089–2094 [DOI] [PubMed] [Google Scholar]
- 23. Intiso D, Amoruso L, Zarrelli M, et al. Long-term functional outcome and health status of patients with critical illness polyneuromyopathy. Acta Neurol Scand. 2011;123:211–219 [DOI] [PubMed] [Google Scholar]
- 24. Heyland DK, Groll D, Caesar M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med. 2005;33:1549–1556 [DOI] [PubMed] [Google Scholar]
- 25. Nordon-Craft A, Schenkman M, Ridgeway K, et al. Physical therapy management and patient outcomes following ICU-acquired weakness: a case series. J Neurol Phys Ther. 2011;35:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brummel NE, Jackson JC, Girard TD, et al. A combined early cognitive and physical rehabilitation program for people who are critically ill: the Activity and Cognitive Therapy in the Intensive Care Unit (ACT-ICU) Trial. Phys Ther. 2012:92:1580–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herridge MS, Tansey CM, Matté A, et al. , Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304 [DOI] [PubMed] [Google Scholar]
- 28. Alison JA, Kenny P, King MT, et al. Repeatability of the Six-Minute Walk Test and relation to physical function in survivor of a critical illness. Phys Ther. 2012;92:1556–1563 [DOI] [PubMed] [Google Scholar]
- 29. Denehy L, Skinner EH, Edbrooke L, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months follow-up. Crit Care. 2013;17:R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Schaaf M, Beelen A, Dongelmans DA, et al. Poor functional recovery after a critical illness: a longitudinal study. J Rehabil Med. 2009;41:1041–1048 [DOI] [PubMed] [Google Scholar]
- 31. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509 [DOI] [PubMed] [Google Scholar]
- 32. Pawlick AJ, Kress JP. Issues affecting the delivery of physical therapy services for individuals with critical illness. Phys Ther. 2013:93:256–265 [DOI] [PubMed] [Google Scholar]