Abstract
Significance: Angiogenesis, the growth of new blood vessels from existing vessels, is an important aspect of the repair process. Restoration of blood flow to damaged tissues provides oxygen and nutrients required to support the growth and function of reparative cells. Vascular endothelial growth factor (VEGF) is one of the most potent proangiogenic growth factors in the skin, and the amount of VEGF present in a wound can significantly impact healing.
Recent Advances: The activity of VEGF was once considered to be specific for endothelial cells lining the inside of blood vessels, partly because VEGF receptor (VEGFR) expression was believed to be restricted to endothelial cells. It is now known, however, that VEGFRs can be expressed by a variety of other cell types involved in wound repair. For example, keratinocytes and macrophages, which both carry out important functions during wound healing, express VEGFRs and are capable of responding directly to VEGF.
Critical Issues: The mechanisms by which VEGF promotes angiogenesis are well established. Recent studies, however, indicate that VEGF can directly affect the activity of several nonendothelial cell types present in the skin. The implications of these extra-angiogenic effects of VEGF on wound repair are not yet known, but they suggest that this growth factor may play a more complex role during wound healing than previously believed.
Future Directions: Despite the large number of studies focusing on VEGF and wound healing, it is clear that the current knowledge of how VEGF contributes to the repair of skin wounds is incomplete. Further research is needed to obtain a more comprehensive understanding of VEGF activities during the wound healing process.

Kelly E. Johnson, PhD
Scope and Signficance
The repair of skin wounds proceeds in a step-wise manner beginning with hemostasis and inflammation during the acute stages of healing. This is followed by periods of robust cellular proliferation, extracellular matrix (ECM) deposition and remodeling, and ultimately scar formation.1–3 Angiogenesis, the formation of new blood vessels from preexisting vasculature, is a prominent feature of the proliferative phase of healing. This process leads to a temporary increase in the number of blood vessels at the site of injury. The delivery of oxygen and nutrients from these new blood vessels is a critical part of the repair process, and defects in angiogenesis are frequently associated with delays in wound healing. A variety of growth factors, cytokines, and lipid mediators produced in response to injury can stimulate angiogenesis. One of the most important proangiogenic mediators is vascular endothelial growth factor (VEGF or VEGF-A), and sufficient VEGF levels are believed to be essential for proper wound healing. Recent studies have started to uncover novel functions for VEGF in the skin outside of its role as a proangiogenic mediator. This article will provide a general overview of angiogenesis and VEGF in the context of wound repair and will discuss potential new biological activities for VEGF during wound healing.
Translational Relevance
The function of VEGF in wound repair has been extensively studied. VEGF stimulates angiogenesis and also influences wound closure and epidermal repair, granulation tissue formation, and the quality of repair—both in terms of the strength of the healed wound and the amount of scar tissue that is deposited. Despite the large number of studies indicating that VEGF can affect multiple aspects of the wound healing process, most of the effects of VEGF during wound repair have been attributed to its proangiogenic activity; however, newer studies have indicated that in addition to vascular endothelial cells, other cells, such as keratinocytes and macrophages, express VEGFRs and can respond directly to VEGF. More detailed studies are needed to define the full range of VEGF activities within a healing wound. The importance of the extra-angiogenic effects of VEGF on wound repair must be established and the potential usefulness of modulating these effects for therapeutic purposes must be determined.
Clinical Relevance
High levels of VEGF are produced during the course of normal wound repair, which results in a vigorous angiogenic response. The clinical significance of adequate VEGF production during wound repair has been repeatedly demonstrated. The levels of active VEGF protein tend to be abnormally low in individuals with chronic, nonhealing wounds like those commonly observed in diabetic patients. Insufficient wound vascularization stemming from low VEGF activity likely contributes to these delays in the repair process. In addition, drugs that block the activity of VEGF, which are used to treat multiple types of cancer, pose a significant risk for wound healing complications. Overall, data from both basic science and clinical studies indicate that appropriate levels of VEGF are needed for efficient wound repair.
Discussion of Findings and Relevant Literature
Neovascularization
An adequate blood supply and sufficient vascularization are necessary to maintain healthy tissues. In order to survive and grow, tissues rely on oxygen and nutrients provided by the blood. Neovascularization, the growth of new blood vessels, is an important component of many physiologic and pathologic processes. Neovascularization is essential for reproduction and embryonic development, it stimulates tissue survival after ischemic events, and it aids in the repair of damaged tissues.4 This process must be tightly regulated, however, as uncontrolled blood vessel growth encourages the development of diseases such as arthritis, macular degeneration, psoriasis, and cancer.5
Mechanisms of neovascularization
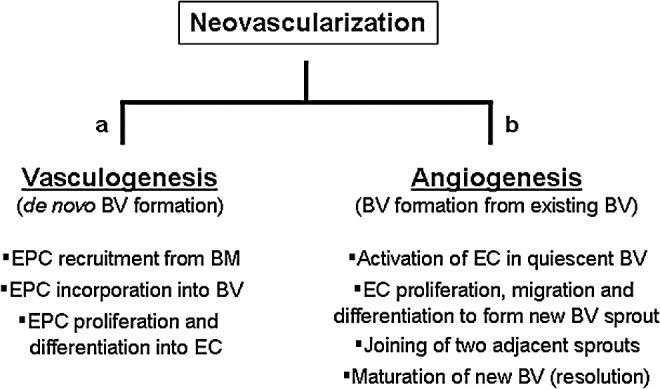
The two most common mechanisms of blood vessel expansion are vasculogenesis and angiogenesis (sometimes referred to as “sprouting angiogenesis”; Fig. 1).4 Vasculogenesis is the de novo formation of new blood vessels by endothelial progenitor cells (EPCs). At early stages of development blood vessels are formed from angioblasts, which differentiate into endothelial cells. This process eventually leads to the formation of a complex vascular network required for embryonic development. Vasculogenesis can also occur in adult tissues, especially in response to ischemia.6,7 During postnatal vasculogenesis, bone marrow–derived EPCs are recruited and become incorporated into the new vessels.8 Local proliferation and differentiation of EPCs results in the formation of functional blood vessels.7 In addition to de novo vessel formation, new blood vessels can also be formed from preexisting vessels through angiogenesis. During this process, new vessels “sprout” from established vessels, which requires endothelial cells to proliferate, migrate, and differentiate into new vascular structures.4 Although both vasculogenesis and angiogenesis are known to occur during the repair of cutaneous wounds, angiogenesis is studied more frequently as a mechanism of neovascularization in wounds.
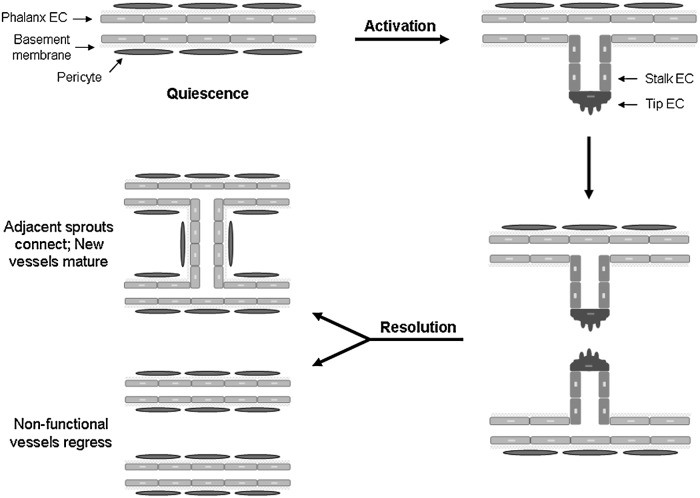
Figure 1.
Mechanisms of neovascularization. The two main mechanisms of blood vessel growth that occur during wound healing are vasculogenesis and angiogenesis. (a) Vasculogenesis is the de novo formation of new blood vessels by EPCs. EPCs are recruited from the bone marrow and become incorporated into the new vessels. Local proliferation and differentiation of EPCs into ECs results in the formation of new, functional blood vessels. (b) Angiogenesis is the formation of new blood vessels from pre-existing vessels. During this process, endothelial cells in quiescent blood vessels are activated, resulting in a vessel “sprout.” This requires endothelial cells to proliferate, migrate and differentiate to form new vascular structures. Two adjacent sprouts will fuse to facilitate perfusion and eventually the new, functional vessel will mature. BV, blood vessel; BM, bone marrow; EC, endothelial cell; EPC, endothelial progenitor cells.
Angiogenesis
Sprouting angiogenesis is a complex process that is observed frequently during tissue repair and in various disease states. Angiogenesis can be divided into phases of quiescence, activation, and resolution (Fig. 2).4 In normal, healthy tissues, blood vessels are maintained in a quiescent state. Quiescent vessels are lined on the inner surface with endothelial cells known as phalanx cells.4 Tight cell–cell adhesion between these cells creates a barrier that helps maintains blood flow. Mature vessels are surrounded by a basement membrane made up mainly of collagen IV and laminin and are coated with pericytes, which promote endothelial cell survival and help maintain vessel stability.5 When quiescent vessels are exposed to a proangiogenic stimulus, endothelial cells within the vessel become activated and begin loosening their cell–cell contacts.4 In addition, detachment of pericytes from the outside of the vessel, along with enzymatic degradation of the basement membrane by matrix metalloproteinases, provides a route for the development of a new vascular sprout.4 Growth of the new vessel is led by a single endothelial cell known as the tip cell.9 Tip cells direct vascular outgrowth by sensing a gradient of proangiogenic mediators like VEGF.9 Adjacent endothelial cells become stalk cells, which proliferate and migrate in the direction of the tip cell, resulting in elongation of the sprouting vessel.4 During the resolution phase of angiogenesis, vascular sprouts will fuse with neighboring sprouts to establish blood flow, while nonfunctional (unperfused) vascular sprouts will regress. The blood vessels will then return to a quiescent state—the phalanx cell phenotype will be restored, a new basement membrane will be formed, and pericytes will cover the vessel.4
Figure 2.
Steps of sprouting angiogenesis. During angiogenesis, new blood vessels are created from established quiescent vessels. Quiescent vessels exhibit a mature phenotype, with phalanx ECs lining the inner surface of the vessel. Quiescent vessels are covered by a basement membrane and are typically surrounded by pericytes. When endothelial cells become activated in response to a proangiogenic stimulus like VEGF, the basement membrane is degraded, pericytes detach, and a specialized endothelial cell called a tip cell is selected. This cell guides elongation of the new vascular sprout. Stalk cells follow behind the tip cell, migrating and proliferating to lengthen the new vessel. During the resolution phase of angiogenesis, two adjacent new vessels will join one another to establish blood flow and nonperfused vessels will regress. Eventually the basement membrane will be re-created, pericyte coverage will be restored, and the vessels will return to a quiescent state. VEGF, vascular endothelial growth factor
In general, the process of angiogenesis is believed to be controlled by changes in the levels of proangiogenic and antiangiogenic molecules present within the microenvironment surrounding the vasculature. Many proangiogenic and antiangiogenic mediators have been identified. Basic fibroblast growth factor, interleukin-8, platelet-derived growth factor, placental growth factor (PlGF), transforming growth factor-β, and VEGF are proteins that stimulate angiogenesis, whereas angiostatin (a fragment of plasminogen), endostatin (a fragment of collagen XVIII), and thrombospondin-1 are examples of mediators that inhibit angiogenesis. The maintenance of vessels in a quiescent state is thought to occur when the level of antiangiogenic signals outweigh proangiogenic signals; however, periods of active angiogenesis occur when endothelial cells sense a shift in the balance of these mediators, with proangiogenic signals predominating over antiangiogenic signals. This idea, known as the angiogenic switch, was proposed by Folkman and Hanahan, who first used it to describe the regulation of tumor angiogenesis.10 When the skin is injured, angiogenesis is stimulated due to an increase in the production of several proangiogenic mediators, including VEGF, at the wound site.
Neovascularization in healing wounds
Effective wound healing requires vascularization of the newly formed tissue. As granulation tissue forms, the number of blood vessels in the dermis increases. A significant increase in the density of blood vessels compared to uninjured skin is commonly observed during the proliferative phase of healing as a result of angiogenesis (Fig. 3).11 As granulation tissue is converted into mature scar tissue, some of the new vessels regress. Eventually the number of vessels normalizes and returns to a level close to what is observed in uninjured skin.12 Although not much is known about how vessel regression is regulated in the skin, simply keeping the levels of proangiogenic mediators elevated cannot prevent capillary regression.13 Studies have suggested that the chemokine ligand 10 (CXCL10), which is elevated in the late stages of wound healing, could be involved in the regression of newly formed vessels by signaling through chemokine receptor 3 (CXCR3).14 The majority of blood vessels created over the course of normal wound healing are believed to be created through angiogenesis,15 and this is the most frequently studied mode of neovascularization in cutaneous repair. However, vasculogenesis has also been demonstrated in excisional wounds and ischemic skin flaps through the identification of bone marrow–derived cells that have incorporated into the new vasculature.7,8 The clinical significance of vasculogenesis in a normally healing wound is not yet clear.
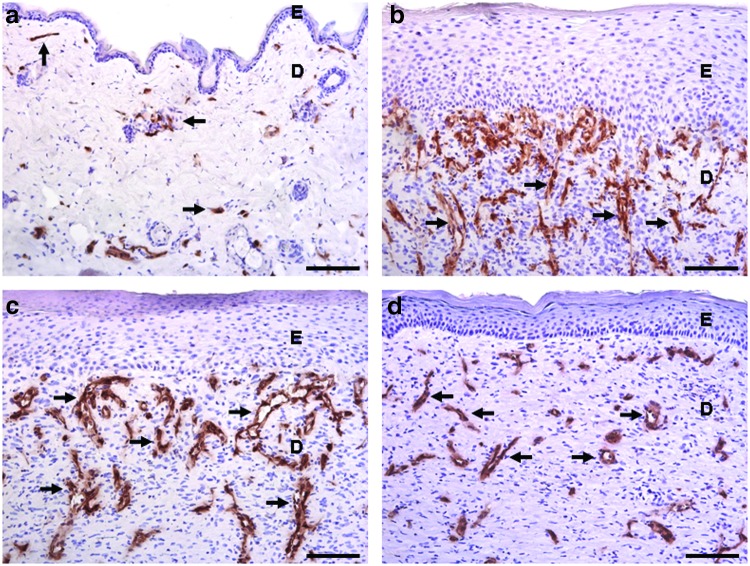
Figure 3.
Blood vessel staining in murine skin wounds. CD-31 (PECAM-1) is expressed by endothelial cells and CD-31 immunostaining is commonly used to identify blood vessels (indicated by arrows, brown staining). Representative images are shown of normal skin or the center of 3-mm excisional wounds after 5, 7, or 14 days of healing in FVB mice. This is a standard laboratory mouse strain named for its sensitivity to Friend leukemia virus B. (a) A basal number of blood vessels are present in normal skin to maintain homeostasis. (b) After 5 days of healing, an increase in CD-31 staining can be seen and new vascular structures are starting to form. (c) At 7 days, vessels with a more defined structure can be observed. (d) By 14 days post injury, the number of blood vessels has started to decline and eventually the density of blood vessels will return close to the level found in uninjured skin. Scale bar=100 μm. D, dermis; E, epidermis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The importance of new blood vessel growth during wound healing is highlighted by the fact that insufficient vascularization is a common feature of chronic, nonhealing wounds. This is also apparent in animal models of delayed wound healing, such as diabetic mice, in which reduced vascularization has been linked to delayed wound closure, reduced reepithelialization, and deficient granulation tissue formation.16 While the majority of evidence points to robust angiogenesis being beneficial for repair in models of delayed healing or severe skin injury (i.e., skin flaps),17,18 the correlation between angiogenesis and repair is less clear in standard wounds made in healthy animals. There are several examples of conflicting results in the literature, with some studies suggesting that inhibiting angiogenesis has no effect or only modest effects on wound closure,19–23 and others indicating that blocking angiogenesis significantly delays wound repair.24–26 One reason for the variability in results could be based on the fact that many of these studies examine blood vessel number or blood vessel density as a measure of angiogenesis without assessing the functionality of the blood vessels. Because a significant number of the new blood vessels created during wound healing are not perfused,27 reducing the overall density of blood vessels within a wound by selective elimination of nonfunctional blood vessels may not be detrimental to the healing process.
VEGF
VEGF (used to refer to VEGF-A throughout this article) is one of the most important proangiogenic molecules in the skin. It has been shown to play a role in hair growth28 as well as the development of skin diseases such as psoriasis29 and skin cancer.26,30 Numerous studies have shown that VEGF is also important for wound healing in the skin. VEGF-A is a 45 kDa heterodimeric heparin-binding protein. It belongs to a family of vascular endothelial growth factors that also includes VEGF-B, VEGF-C, VEGF-D, and PlGF. Multiple isoforms of VEGF-A can be generated through alternative splicing.31 VEGF, which was originally identified as a vascular permeability factor,32 is capable of inducing vascular permeability with a potency several thousand-fold higher than histamine. Subsequent studies found that VEGF is a strong positive regulator of angiogenesis33 and stimulates endothelial cell functions needed for new blood vessel formation, such as proliferation, migration, differentiation, and survival.33–35 The importance of VEGF as a mediator of neovascularization is highlighted by studies showing that the loss of even a single copy of the VEGF gene results in embryonic lethality at early stages of development.36,37
VEGF family members elicit their effects on endothelial cells by binding to and activating tyrosine kinase receptors located on the cell surface (Fig. 4).38 VEGF-A is capable of binding to multiple receptors, including VEGF receptor-1 (VEGFR-1) and VEGF receptor-2 (VEGFR-2). These are tyrosine kinase receptors that contain seven immunoglobulin-like domains on the extracellular portion of the receptor, as well as a single transmembrane region and an intracellular tyrosine kinase domain.39 These two receptors differ in their ligand-binding properties and tyrosine kinase activity. VEGFR-1 binds to the VEGF ligand with higher affinity, whereas VEGFR-2 exhibits stronger inherent tyrosine kinase activity.40 VEGFR-2 is believed to be the more important of the two receptors in terms of controlling endothelial cell function and regulating angiogenesis based on its superior ability to stimulate downstream signaling cascades. Upon binding to VEGF, phosphorylation of tyrosine residues on VEGFR stimulates activation of protein kinase B, which inhibits apoptosis, and the mitogen-activated protein kinase (MAPK) pathway, which induces proliferation (Fig. 5).41 Activation of Src kinase, focal adhesion kinase, and p38 MAPK also occurs and leads to cell migration.41 In addition to the membrane form of VEGFR-1, a soluble form of VEGFR-1 (sVEGFR-1) also exists; this form is generated by alternative splicing of the VEGFR-1 gene.42 sVEGFR-1 contains the extracellular ligand-binding domain but lacks the transmembrane and signaling domains; therefore, it acts as a negative regulator of angiogenesis by binding to VEGF with high affinity and preventing its interaction with membrane-bound forms of VEGFR-1 or VEGFR-2. Some isoforms of VEGF-A can also bind to neuropilins (NRPs), which are single-pass transmembrane proteins that bind to semaphorins as well as some members of the VEGF family.41 NRPs act as coreceptors for VEGF and enhance the activity of VEGFRs.43
Figure 4.
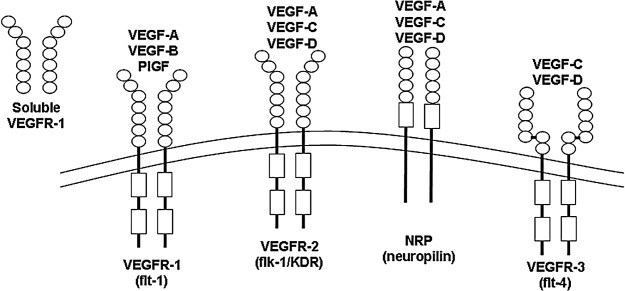
VEGF–VEGFR binding partners. VEGF-A belongs to a family of VEGF proteins that also includes VEGF-B, VEGF-C, VEGF-D, and PlGF. Each family member binds to one or more tyrosine kinase receptors of the VEGFR family (VEGFR-1, -2, and -3). Soluble forms of some VEGFRs exist, including sVEGFR-1, which binds to VEGF and blocks its ability to signal through the membrane-bound forms of the receptors. Some VEGF family members can also bind to NRPs, which function as coreceptors. NRP, neuropilin; VEGFR, VEGF receptor.
Figure 5.
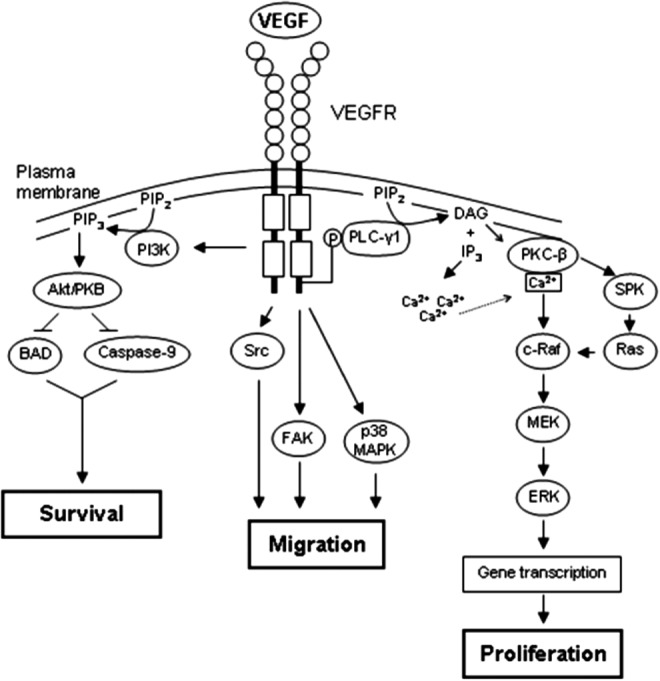
VEGFR signaling. VEGF–VEGFR binding causes dimerization and autophosphorylation of the VEGFR. VEGFR phosphorylation triggers PI3K activation and phosphorylation of Akt. Active Akt blocks the proapoptotic molecules BAD and caspase-9, resulting in cell survival (left). VEGFR signaling also activates several kinases that mediate cell migration, including Src, FAK, and p38 MAPK (middle). Activation of multiple pathways following VEGFR phosphorylation leads to MAPK signaling (phosphorylation of MEK/ERK), which stimulates cellular proliferation (right). Akt/PKB, protein kinase B; BAD, Bcl-2-associated death promoter; DAG, diacylglycerol; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; IP3, inositol 1,4,5-triphosphate; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; PLC, phospholipase C; SPK, sphingosine kinase.
VEGF in wound healing
Angiogenesis is a prominent feature of the wound healing response. Studies examining VEGF in wound repair began soon after the initial discovery and characterization of VEGF as a proangiogenic factor. Since that time, VEGF has been shown to play a role in several facets of the repair process (Fig. 6). VEGF mRNA44 and protein (Fig. 7) increase at early time points post injury in the skin, and VEGF protein levels increase and remain elevated in wound fluid for at least a week in surgical wounds.45 Although several different mediators regulate angiogenesis, VEGF is believed to be one of the most important proangiogenic mediators during wound healing.45 Multiple cellular sources likely contribute to the increase in VEGF after injury (Fig. 6). VEGF, which is normally expressed at low levels by epidermal keratinocytes, is upregulated in these cells in injured skin.44 Studies in human wounds and animal models have indicated that VEGF is produced by keratinocytes early in the wound-healing process;44,46 however, more recent evidence suggests that keratinocytes also produce VEGF at later stages of healing.47 Activated fibroblasts, mast cells, and macrophages also express VEGF in injured skin.44,45,48 In fact, myeloid cells (i.e., monocytes and macrophages) were recently found to be a major source of VEGF at certain time points post injury,47 and both keratinocyte-derived and myeloid cell–derived VEGF have been shown to affect some components of the repair process.26,47,49 For example, delayed wound closure, reduced vessel density, and decreased granulation tissue formation have been reported in mice lacking VEGF in myeloid cells,47,49 and delayed wound closure and reduced vessel density have been reported in mice lacking VEGF in keratinocytes.26 Hypoxia is one reason VEGF increases during wound healing. Low oxygen levels in wounded skin causes activation of the transcription factor HIF, which results in transcription of the VEGF gene.50 Oxidants produced in response to injury, such as hydrogen peroxide, and a variety of other mediators produced at the wound site, such as epidermal growth factor, keratinocyte growth factor, transforming growth factor, and tumor necrosis factor, have also been shown to stimulate the production of VEGF by keratinocytes.51–53 Less is known about what shuts down VEGF expression; however, Fra-1, a member of the Fos transcription factor family, was recently shown to act as a negative regulator of VEGF transcription in healing skin flaps.54
Figure 6.
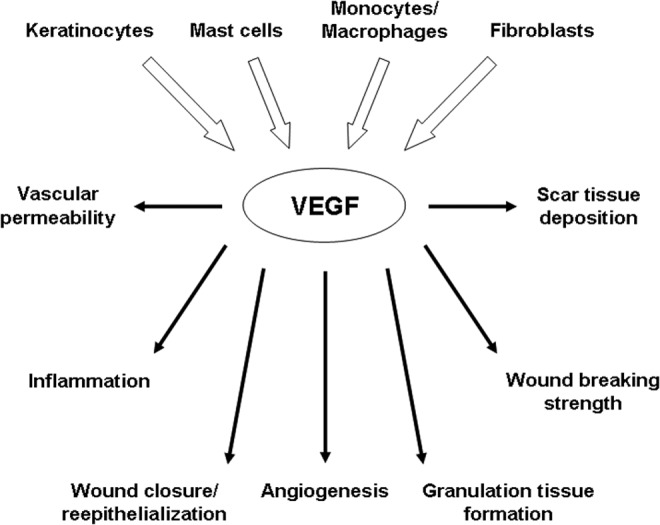
Cellular sources and actions of VEGF during wound healing. During wound healing, VEGF is produced by a variety of cell types, including keratinocytes, mast cells, macrophages, and fibroblasts (top). The VEGF produced in response to injury plays a role in many aspects of wound healing (bottom). During the acute phases of healing, VEGF stimulates vascular permeability and adhesion molecule expression, which aids in the recruitment of inflammatory cells. VEGF levels can influence the rate of wound closure/re-epithelialization, angiogenesis, granulation tissue formation, and the strength of the healed wound during the proliferative phase. VEGF can also promote the formation of scar tissue during the scar formation/remodeling phase.
Figure 7.
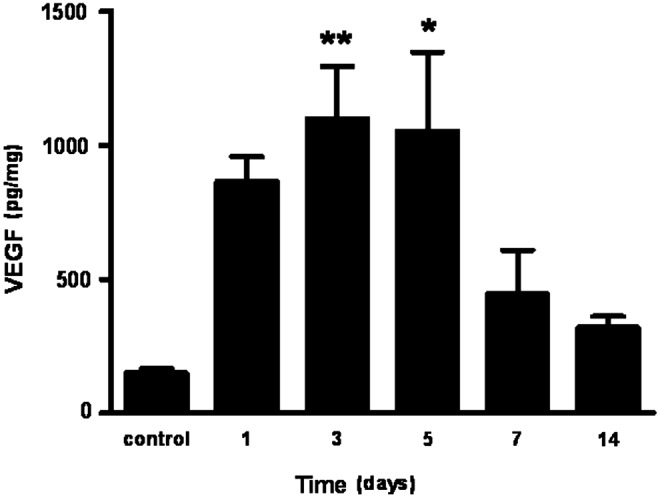
VEGF production in murine skin wounds. VEGF levels were measured by ELISA in 3-mm excisional skin wounds in FVB mice to illustrate the pattern of VEGF protein expression during wound healing. VEGF protein levels start to increase in wounded tissue 1 day after injury compared to control uninjured skin. VEGF levels are significantly elevated compared to control skin at 3 and 5 days post wounding. Protein levels begin to normalize between 7 and 14 days. Bars represent mean amount of VEGF (pg per mg of total protein)±standard error of the mean. (n=5 wounds from separate mice per time point). *p<0.05, **p<0.01 by one-way analysis of variance with Bonferroni post hoc testing.
VEGF and the acute wound-healing response
Endothelial cells play a crucial role in the regulation of inflammation during the early stages of wound healing. The loosening of cell–cell contacts and the expression of adhesion molecules allows endothelial cells to facilitate the movement of circulating inflammatory cells into the tissue at the site of injury. VEGF is a strong inducer of vascular permeability in the skin,30,55 and the correlation between VEGF expression patterns and permeable blood vessels in injured skin44 suggests that VEGF contributes to vascular permeability at early stages of healing. VEGF also affects the interactions between endothelial cells and circulating inflammatory cells. Detmar and colleagues have shown that VEGF increases leukocyte rolling and adhesion on the endothelium by affecting the expression of selectins and intercellular adhesion molecules on endothelial cells.56 This is critical for the ability of circulating inflammatory cells to move from the bloodstream into the tissue, a hallmark of the inflammatory response. VEGF also increases the number of dermal mast cells,56 a cell type involved in multiple phases of wound healing.57 Additionally, an increase in the density of macrophages has been observed in wounds created in transgenic mice that overexpress VEGF in the epidermis, suggesting that VEGF plays a role in recruiting macrophages to damaged skin.58
VEGF activity in the proliferative phase of healing
Several cell types work together to repair the epidermal and dermal layers of the skin during the proliferative phase of healing, ultimately leading to complete healing of the wound. Keratinocytes proliferate and migrate to repair the epidermal barrier during re-epithelialization. Repair of the dermis also begins during this phase with the formation of granulation tissue, which is rich in inflammatory cells, fibroblasts, and new blood vessels. These new vessels supply blood containing the oxygen and nutrients required to support the activity of the various cell types involved in this phase of repair. VEGF has been shown to regulate several aspects of the proliferative phase, including repair of the epidermal barrier as well as the underlying dermis.
The contribution of VEGF to overall wound closure and epidermal repair has been examined extensively in animal studies. With the exception of one study using adeno-associated virus–mediated VEGF expression,59 most of the results suggest that enhancing VEGF levels in healthy animals does not significantly accelerate wound closure or increase re-epithelialization.58,60,61 This may be because VEGF levels are already high in normally healing wounds. However, reducing VEGF activity by treating with neutralizing antibodies or small molecule inhibitors of VEGF signaling or conditional genetic deletion of VEGF lead to delayed healing.26,49,62,63 For example, Rossiter and colleagues performed wound-healing studies in mice with VEGF-deficient keratinocytes and showed that the wounds contained fewer blood vessels beneath the epidermis and healed more slowly.26 Additionally, topical treatment with neutralizing antibodies to VEGFR-1 results in reduced re-epithelialization rates.62 More recent studies by Stockmann and colleagues showed delayed healing in mice lacking VEGF in myeloid cells.49
Additional support for the importance of VEGF in wound closure comes from models of impaired healing or severe injury. Wounds generated in diabetic mice, which heal significantly more slowly than healthy mice, have fewer blood vessels and contain less VEGF.16,53,64 Enhancing VEGF levels in diabetic wounds by treating topically with recombinant VEGF or by using viral vector-mediated or liposome-mediated gene transfer accelerates wound closure, increases granulation tissue formation, and enhances the quality of the healed wound (i.e., improved breaking strength).17,64–66 A strong role for VEGF has also been demonstrated in ischemic skin flap models. Augmenting VEGF levels leads to significantly improved vascularization and enhanced skin flap survival.18,67–69
VEGF has also been implicated in repair of the dermis in both normal wound-healing models and models of delayed healing. Based on the results of multiple studies, there appears to be a strong correlation between VEGF levels or VEGF activity and the amount of granulation tissue formed in both healthy and diabetic animals.17,47,59,60,64–66 Additionally, VEGF has been shown to affect wound breaking strength in some cases.66,70 This could result from alterations in collagen production or the arrangement of the deposited collagen.
As with animal studies, clinical studies also support the idea that sufficient levels of VEGF are required for effective healing. Poor vascularization is a hallmark of chronic wounds, such as diabetic or venous ulcers. This likely results in part from inadequate VEGF activity in these wounds, which leads to inefficient angiogenesis. This could be due to degradation of VEGF by the abnormally high protease activity in chronic wounds71 or neutralization of VEGF due to high levels of soluble VEGFR-1.72
VEGF and scar formation
In the final stages of the repair process, fibroblasts continue to deposit collagen and other ECM proteins and also remodel the immature collagen matrix into mature scar tissue. Several studies have suggested that VEGF contributes to scar tissue production. Our laboratory has shown that VEGF levels correlate with the amount of scar tissue produced in mouse models of fetal and adult wound healing. Systemic treatment with neutralizing VEGF antibodies led to a reduction in scar size and normalization of the collagen fibril structure in adult incisional wounds.73 In addition, injection of recombinant VEGF resulted in the formation of large scars in embryonic day 15 fetal wounds, which normally heal without scars.73 Abnormal scars, such as hypertrophic scars74,75 and keloids,76,77 have been shown to express high levels of VEGF. Treatment of hypertrophic scar patients with interferon α2b has been linked to a reduction in angiogenesis and VEGF,78 suggesting that reducing VEGF may improve scars. Similarly, treatments used to induce keloid regression have been shown to reduce VEGF levels in keloid tissue.79,80 Despite the positive association between VEGF levels and scar formation, little is known about the mechanisms responsible. VEGF may promote scar tissue formation indirectly based on its ability to stimulate angiogenesis; however, VEGF can also increase the number of inflammatory cells in the skin56,58 and some studies have suggested that VEGF can directly influence dermal fibroblast behavior.66 It is therefore possible that VEGF promotes scar tissue formation by multiple mechanisms.
Nonendothelial effects of VEGF
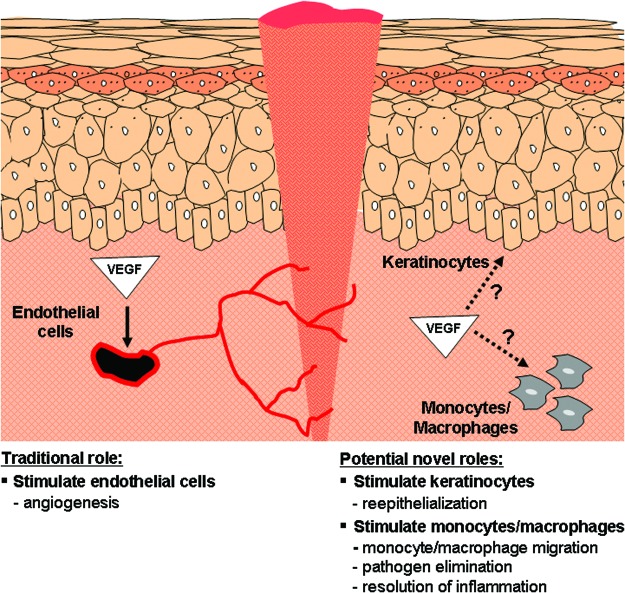
To date, the majority of studies examining VEGF in wound healing have focused on VEGF as a mediator of angiogenesis and, as a result, any findings have been attributed to the effects of VEGF on endothelial cells (Fig. 8). Many studies, however, have now shown that VEGF can directly affect a variety of cells other than endothelial cells that also express one or more functional VEGFRs. Importantly, VEGF has been shown to directly affect several nonendothelial cell types known to play an important role in the wound-healing response, including keratinocytes and macrophages (Fig. 8).
Figure 8.
Endothelial and nonendothelial effects of VEGF during wound healing. To date, most of the beneficial effects of VEGF have been attributed to the ability of VEGF to stimulate angiogenesis by signaling through VEGFRs on endothelial cells (left). Recent studies have shown, however, that VEGFRs are also present on epidermal keratinocytes and myeloid cells (monocytes/macrophages), suggesting that VEGF can directly stimulate these nonendothelial cell types (right). These novel alternative signaling pathways could promote wound re-epithelialization by keratinocytes and could also affect trafficking of circulating leukocytes into the wound, pathogen elimination, or resolution of inflammation. Further work is needed to determine if these alternative pathways are active during wound repair in vivo. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Direct effects of VEGF on keratinocytes
Keratinocytes are the cells that make up the epidermal barrier; they are responsible for resealing this barrier when the skin is damaged. Keratinocytes are also one of the main sources of VEGF during wound healing. Traditionally, VEGF produced by epidermal keratinocytes was thought to act in a paracrine manner, stimulating endothelial cells in blood vessels within the underlying dermis. Functional VEGFRs, however, have been recently identified on keratinocytes,62,81–83 which suggests the possibility of autocrine VEGF signaling in keratinocytes as well as direct effects of VEGF derived from other cellular sources on keratinocytes. Several groups have now shown that keratinocytes express VEGFR-162,82,84,85 and the coreceptor NRP-1.81,83,85 Some groups have also suggested that VEGFR-2 and VEGFR-3 are present on keratinocytes,85,86 although a functional role for these receptors has not yet been demonstrated in vivo. Direct effects of VEGF on keratinocytes have been described in vitro using cultured keratinocytes. For example, VEGF can increase the proliferation of cultured primary human keratinocytes,62 primary mouse keratinocytes,82 and keratinocyte-derived tumor cells82 through VEGFR-1, and VEGF can also stimulate the migration of primary human keratinocytes.66 Furthermore, VEGF has been shown to mediate keratinocyte survival following exposure to ultraviolet light,83,86 and both VEGFR-1 and NRP-1 have been shown to regulate keratinocyte function in vivo.81–83
Overall, the fact that keratinocytes express functional VEGFRs implies that VEGF can directly influence these cells during wound repair. Studies in other systems have suggested possible roles for VEGF in the regulation of keratinocyte proliferation, migration, and survival.62,66,82,83,86 Although the contribution of direct effects of VEGF on keratinocytes has yet to be examined specifically in wound-healing models, together these studies imply that VEGF could directly contribute to the repair of the epidermal barrier during wound healing by stimulating keratinocytes.
Direct effects of VEGF on monocytes/macrophages
In addition to keratinocytes, myeloid cells (monocytes and macrophages) are another important cell type for wound healing known to respond directly to VEGF. In addition to fighting off potential pathogens, macrophages are involved in multiple stages of the wound-healing process. Although the importance of macrophages in wound repair has been the subject of much debate,87,88, recent studies have demonstrated the importance of macrophages for proper wound healing.89–91 Studies using animal models in which macrophages were specifically targeted using diphtheria toxin have been particularly useful for elucidating the role of macrophages in wound healing. Treatment with diphtheria toxin in mice containing macrophages that express the diphtheria toxin receptor has been used to ablate macrophages during the early phases of repair. This caused a delay in wound closure and re-epithelialization as well as reduced angiogenesis and granulation tissue formation.90,91
In addition to defending against infection by intercepting and killing potentially harmful microbes that enter the skin when it is damaged and stimulating early inflammatory processes, macrophages also play an important role in the resolution of inflammation. Macrophages stimulate resolution in part by eliminating apoptotic neutrophils within the wound and producing anti-inflammatory and proresolving mediators. This is usually accompanied by a switch in macrophage henotype from proinflammatory M1 macrophages (classically activated) to anti-inflammatory/prohealing M2 macrophages (alternatively activated).92 Studies have shown that in slowly healing diabetic wounds, macrophages do not effectively remove apoptotic neutrophils89 and the number of M1 macrophages outweigh the M2 macrophages.93,94 This could contribute to the persistent inflammation observed in chronic, nonhealing wounds. Together, these data highlight the importance of macrophages in wound healing.
In order to aid in the wound repair process, an appropriate number of macrophages must be recruited to the wound site. Proinflammatory mediators produced after injury stimulate the activation of resident macrophages and the recruitment of monocytes from circulation, which eventually mature into tissue macrophages. Interestingly, monocytes and macrophages express VEGFR-1 and VEGF has been shown to increase the migration of these cells in vitro through VEGFR-1.95–97 Studies using multiple animal models have also shown that VEGF enhances macrophage recruitment in vivo.98–101 A role for VEGF in macrophage recruitment has also been demonstrated in the skin. A higher density of macrophages has been reported in wounds from VEGF transgenic mice.58 Additionally, in an orthotopic tumor model, subcutaneous injection of a VEGF-transfected keratinocyte cell line caused an increase in the number of macrophages compared to control cells.102 A recent study has shown that Notch-1 influences the number of macrophages recruited to skin wounds and that Notch-1 signaling regulates VEGFR-1 expression on the surface of macrophages.103
In addition to macrophage recruitment, recent studies have also suggested that VEGF stimulates the uptake of apoptotic cells by macrophages through VEGFR-1.104 If this occurs during wound healing, VEGF could aid in the resolution of inflammation. VEGF has also been shown to induce macrophage apoptosis through upregulation of the proapoptotic molecule tumor necrosis factor superfamily member 14, suggesting that VEGF could also help stimulate the resolution of inflammation by enhancing the removal of macrophages during late stages of inflammation via apoptosis.105 Despite the established importance of macrophages in wound healing and the evidence indicating that macrophages respond directly to VEGF, the role of VEGF on macrophage function has not been well studied during the process of wound repair.
Future Directions
Altering VEGF for therapeutic purposes
Given that the levels of VEGF are low in wounds that exhibit delayed healing and that augmenting VEGF accelerates healing in many animal studies, VEGF has been discussed as a potential therapy for recalcitrant wounds. Increasing VEGF should enhance angiogenesis (and vasculogenesis), resulting in the formation of more blood vessels and, as a result, improve blood flow in the area of the wound. Clinical trials have been performed using topical recombinant human VEGF (telburmin, Genentech, South San Fransisco, CA) on diabetic foot ulcers;106 however, even though positive trends in incidence of complete healing and time to complete healing were reported, no published information is available beyond these phase I studies.
While it seems reasonable that VEGF treatment would improve healing, there are some problems with this approach. In general, topical treatment with a single growth factor has not been as effective as predicted for the treatment of chronic wounds. One impediment to the potential healing benefits of growth factor therapy is the hostile environment of chronic wounds. These wounds tend to have very high levels of proteases that cleave and inactivate proteins. Plasmin, which is found at high levels in nonhealing wounds, is capable of cleaving VEGF and inhibiting its biological activity.71 The development of protease-resistant forms of growth factors or delivery of growth factors embedded in ECM components could be used to overcome these obstacles.107,108 Another issue with VEGF in particular is that it has strong effects on vascular permeability, and high VEGF levels are often associated with leaky, immature, poorly perfused vessels.109 It is possible that a strategy to combine VEGF with other factors could be used to facilitate the formation of more stable, mature vessels, which would be more effective in delivering oxygen to the wound site.
While most of the studies published to date have suggested that VEGF is beneficial for wound closure, there is some evidence that heightened VEGF levels might exacerbate scar tissue formation and/or lead to abnormal scarring.73–80 This suggests that inhibiting VEGF might be useful for reducing scar formation. Reducing VEGF and the overall number of blood vessels in a healing wound might seem risky; however, a significant proportion of new blood vessels in a wound are immature and not perfused.27 As immature vessels are more sensitive to anti-VEGF drugs,110 it may be possible to reduce the overall number of blood vessels by selectively pruning nonfunctional vessels without having a negative impact on stable vessels and oxygen delivery. This type of therapy might be useful for nonsevere, superficial wounds in a patient who might be willing to accept slightly slower healing for a better cosmetic outcome or in patients at risk for developing abnormal scars such as keloids. In the future, more work needs to be done to determine whether targeting VEGF is a viable clinical approach to reduce the appearance of scars in humans.
TAKE-HOME MESSAGES.
• New blood vessel formation, or neovascularization, is important for successful tissue restoration during wound repair. The new blood vessels formed during wound healing supply the damaged skin with oxygen and nutrients from the blood. Neovascularization can occur through vasculogenesis (de novo formation of new blood vessels) or angiogenesis (creation of new blood vessels from existing vessels).
• Damage to the skin stimulates the production of mediators that promote neovascularization. VEGF, which stimulates vasculogenesis and angiogenesis, is one of the most important proangiogenic factors in the skin. VEGF is present at high levels in cutaneous wounds, and multiple cells types, including keratinocytes, macrophages, and fibroblasts, produce VEGF in response to injury.
• The importance of VEGF in the repair process is well documented, especially as a proangiogenic factor. Animal studies have shown that augmenting VEGF results in accelerated healing. Conversely, animals that have reduced levels of VEGF tend to heal more slowly. Examination of human samples has suggested that low VEGF activity contributes to chronic, nonhealing wounds.
• While most studies have indicated a beneficial role for VEGF, this growth factor may also promote scar formation. This could mean that optimal VEGF levels may be different depending on the wound type; it may be ideal to supplement VEGF in nonhealing wounds, whereas it may be beneficial to limit VEGF activity in wounds for which cosmetic outcome is a priority.
• Overall, studies have shown that VEGF influences the repair process at multiple stages by stimulating endothelial cells and inducing angiogenesis; however, VEGF likely plays a broader role in wound healing than previously believed due to its ability to affect multiple cells types involved in the repair process, including keratinocytes and macrophages. More work needs to be done to fully characterize the actions of VEGF during wound repair.
Summary
It is clear that VEGF is active during the wound repair process and that VEGF levels can influence the speed and quality of repair. Optimal levels of VEGF may vary depending on the type of wound. Inadequate VEGF levels can contribute to impaired healing and the development of chronic, nonhealing wounds, whereas high VEGF levels could promote overabundant scar tissue formation. Despite the large number of studies focusing on VEGF and wound healing, there is still a lack of information about how VEGF can affect the behavior of cells other than endothelial cells in the wound that are capable of responding to VEGF, such as keratinocytes and macrophages.
Abbreviations and Acronyms
- AAV
adeno-associated virus
- BM
basement membrane
- bFGF
basic fibroblast growth factor
- BAD
bcl-2-associated death promoter
- BV
blood vessel
- BM
bone marrow
- CXCL-10
CXC motif ligand-10
- CXCR3
CXC chemokine receptor 3
- DAG
diacylglycerol
- EC
endothelial cell
- EPC
endothelial progenitor cell
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- IP3
inositol 1,4,5-triphosphate
- IL-8
interleukin-8
- KC
keratinocyte
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase/ extracellular signal-regulated kinase kinase
- NRP
neuropilin
- PI3K
phosphatidylinositol 3-kinase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol 3,4,5-triphosphate
- PLC
phospholipase C
- SPK
sphingosine kinase
- P1GF
placental growth factor
- PDGF
platelet-derived growth factor
- Akt/PKB
protein kinase B
- PKC
protein kinase C
- sVEGFR-1
soluble vascular endothelial growth factor receptor-1
- TGF-β
transforming growth factor-β
- TNFSF14/LIGHT
tumor necrosis factor superfamily member 14
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Acknowledgments and Funding Sources
The authors have received funding from the following sources: National Institutes of Health grants T32GM068412 (K.E.J.) and R01CA127109 (T.A.W.).
Author Disclosure and Ghostwriting
The authors declare that they have no competing financial interests to disclose. The content of this article was expressly written by the authors listed and no ghostwriters were used.
About the Authors
Kelly E. Johnson, PhD, is a recent graduate of the Biomedical Sciences Graduate Program at The Ohio State University and Traci A. Wilgus, PhD, is an Assistant Professor in the Department of Pathology at The Ohio State University. Their research focuses on examining the contribution of inflammation and angiogenesis to wound healing and skin carcinogenesis. Recent interests include understanding the role of VEGF on nonendothelial cell types during wound healing.
References
- 1.Gurtner GC, Werner S, Barrandon Y, and Longaker MT: Wound repair and regeneration. Nature 2008; 453:314. [DOI] [PubMed] [Google Scholar]
- 2.Martin P: Wound healing—aiming for perfect skin regeneration. Science 1997; 276:75. [DOI] [PubMed] [Google Scholar]
- 3.Singer AJ. and Clark RA: Cutaneous wound healing. N Engl J Med 1999; 341:738. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. and Jain RK: Molecular mechanisms and clinical applications of angiogenesis. Nature 2011; 473:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P: Angiogenesis in health and disease. Nat Med 2003; 9:653. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Kalka C, Masuda H, et al. : Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5:434. [DOI] [PubMed] [Google Scholar]
- 7.Tepper OM, Capla JM, Galiano RD, et al. : Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 2005; 105:1068. [DOI] [PubMed] [Google Scholar]
- 8.Asahara T, Masuda H, Takahashi T, et al. : Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85:221. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt H, Golding M, Fruttiger M, et al. : VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003; 161:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. and Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86:353. [DOI] [PubMed] [Google Scholar]
- 11.Brown NJ, Smyth EA, Cross SS, and Reed MW: Angiogenesis induction and regression in human surgical wounds. Wound Repair Regen 2002; 10:245. [DOI] [PubMed] [Google Scholar]
- 12.Swift ME, Kleinman HK, and DiPietro LA: Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest 1999; 79:1479. [PubMed] [Google Scholar]
- 13.Gosain A, Matthies AM, Dovi JV, Barbul A, Gamelli RL, and DiPietro LA: Exogenous pro-angiogenic stimuli cannot prevent physiologic vessel regression. J Surg Res 2006; 135:218. [DOI] [PubMed] [Google Scholar]
- 14.Bodnar RJ, Yates CC, Rodgers ME, Du X, and Wells A: IP-10 induces dissociation of newly formed blood vessels. J Cell Sci 2009; 122:2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluff JE, Ferguson MW, O'Kane S, and Ireland G: Bone marrow-derived endothelial progenitor cells do not contribute significantly to new vessels during incisional wound healing. Exp Hematol 2007; 35:500. [DOI] [PubMed] [Google Scholar]
- 16.Stallmeyer B, Pfeilschifter J, and Frank S: Systemically and topically supplemented leptin fails to reconstitute a normal angiogenic response during skin repair in diabetic ob/ob mice. Diabetologia 2001; 44:471. [DOI] [PubMed] [Google Scholar]
- 17.Galeano M, Deodato B, Altavilla D, et al. : Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia 2003; 46:546. [DOI] [PubMed] [Google Scholar]
- 18.Liu PY, Tong W, Liu K, et al. : Liposome-mediated transfer of vascular endothelial growth factor cDNA augments survival of random-pattern skin flaps in the rat. Wound Repair Regen 2004; 12:80. [DOI] [PubMed] [Google Scholar]
- 19.Jacobi J, Tam BY, Sundram U, et al. : Discordant effects of a soluble VEGF receptor on wound healing and angiogenesis. Gene Ther 2004; 11:302. [DOI] [PubMed] [Google Scholar]
- 20.Berger AC, Feldman AL, Gnant MF, et al. : The angiogenesis inhibitor, endostatin, does not affect murine cutaneous wound healing. J Surg Res 2000; 91:26. [DOI] [PubMed] [Google Scholar]
- 21.Lange-Asschenfeldt B, Velasco P, Streit M, et al. : The angiogenesis inhibitor vasostatin does not impair wound healing at tumor-inhibiting doses. J Invest Dermatol 2001; 117:1036. [DOI] [PubMed] [Google Scholar]
- 22.Jang YC, Arumugam S, Gibran NS, and Isik FF: Role of alpha(v) integrins and angiogenesis during wound repair. Wound Repair Regen 1999; 7:375. [DOI] [PubMed] [Google Scholar]
- 23.Roman CD, Choy H, Nanney L, et al. : Vascular endothelial growth factor-mediated angiogenesis inhibition and postoperative wound healing in rats. J Surg Res 2002; 105:43. [DOI] [PubMed] [Google Scholar]
- 24.Klein SA, Bond SJ, Gupta SC, Yacoub OA, and Anderson GL: Angiogenesis inhibitor TNP-470 inhibits murine cutaneous wound healing. J Surg Res 1999; 82:268. [DOI] [PubMed] [Google Scholar]
- 25.Streit M, Velasco P, Riccardi L, et al. : Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. Embo J 2000; 19:3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossiter H, Barresi C, Pammer J, et al. : Loss of vascular endothelial growth factor a activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res 2004; 64:3508. [DOI] [PubMed] [Google Scholar]
- 27.Bluff JE, O'Ceallaigh S, O'Kane S, Ferguson MW, and Ireland G: The microcirculation in acute murine cutaneous incisional wounds shows a spatial and temporal variation in the functionality of vessels. Wound Repair Regen 2006; 14:434. [DOI] [PubMed] [Google Scholar]
- 28.Yano K, Brown LF, and Detmar M: Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest 2001; 107:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detmar M, Brown LF, Claffey KP, et al. : Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 1994; 180:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larcher F, Murillas R, Bolontrade M, Conti CJ, and Jorcano JL: VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene 1998; 17:303. [DOI] [PubMed] [Google Scholar]
- 31.Park JE, Keller GA, and Ferrara N: The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 1993; 4:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, and Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983; 219:983. [DOI] [PubMed] [Google Scholar]
- 33.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, and Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246:1306. [DOI] [PubMed] [Google Scholar]
- 34.Gerber HP, McMurtrey A, Kowalski J, et al. : Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998; 273:30336. [DOI] [PubMed] [Google Scholar]
- 35.Keck PJ, Hauser SD, Krivi G, et al. : Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989; 246:1309. [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P, Ferreira V, Breier G, et al. : Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380:435. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N, Carver-Moore K, Chen H, et al. : Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380:439. [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P. and Ruiz de Almodovar C: VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci 2013; 70:1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrara N, Gerber HP, and LeCouter J: The biology of VEGF and its receptors. Nat Med 2003; 9:669. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya M: Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 2013; 153:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch S. and Claesson-Welsh L: Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2012; 2:a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kendall RL. and Thomas KA: Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993; 90:10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soker S, Miao HQ, Nomi M, Takashima S, and Klagsbrun M: VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem 2002; 85:357. [DOI] [PubMed] [Google Scholar]
- 44.Brown LF, Yeo KT, Berse B, et al. : Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992; 176:1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nissen NN, Polverini PJ, Gamelli RL, and DiPietro LA: Basic fibroblast growth factor mediates angiogenic activity in early surgical wounds. Surgery 1996; 119:457. [DOI] [PubMed] [Google Scholar]
- 46.Kishimoto J, Ehama R, Ge Y, et al. : In vivo detection of human vascular endothelial growth factor promoter activity in transgenic mouse skin. Am J Pathol 2000; 157:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willenborg S, Lucas T, van Loo G, et al. : CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012; 120:613. [DOI] [PubMed] [Google Scholar]
- 48.Shiota N, Nishikori Y, Kakizoe E, et al. : Pathophysiological role of skin mast cells in wound healing after scald injury: study with mast cell-deficient W/W(V) mice. Int Arch Allergy Immunol 2010; 151:80. [DOI] [PubMed] [Google Scholar]
- 49.Stockmann C, Kirmse S, Helfrich I, et al. : A wound size-dependent effect of myeloid cell-derived vascular endothelial growth factor on wound healing. J Invest Dermatol 2011; 131:797. [DOI] [PubMed] [Google Scholar]
- 50.Elson DA, Ryan HE, Snow JW, Johnson R, and Arbeit JM: Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res 2000; 60:6189. [PubMed] [Google Scholar]
- 51.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, and Roy S: Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem 2002; 277:33284. [DOI] [PubMed] [Google Scholar]
- 52.Brauchle M, Funk JO, Kind P, and Werner S: Ultraviolet B and H2O2 are potent inducers of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem 1996; 271:21793. [DOI] [PubMed] [Google Scholar]
- 53.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, and Werner S: Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem 1995; 270:12607. [DOI] [PubMed] [Google Scholar]
- 54.Seitz O, Schurmann C, Pfeilschifter J, Frank S, and Sader R: Identification of the Fra-1 transcription factor in healing skin flaps transplants: a potential role as a negative regulator of VEGF release from keratinocytes. J Craniomaxillofac Surg 2012; 40:379. [DOI] [PubMed] [Google Scholar]
- 55.Collins PD, Connolly DT, and Williams TJ: Characterization of the increase in vascular permeability induced by vascular permeability factor in vivo. Br J Pharmacol 1993; 109:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Detmar M, Brown LF, Schon MP, et al. : Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998; 111:1. [DOI] [PubMed] [Google Scholar]
- 57.Wulff BC. and Wilgus TA: Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol 2013; 22:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong YK, Lange-Asschenfeldt B, Velasco P, et al. : VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J 2004; 18:1111. [DOI] [PubMed] [Google Scholar]
- 59.Deodato B, Arsic N, Zentilin L, et al. : Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther 2002; 9:777. [DOI] [PubMed] [Google Scholar]
- 60.Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, and Mustoe TA: Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg 1999; 134:200. [DOI] [PubMed] [Google Scholar]
- 61.Vranckx JJ, Yao F, Petrie N, et al. : In vivo gene delivery of Ad-VEGF121 to full-thickness wounds in aged pigs results in high levels of VEGF expression but not in accelerated healing. Wound Repair Regen 2005; 13:51. [DOI] [PubMed] [Google Scholar]
- 62.Wilgus TA, Matthies AM, Radek KA, et al. : Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol 2005; 167:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobi J, Tam BY, Sundram U, et al. : Discordant effects of a soluble VEGF receptor on wound healing and angiogenesis. Gene Ther 2004; 11:302. [DOI] [PubMed] [Google Scholar]
- 64.Romano Di Peppe S, Mangoni A, Zambruno G, et al. : Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther 2002; 9:1271. [DOI] [PubMed] [Google Scholar]
- 65.Galiano RD, Tepper OM, Pelo CR, et al. : Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 2004; 164:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brem H, Kodra A, Golinko MS, et al. : Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol 2009; 129:2275. [DOI] [PubMed] [Google Scholar]
- 67.Wang XT, Avanessian B, Ma Q, Durfee H, Tang YQ, and Liu PY: Enhancement of flap survival and changes in angiogenic gene expression after AAV2-mediated VEGF gene transfer to rat ischemic flaps. Wound Repair Regen 2011; 19:498. [DOI] [PubMed] [Google Scholar]
- 68.Giunta RE, Holzbach T, Taskov C, et al. : AdVEGF165 gene transfer increases survival in overdimensioned skin flaps. J Gene Med 2005; 7:297. [DOI] [PubMed] [Google Scholar]
- 69.Taub PJ, Marmur JD, Zhang WX, et al. : Locally administered vascular endothelial growth factor cDNA increases survival of ischemic experimental skin flaps. Plast Reconstr Surg 1998; 102:2033. [DOI] [PubMed] [Google Scholar]
- 70.Christoforidis JB, Wang J, Jiang A, et al. : The effect of intravitreal bevacizumab and ranibizumab on cutaneous tensile strength during wound healing. Clin Ophthalmol 2013; 7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lauer G, Sollberg S, Cole M, et al. : Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol 2000; 115:12. [DOI] [PubMed] [Google Scholar]
- 72.Eming SA, Lauer G, Cole M, et al. : Increased levels of the soluble variant of the vascular endothelial growth factor receptor VEGFR-1 are associated with a poor prognosis in wound healing. J Invest Dermatol 2004; 123:799. [DOI] [PubMed] [Google Scholar]
- 73.Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, and Dipietro LA: Regulation of scar formation by vascular endothelial growth factor. Lab Invest 2008; 88:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hakvoort T, Altun V, van Zuijlen PP, de Boer WI, van Schadewij WA, and van der Kwast TH: Transforming growth factor-beta(1), -beta(2), -beta(3), basic fibroblast growth factor and vascular endothelial growth factor expression in keratinocytes of burn scars. Eur Cytokine Netw 2000; 11:233. [PubMed] [Google Scholar]
- 75.Zhu KQ, Engrav LH, Armendariz R, et al. : Changes in VEGF and nitric oxide after deep dermal injury in the female, red Duroc pig-further similarities between female, Duroc scar and human hypertrophic scar. Burns 2005; 31:5. [DOI] [PubMed] [Google Scholar]
- 76.Gira AK, Brown LF, Washington CV, Cohen C, and Arbiser JL: Keloids demonstrate high-level epidermal expression of vascular endothelial growth factor. J Am Acad Dermatol 2004; 50:850. [DOI] [PubMed] [Google Scholar]
- 77.Wu Y, Zhang Q, Ann DK, et al. : Increased vascular endothelial growth factor may account for elevated level of plasminogen activator inhibitor-1 via activating ERK1/2 in keloid fibroblasts. Am J Physiol Cell Physiol 2004; 286:C905. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Chen H, Shankowsky HA, Scott PG, and Tredget EE: Improved scar in postburn patients following interferon-alpha2b treatment is associated with decreased angiogenesis mediated by vascular endothelial cell growth factor. J Interferon Cytokine Res 2008; 28:423. [DOI] [PubMed] [Google Scholar]
- 79.Salem A, Assaf M, Helmy A, et al. : Role of vascular endothelial growth factor in keloids: a clinicopathologic study. Int J Dermatol 2009; 48:1071. [DOI] [PubMed] [Google Scholar]
- 80.Wu WS, Wang FS, Yang KD, Huang CC, and Kuo YR: Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol 2006; 126:1264. [DOI] [PubMed] [Google Scholar]
- 81.Beck B, Driessens G, Goossens S, et al. : A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011; 478:399. [DOI] [PubMed] [Google Scholar]
- 82.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, and Sibilia M: Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010; 140:268. [DOI] [PubMed] [Google Scholar]
- 83.Riese A, Eilert Y, Meyer Y, et al. : Epidermal expression of neuropilin 1 protects murine keratinocytes from UVB-induced apoptosis. PLoS One 2012; 7:e50944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith JR, Lanier VB, Braziel RM, Falkenhagen KM, White C, and Rosenbaum JT: Expression of vascular endothelial growth factor and its receptors in rosacea. Br J Ophthalmol 2007; 91:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Man XY, Yang XH, Cai SQ, Yao YG, and Zheng M: Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Mol Med 2006; 12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu JW, Wu XJ, Luo D, Lu ZF, Cai SQ, and Zheng M: Activation of VEGFR-2 signaling in response to moderate dose of ultraviolet B promotes survival of normal human keratinocytes. Int J Biochem Cell Biol 2012; 44:246. [DOI] [PubMed] [Google Scholar]
- 87.Leibovich SJ. and Ross R: The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975; 78:71. [PMC free article] [PubMed] [Google Scholar]
- 88.Martin P, D'Souza D, Martin J, et al. : Wound healing in the PU. 1 null mouse–tissue repair is not dependent on inflammatory cells. Curr Biol 2003; 13:1122. [DOI] [PubMed] [Google Scholar]
- 89.Khanna S, Biswas S, Shang Y, et al. : Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 2010; 5:e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lucas T, Waisman A, Ranjan R, et al. : Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184:3964. [DOI] [PubMed] [Google Scholar]
- 91.Mirza R, DiPietro LA, and Koh TJ: Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009; 175:2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novak ML. and Koh TJ: Macrophage phenotypes during tissue repair. J Leukoc Biol 2013; 93:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mirza R. and Koh TJ: Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011; 56:256. [DOI] [PubMed] [Google Scholar]
- 94.Mirza RE, Fang MM, Ennis WJ, and Koh TJ: Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013; 62:2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen H, Clauss M, Ryan J, et al. : Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood 1993; 81:2767. [PubMed] [Google Scholar]
- 96.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, and Marme D: Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996; 87:3336. [PubMed] [Google Scholar]
- 97.Sawano A, Iwai S, Sakurai Y, et al. : Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 2001; 97:785. [DOI] [PubMed] [Google Scholar]
- 98.Murakami M, Iwai S, Hiratsuka S, et al. : Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood 2006; 108:1849. [DOI] [PubMed] [Google Scholar]
- 99.Murakami M, Zheng Y, Hirashima M, et al. : VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol 2008; 28:658. [DOI] [PubMed] [Google Scholar]
- 100.Muramatsu M, Yamamoto S, Osawa T, and Shibuya M: Vascular endothelial growth factor receptor-1 signaling promotes mobilization of macrophage lineage cells from bone marrow and stimulates solid tumor growth. Cancer Res 2010; 70:8211. [DOI] [PubMed] [Google Scholar]
- 101.Kerber M, Reiss Y, Wickersheim A, et al. : Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res 2008; 68:7342. [DOI] [PubMed] [Google Scholar]
- 102.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, and Mueller MM: Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol 2012; 227:17. [DOI] [PubMed] [Google Scholar]
- 103.Outtz HH, Wu JK, Wang X, and Kitajewski J: Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. J Immunol 2010; 185:4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kearns MT, Dalal S, Horstmann SA, et al. : Vascular endothelial growth factor enhances macrophage clearance of apoptotic cells. Am J Physiol Lung Cell Mol Physiol 2012; 302:L711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petreaca ML, Yao M, Ware C, and Martins-Green MM: Vascular endothelial growth factor promotes macrophage apoptosis through stimulation of tumor necrosis factor superfamily member 14 (TNFSF14/LIGHT). Wound Repair Regen 2008; 16:602. [DOI] [PubMed] [Google Scholar]
- 106.Hanft JR, Pollak RA, Barbul A, et al. : Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care 2008; 17:30. [DOI] [PubMed] [Google Scholar]
- 107.Lauer G, Sollberg S, Cole M, Krieg T, and Eming SA: Generation of a novel proteolysis resistant vascular endothelial growth factor165 variant by a site-directed mutation at the plasmin sensitive cleavage site. FEBS Lett 2002; 531:309. [DOI] [PubMed] [Google Scholar]
- 108.Upton Z, Wallace HJ, Shooter GK, et al. : Human pilot studies reveal the potential of a vitronectin: growth factor complex as a treatment for chronic wounds. Int Wound J 2011; 8:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thurston G, Suri C, Smith K, et al. : Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999; 286:2511. [DOI] [PubMed] [Google Scholar]
- 110.Benjamin LE, Golijanin D, Itin A, Pode D, and Keshet E: Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 1999; 103:159. [DOI] [PMC free article] [PubMed] [Google Scholar]