Abstract
The FDA-approved glucagon-like-peptide-1 receptor (GLP-1R) agonists exendin-4 and liraglutide reduce food intake and body weight. Nausea is the most common adverse side effect reported with these GLP-1R agonists. Whether food intake suppression by exendin-4 and liraglutide occurs independently of nausea is unknown. Further, the neurophysiological mechanisms mediating the nausea associated with peripheral GLP-1R agonist use are poorly understood. Using two established rodent models of nausea [conditioned taste avoidance (CTA) and pica (ingestion of non-nutritive substances)], results show that all peripheral doses of exendin-4 that suppress food intake also produce CTA, whereas one dose of liraglutide suppresses intake without producing CTA. Chronic (12 days) daily peripheral administration of exendin-4 produces a progressive increase in pica coupled with stable, sustained food intake and body weight suppression, whereas the pica response and food intake reduction by daily liraglutide are more transient. Results demonstrate that the nausea response accompanying peripheral exendin-4 occurs via a vagal-independent pathway involving GLP-1R activation in the brain as the exendin-4-induced pica response is attenuated with CNS co-administration of the GLP-1R antagonist exendin-(9-39), but not by vagotomy. Direct administration of exendin-4 to the medial subnucleus of the nucleus tractus solitarius (mNTS), but not to the central nucleus of the amygdala, reduced food intake and produced a pica response, establishing the mNTS as a potential GLP-1R-expressing site mediating nausea responses associated with GLP-1R agonists.
Keywords: GLP-1, diabetes, malaise, obesity, Victoza, Byetta, exenatide, vomiting
1. Introduction
The prevalence of obesity in the USA has increased by 75% since the early 1980s, with over 1/3 of adults now classified as obese (2011; Ogden et al., 2007). Despite this alarming trend, the development of effective pharmacological treatments for obesity has thus far proven unsuccessful. Recent attention has been devoted to glucagon-like-peptide-1 (GLP-1) and its receptor (GLP-1R) as a promising target for obesity treatment. GLP-1 is an incretin hormone synthesized and secreted principally from two locations: the L cells in the small intestine and neurons in the nucleus tractus solitarius (NTS) of the hindbrain (Holst, 2007). In addition to glycemic control, the GLP-1 system is also involved in regulating food intake in both human and animal models [see (Hayes et al., 2010; Holst and Seino, 2009; Knudsen, 2010) for review]; however, clinical use of the native peptide for obesity and hyperglycemia is limited by its rapid degradation rate by the dipeptidyl-peptidase-4 (DPP-IV) enzyme and other endopeptidases. Longer-acting pharmacological GLP-1R agonists that are resistant to enzymatic degradation, including exendin-4 (trade name Byetta) and liraglutide (trade name Victoza), are currently approved for human Type 2 Diabetes Mellitus (T2DM) treatment and may also hold promise for obesity treatment. While GLP-1R ligand-based pharmacotherapies present negligible risk of life-threatening adverse events (Blonde et al., 2006a; Buse et al., 2009; Montanya and Sesti, 2009; Pinkney et al., 2010; Russell-Jones, 2010), unfortunately GLP-1R agonists are not devoid of side effects that negatively impact quality of life and produce treatment attrition. Most notably, nausea and malaise is reported in up to ~50% of subjects prescribed exenatide (synthetic version of exendin-4) (Bergenstal et al., 2010; Buse et al., 2004; Buse et al., 2009; DeFronzo et al., 2005) and up to ~30% of subjects prescribed liraglutide (Astrup et al., 2009; Buse et al., 2009) in clinical trials, which contributes to discontinuation of drug treatment in ~6–10% and reduced dose tolerance in another ~15% (Bergenstal et al., 2010; Buse et al., 2004; DeFronzo et al., 2005; John et al., 2007; Kendall et al., 2005) of patients. Thus, it is clear that a better understanding of the neurophysiological mechanisms that mediate nausea and related side effects produced by GLP-1R ligand therapy is needed to provide a foundation for improved GLP-1R-based treatments for T2DM and obesity.
Whether the food intake reduction by GLP-1 and GLP-1R ligands is only secondary to the induction of nausea is a concept worthy of consideration, but one poorly understood. This lack of knowledge is partially attributable to the fact that unlike emesis (i.e., retching and vomiting), the subjective experience of nausea cannot be overtly measured in humans, which is underscored by the fact that available patient self-reporting tools for nausea have poor validity and reliability (Brearley et al., 2008; Wood et al., 2011). However, in rodents which lack the anatomy and physiology necessary for vomiting (Andrews and Horn, 2006), two quantitative experimental models are available for the behavioral study of nausea/malaise: conditioned taste aversion/avoidance (CTA), which is the aversion to or avoidance of flavors or foods paired with illness (Garcia et al., 1955), and pica, which is the consumption of nonnutritive substances (e.g., kaolin clay) in response to nausea-inducing agents (De Jonghe and Horn, 2008; De Jonghe et al., 2009; Mitchell et al., 1976), a behavior which may represent an adaptive response to reduce the adverse effects of toxins on the organism. Previous reports show that GLP-1 administered directly to the central nucleus of the amygdala (CeA) produces a CTA in rats, and that the CTA and the food intake suppression produced by the toxin LiCl is attenuated with CeA GLP-1R blockade (Kinzig et al., 2002b; Rinaman, 1999). Kinzig and colleagues further reported that hindbrain GLP-1 administration reduces food intake without producing a CTA (Kinzig et al., 2002b), suggesting there may be a GLP-1R pathway capable of reducing food intake independent of nausea. These findings signify that CNS GLP-1R signaling appears to be involved with the neural processing of visceral malaise; however, the mechanisms mediating the nausea produced from peripheral administration of GLP-1R agonists remain unexplored. Understanding the physiological and neuronal pathways mediating the nausea response by peripheral GLP-1R agonists is very clinically relevant given that 1) long-acting GLP-1R agonists exendin-4 and liraglutide are administered peripherally for human T2DM treatment, and 2) these GLP-1R ligands produce nausea in a substantial percentage of patients.
Native GLP-1, secreted endogenously from intestinal L cells, appears to reduce food intake by acting in a paracrine-like fashion on adjacent GLP-1R expressed on vagal afferents innervating the GI tract instead of acting directly on GLP-1R in the brain (Hayes et al., 2010). This notion is supported by findings that circulating levels of GLP-1 are minimal due to rapid enzymatic degradation after release (Holst, 2007), and that peripheral (IP) GLP-1 administration, resembling the physiological profile of action for intestinally-derived peptides (Lo et al., 2009; Lukas et al., 1971), does not reduce food intake following selective ablation of subdiaphragmatic vagal afferent fibers (Ruttimann et al., 2009). This is not the case, however, for longer-acting GLP-1R agonists. A recent report demonstrated that food intake suppression by IP administration of exendin-4 and liraglutide is mediated by both a vagal afferent-dependent, as well as a vagal afferent-independent pathway that likely involves blood-brain barrier (BBB) penetrance and subsequent direct CNS GLP-1R activation (Kanoski et al., 2011). The extent to which the nausea response following peripheral administration of longer-acting GLP-1R agonists involves vagal afferent signaling, direct CNS GLP-1R activation, or both is unknown and is a focus of this report.
Here, we employ two established rat models of nausea, CTA and pica, to explore whether food intake suppression by exendin-4 and liraglutide occurs independent of nausea or malaise and to establish whether nausea produced by the GLP-1R agonists requires vagal communication and/or action of CNS GLP-1R. Overall, results show that the food intake suppressive effects of the peripherally administered liraglutide and exendin-4 appear to be based, in part, on nausea mediated by CNS GLP-1R activation. Activation of GLP-1R expressed in the mNTS, but not the CeA, is shown to reduce food intake with concomitant pica.
2. Materials and Methods
2.1. Animals and Drugs
Adult male Sprague-Dawley rats (Charles River Laboratories; 250–265 at time of purchase), housed individually in hanging metal cages under a 12h light/dark cycle (lights on 0900 h), had ad libitum access to rodent chow (Purina 5001; St. Louis, MO) and water except where noted. For one experiment (Experiment 1b), rats were maintained in a reverse 12h light/dark cycle (lights on 2100 h). All procedures conformed to and receive approval from the institutional standards of The University of Pennsylvania Animal Care and Use Committee.
Exendin-4 (American Peptide Company), Liraglutide (Bachem), and LiCl (Fischer Pharmaceuticals) were dissolved in sterile 0.9% NaCl. Exendin-(9-39) and exendin-4 (American Peptide Company) were dissolved in artificial cerebrospinal fluid (aCSF) for ICV injections. The volume for IP administrations was 1ml/kg body weight, except for LiCl, which was given 1.33ml/100g body weight.
2.2. Surgeries
2.2.1. Cannula implantation
Under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (Metacam 2mg/kg), guide cannulae (Plastics One; 26-guage) cemented to the skull using four jewelers screws were implanted at the following coordinates: for 3rd ICV, 2.0 mm caudal to bregma, on midline, 7.7 mm below skull surface; for mNTS, 1 mm caudal to occipital suture, 0.75 mm from midline, 6.9 mm below skull surface; for CeA, 2.2 mm caudal to bregma, 4 mm from midline, 6.4 mm below skull surface. Injectors for drug administration project 2 mm beyond guide cannulae. Following a 7-day surgical recovery period, 3rd ICV and mNTS cannula placements were assessed by measurement of the sympathoadrenal-mediated hyperglycemic response to the cytoglucopenia induced by 5-thio-D-glucose (210 μg in 2μl aCSF for 3rd ICV; 24 μg in 100nl for mNTS) (Ritter et al., 1981). A post-injection elevation of at least 100% of baseline glycemia was required for subject inclusion. Coordinates for CeA cannulae placements were assessed through verification of the position of 100nl pontamine sky blue injections.
2.2.2. Subdiaphragmatic Vagotomy
Rats were habituated to liquid diet (Research Diets; AIN76A) for five days prior to surgery. Under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (Metacam 2mg/kg), the trunks of the subdiaphragmatic vagus were transected as described (Williams et al., 2000). Briefly, a midline abdominal incision was made and the dorsal and ventral branches of the vagus were dissected from the esophagus. Each branch was tied with a surgical suture at two points separated by approximately 1–2cm, and then cauterized between the sutures. Sham surgeries were also performed, in which the trunks were exposed but not tied or cauterized. The incision was closed and rats were allowed at least 1-week recovery until liquid diet intake stabilized. After the experiment, vagotomies were visually verified postmortem and functionally verified with a test of food intake suppression by IP cholecystokinin (CCK-8 4μg/kg, Bachem) or saline (treatments given counterbalanced on separate days) following an overnight fast. Vagotomy rats showing CCK-induced food intake suppression of 20% or greater relative to saline treatment were removed from all analyses.
2.3. Procedures
2.3.1. Experiment 1: Dose-response conditioned taste avoidance (CTA) and food intake suppression following IP exendin-4 and liraglutide
2.3.1.1. Experiment 1a
For conditioned taste avoidance learning [modified from (Sclafani, 2001)], rats were assigned to one of the following groups based on drug treatment (n=8/group): 1) LiCl 0.15M, 2) exendin-4 0.1 μg/kg, 3) exendin-4 0.25 μg/kg, 4) exendin-4 0.5 μg/kg, 5) exendin-4 0.75 μg/kg, 6) exendin-4 1.33 μg/kg, 7) exendin-4 3.0 μg/kg, 8) liraglutide 10 μg/kg, 9) liraglutide 50 μg/kg, and 10) liraglutide 100 μg/kg. Food was available ad libitum throughout the experiment. Rats were first habituated to a water deprivation schedule for 7 days in which water access was given once daily for 90 min in two water bottles. Water intake was monitored on day 6 to ensure that rats were drinking from each bottle. For training, IP drug or vehicle treatments were counterbalanced (within-subjects design) across two training days separated by an intervening day. On each training day during the normal 90-min water access period, rats were given two bottles containing approximately 100ml of a Kool-Aid mix (0.16 oz unsweetened Kool-Aid, 10ml saccharin, 3.5Lwater). Rats received one flavor of Kool-Aid, cherry or grape, on each training day. These flavors have previously been shown to be equally preferred in rats [e.g., (Lucas and Sclafani, 1996)]. Immediately after the 90-minute flavor exposure period, rats were given IP injections of vehicle or drug (flavor/drug pairings counterbalanced with respect to groups). Two days after the second training day, rats were given a 2-bottle preference test during the normal 90-min water access period; one bottle was presented containing the cherry Kool-Aid mix, the other containing the grape Kool-Aid mix. The side (left vs. right) of the initial flavor presentation was counterbalanced with respect to groups and treatment orders. At 45 min fluid intake was recorded and the side of flavor presentation was switched. Fluid intake was recorded again at 90 min.
2.3.1.2. Experiment 1b
For food intake analysis, a separate cohort of rats was divided into two groups to receive either liraglutide (n=9) or exendin-4 (n=9). The rats in the liraglutide group received the following six treatments using a within-subjects, Latin square design: vehicle, liraglutide 5 μg/kg, liraglutide 10 μg/kg, liraglutide 25 μg/kg, liraglutide 50 μg/kg, and liraglutide 100 μg/kg. Rats in the exendin-4 group received the following six treatments using a within-subjects, Latin square design: vehicle, exendin-4 0.1 μg/kg, exendin-4 0.25 μg/kg, exendin-5 0.5 μg/kg, exendin-4 1.33 μg/kg, and exendin-4 3.0 μg/kg. IP injections were given immediately before dark onset to nondeprived rats. Food intake (spillage accounted for) was recorded at 1, 3, 6, and 24h following injections, whereas body weight was recorded at 24h post injections. Treatments were separated by 3–4 intervening days.
2.3.2. Experiment 2: Pica, food intake, and body weight following chronic daily IP exendin-4 and liraglutide
2.3.2.1. Experiment 2a
Rats were divided into three groups (n=9/group): vehicle, exendin-4 3.0 μg/kg twice daily (b.i.d.), and liraglutide 50 μg/kg once daily (q.d.). Drug regiments were based on human clinical use for liraglutide (q.d.) and exenatide (b.i.d.) [e.g., (Astrup et al., 2009; Buse et al., 2009)], and doses were selected based on the following criteria, 1) being suprathreshold for CTA (Experiment 1a), 2) being suprathreshold for 3, 6, and 24h intake suppression following acute q.d. administration (Experiment 1b), and 3) producing comparable magnitude of 3h intake suppression relative to vehicle following acute q.d. administration (Experiment 1b). Rats were first habituated to ad libitum kaolin pellet (Research Diets; K50001) access for one week. Probe recordings of kaolin intake conducted during the habituation phase demonstrated that baseline kaolin intake was negligible (average < 0.5g; data not shown). After the habituation period, two IP injections were given each day for 12 days; the first injection occurred 90 minutes after lights onset, the second 6h later. The second injection was vehicle for the liraglutide and vehicle groups and exendin-4 for the exendin-4 group. Each day body weight, food intake, and kaolin intake were recorded prior to the first daily injection.
2.3.2.2. Experiment 2b
A separate study was conducted to determine if the linear increase in kaolin intake observed for the exendin-4 group across days (see Figure 2a) is based on a progressive increase in the magnitude of exendin-4-induced nausea, or rather, that the increase in kaolin intake is based on exendin-4-treated rats learning across days about the potential reinforcing effects of kaolin intake. To discriminate between these two possibilities, a separate cohort of rats was divided into three groups: vehicle with kaolin access beginning on day 1 (n=9), exendin-4 3.0μg/kg b.i.d. with kaolin access beginning on day 1 (n=8), and exendin-4 3.0μg/kg b.i.d. with kaolin access only on day 11 (n=8). The first two groups were treated identical to the vehicle and exendin-4 groups, respectively, in Experiment 2a. The exendin-4 3.0μg/kg b.i.d. kaolin day #11 group was habituated to the kaolin pellets before experimental injections as previously described; however, kaolin was removed from the cage for the first 10 days of treatments and returned on day #11. If the magnitude of nausea increased across days with chronic treatment, the amount of kaolin consumed on day 11 should be similar between the two exendin-4 groups.
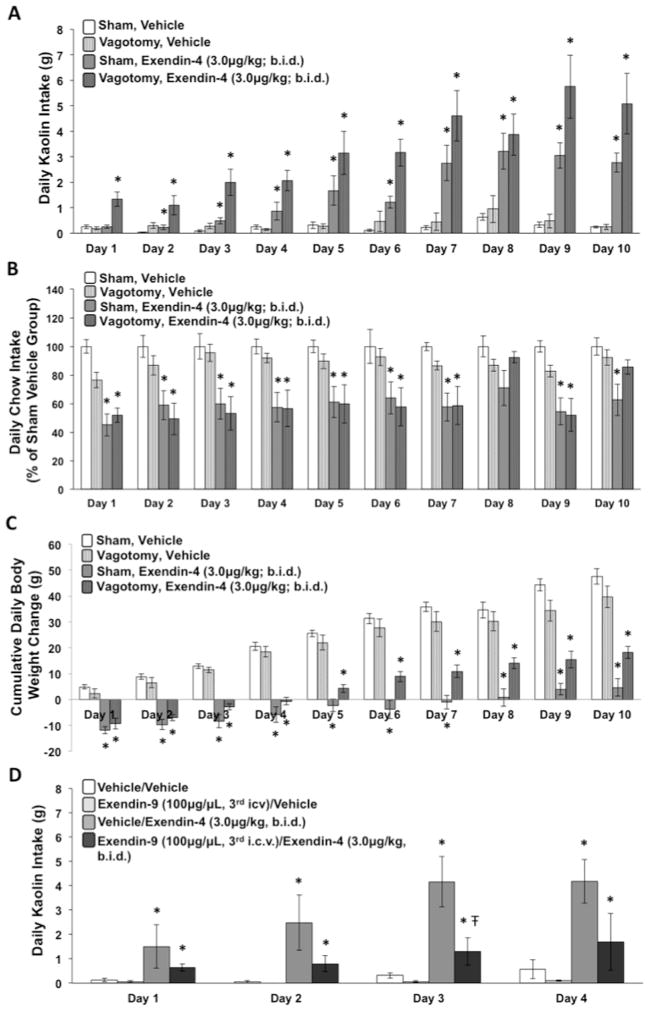
Figure 2.
Daily kaolin-clay intake (A), suppression of chow intake (B), and cumulative daily body weight change (C) in rats that received daily IP administration of exendin-4 (3.0μg/kg, b.i.d.), liraglutide (50μg/kg, q.d.), or vehicle for 12 consecutive days. Trendlines are included in (C) to show the slope of body weight change for the three groups of rats across the 12 days. (D) Cumulative intake from days 1–12 and from days 3–12 of the daily treatment period. Data are mean ± SEM, * = p < 0.05 vs. vehicle group.
2.3.3. Experiment 3: Pica, food, intake, and body weight following chronic daily IP exendin-4 in subdiaphragmatic vagotomized and control rats
To determine the role of the vagus nerve in mediating the pica response and food intake suppressive effects following chronic IP exendin-4 treatment, we examined the effects of total subdiaphragmatic vagotomy and sham lesions on kaolin and food intake during chronic daily exendin-4 3.0μg/kg b.i.d. or vehicle treatments. Treatment groups were as follows: sham vehicle (n=6), vagotomy vehicle (n=9), sham exendin-4 3.0 μg/kg b.i.d. (n=6), and vagotomy exendin-4 3.0 μg/kg b.i.d. (n=8). Procedures were identical to those described for Experiment 2a except that both groups were maintained on liquid diet instead of solid chow and the kaolin was powdered. Powdered kaolin was given instead of pelleted to reduce the likelihood of gastric stasis in vagotomy rats given that total subdiaphragmatic vagotomy is associated with impaired gastric emptying (Gutierrez et al., 1971; Tinker et al., 1970).
2.3.4. Experiment 4: Pica with combined IP exendin-4 and CNS GLP-1R blockade [3rd ICV exendin-(9-39)]
Rats were divided into four groups based on 3rd ICV treatments/IP (2μl/1ml/kg): vehicle/vehicle (n=5), vehicle/exendin-4 3.0 μg/kg b.i.d. (n=6), exendin-(9-39) 100 μg/vehicle (n=6), exendin-(9-39) 100 μg/exendin-4 3.0 μg/kg b.i.d. (n=5). ICV injections were given once daily approximately 90 minutes after lights onset; IP injections were given twice daily, 15 minutes after ICV injections and again 6h later. Treatments were given for four consecutive days and kaolin intake was recorded daily.
2.3.5. Experiment 5: Pica following ICV exendin-4
Rats were divided into two groups, vehicle (n=11) or exendin-4 0.15 μg/1 μl (n=7), for acute 3rd ICV treatment. Dose selection of ICV exendin-4 is based on our previous reports examining food intake suppression (Hayes et al., 2011b). Injections were given approximately 90 minutes after lights onset to non-food-deprived rats. Food and kaolin intake were recorded as previously described.
2.3.6. Experiment 6: Pica and CTA following NTS and CeA administration of exendin-4
2.3.6.1. Experiment 6a
Rats with cannula targeting either the mNTS (n=16) or the CeA (n=18) received intraparenchymal injections of vehicle or exendin-4 0.05 μg/100nl on two separate days (counterbalanced within-subjects design, injections separated by 3 days). Dose selection of intraparenchymal exendin-4 is based on our previous report examining food intake suppression (Hayes et al., 2011b). Injections were given approximately 90 minutes after lights onset, and food and kaolin intake were recorded 24h later.
2.3.6.2. Experiment 6b
Five days following the conclusion of Experiment 6a, a subset of the CeA rats (n = 8) and the mNTS rats (n = 10) were water restricted for the CTA paradigm. Procedures for CTA were identical to those described previously; the dose of exendin-4 was the same as Experiment 6a (0.05 μg/100nl in aCSF). Given the observed low levels of fluid intake of both the drug-paired and the vehicle-paired flavors in the two-bottle test day for the mNTS-injected rats (see Figure 7d), a separate group of rats with mNTS cannulae (n=7) was used to assess the hypothesis that this effect was based on a competing taste avoidance produced by mNTS vehicle injections. For this group the procedures for the CTA were identical to those previously described, with the exception that the rats received either mNTS vehicle (aCSF) injections or no injection counterbalanced across the two training days.
Figure 7.
24hr kaolin intake (A), chow intake (B), and body weight change (C) following an acute intraparenchymal mNTS administration of exendin-4 (0.05μg/100nl) or vehicle. In a two-bottle CTA learning paradigm, mNTS administration of exendin-4 did not produce a CTA when compared to vehicle injections (D); however, fluid intake for the vehicle-paired flavor was suppressed in the testing session compared to previous CTA testing sessions in this report. In separate rats, a CTA was produced following mNTS injection of vehicle compared to a non-injection condition (E). Data are mean ± SEM, * = p < 0.05 vs. vehicle.
2.4. Statistical Analysis
CTA and food intake were analyzed via repeated measures ANOVA with drug as a within-subjects variable. For food intake analysis Newman Keuls posthoc tests were used to compare individual doses to vehicle treatment when main effects of drug were significant. All chronic pica studies were analyzed with repeated measures ANOVA using within-subjects (days) and between-subjects (groups) factors. Separate repeated measures ANOVAs were conducted to compare each drug group to the vehicle group for Experiment 2a and to compare the two exendin-4-treated groups for Experiment 2b. Experiment 3 was analyzed with repeated-measures ANOVA using drug and surgical group as separate between-subjects factors. Experiment 4 was analyzed with repeated-measures ANOVA using ICV and IP drug group as separate between-subjects factors. Planned comparisons against vehicle treatment were also performed for food and kaolin intake for each day for all chronic studies, and to compare the two exendin-4 treated groups on each day for Experiment 4. Acute pharmacology studies were analyzed with either one-way ANOVA for between-subjects design (Experiment 5) or with repeated measures ANOVA for within-subjects design (Experiment 6).
3. Results
3.1. Experiment 1a and 1b
IP Exendin-4 administration produced a robust CTA at all doses that were also effective in suppressing food intake (Figures 1a, 1c). The lowest dose examined (0.1 μg/kg) did not produce a CTA or a significant suppression of food intake at any time point examined relative to vehicle, whereas 0.25 μg/kg exendin-4 produced a CTA [F(1,7) = 7.36, p < 0.05] and suppressed 3h and 24h food intake [Fs(1,8) > 6.17, ps < 0.05]. The highest doses of IP liraglutide (50 and 100 μg/kg) produced a CTA (Fs(1,7) > 5.96, ps < 0.05] and suppressed food intake at 3, 6, and 24h [Fs(1,8) > 12.04, ps < 0.01]; however, a dose was obtained (10 μg/kg) that significantly suppressed 24h food intake relative to vehicle [F(1,8) = 6.45, p < 0.05] without producing a significant CTA (Figures 1b, 1d).
Figure 1.
Dose-response suppression of cumulative chow intake following IP administration of exendin-4 (A) or liraglutide (B). In a two-bottle CTA learning paradigm, IP exendin-4 produced a CTA at all doses capable of reducing food intake (0.25, 0.5, 0.75, 1.33, 3.0 μg/kg) (C); only the lowest dose of exendin-4 (0.1 μg/kg), ineffective at reducing food intake, did not produce a CTA. IP liraglutide produced a CTA at 50 and 100μg/kg; however, a CTA was not obtained with the 10μg/kg dose of liraglutide that was effective in reducing 24hr food intake (D). Data are mean ± SEM, * = p < 0.05 vs. vehicle.
3.2. Experiment 2a and 2b
IP b.i.d. exendin-4 produced a pica response [F(1,16) = 4.88, p < 0.05], as well as food intake [F(1,16) = 15.50, p < 0.01] and body weight suppression [F(1,16) = 5.24, p < 0.05] on the first day of administration relative to vehicle group. Kaolin intake increased progressively across the entire treatment period, plateauing at day 10 (Figure 2a) [group x day interaction, F(11,176) = 7.63, p < 0.0001; group main effect, F(1,16) = 28.42, p < 0.0001]. The magnitude of food intake suppression remained relatively stable across the treatment period [nonsignificant group x day interaction; group main effect, F(1,16) = 10.12, p < 0.01], whereas body weight loss relative to vehicle group gradually increased over time (Figures 2b, 2c) [group x day interaction, F(11,176) = 2.80, p < 0.01; group main effect, F(1,16) = 6.36, p < 0.05].
IP q.d. liraglutide produced a significant pica response relative to the vehicle group only on the first day of administration (Figure 2a) [F(1,16) = 5.30, p < 0.05]. Food intake suppression was robust on day 1 [F(1,16) = 18.39, p < 0.001], modest on day 2 [F(1,16) = 5.53, p < 0.05], and not significantly different from vehicle for days 3–12, with the exception of day 8 [F(1,16) = 4.93, p < 0.05] (Figure 2b). Body weight was significantly reduced relative to the vehicle group on all days [Fs(1,16) > 7.03, ps < 0.05], which appeared to be largely due to the initial body weight drop on the first two days, as the slope of body weight gain from days 3–12 was comparable to that of the vehicle group (see trend lines from day 1–12, Figure 2c). For liraglutide vs. saline, there was a significant main effect of group for kaolin intake and body weight [Fs(1,16) > 9.70, ps < 0.01]; a significant drug x day interaction was also obtained for kaolin intake, food intake, and for body weight [Fs(11,176) > 2.09, ps < 0.05]. An additional analysis of cumulative food intake from days 1–12 and from days 3–12 was also conducted to more carefully examine whether liraglutide significantly reduced food intake after day 3 of administration. Results demonstrated that both liraglutide and exendin-4 significantly reduced cumulative food intake relative to the vehicle group from days 1–12 [Fs(1,16) > 4.62, ps < 0.05], whereas from days 3–12, cumulative intake was only reduced for the exendin-4 group [F(1,16) = 9.08, p < 0.01].
For Experiment 2b, b.i.d. exendin-4 treatment again produced a linear increase across days in the magnitude of the pica response for the kaolin access day 1 group [group x day interaction, F(10,70) = 2.53, p < 0.05; group main effect, F(1,7) = 43.18, p < 0.001] (Figure 3a). For the group that received kaolin access on day #11 of exendin-4 treatment, the amount of kaolin consumed was nearly identical to that observed in the exendin-4-treated group that had kaolin access from day 1. The magnitude of food intake and body weight suppression over the treatment period were comparable between the two exendin-4-treated groups (Figures 3b, 3c), although a non-significant trend was observed towards greater body weight loss for the exendin-4 group that received kaolin access on day #11 compared to the exendin-4 group with access on day 1. When comparing either exendin-4 group to the vehicle group, significant main effects for food intake and body weight were observed [Fs(1,15) > 10.23, ps < 0.01], as was a significant group x day interaction for body weight [F(10,150) = 2.61, p < 0.01].
Figure 3.
Daily kaolin-clay intake (A), suppression of chow intake (B), and cumulative daily body weight change (C) in three groups of rats that received either twice-daily (b.i.d.) IP administration of exendin-4 (3.0μg/kg, with kaolin access beginning on day 1), exendin-4 (3.0μg/kg, with kaolin access only on day 11), or vehicle (with kaolin access beginning on day 1) for 11 consecutive days. Data are mean ± SEM, * = p < 0.05 vs. vehicle group.
3.3. Experiment 3
A progressive increase in kaolin intake across days was observed for both vagotomy and sham controls treated with b.i.d. exendin-4 compared to respective vehicle groups (group x day interaction, Fs (9,99) > 8.52, ps < .0001; group main effect, Fs (1,11) > 46.98, ps < 0.0001]. The overall magnitude of the pica response was larger for the vagotomy exendin-4 group compared to the sham exendin-4 group [F(1,12) = 10.55, p < 0.01], however, this group difference did not differ by day. Food intake suppression relative to vehicle groups was observed in both vagotomy and sham controls [Fs(1,11) > 37.12, ps < 0.0001] (Figure 4b). The magnitude of exendin-4-induced food intake suppression was larger for sham compared to vagotomy rats; an outcome that is likely due to the lower baseline intake for vagotomy rats [e.g., vehicle vagotomy vs. vehicle sham groups, F(1,14) = 4.43, p = 0.054]. Similar effects were obtained for body weight change (Figure 4c). For kaolin intake, overall significant main effects of drug group [F(1,26) = 56.53, p < 0.0001] and surgical group [F(1,26) = 11.98, p < 0.01], and a significant drug x surgical group interaction [F(1,26) = 9.23, p < 0.01] were observed. The drug x surgical group x day interaction was not significant for kaolin intake. For food intake and body weight, there were significant main effects of drug [Fs(1,26) > 8.46, ps < 0.01] and a significant surgical x drug group interaction for food intake [F(1,26) = 8.33, p < 0.01].
Figure 4.
Daily powdered kaolin-clay intake (A), suppression of maintenance liquid diet (B), and cumulative daily body weight change (C) following b.i.d. IP administration of exendin-4 (3μg/kg) or vehicle in rats that had undergone either complete subdiaphragmatic vagotomy or sham surgery. Data are mean ± SEM, * = p < 0.05 from within-surgery vehicle-group. Having established that vagal signaling is not required for the pica response produced by IP exendin-4, a separate groups of rats received daily 3rd icv administration of the GLP-1R antagonist exendin-(9-39) (100μg/2μl, q.d.) or vehicle in combination with IP exendin-4 (3μk/kg, b.i.d.) or vehicle and kaolin intake was recorded for 4 consecutive days (D). Data are mean ± SEM, * = p < 0.05 vs. vehicle/vehicle group, Ŧ = p < 0.05 vs. vehicle/exendin-4 group.
3.4. Experiment 4
Exendin-4 b.i.d. treatment produced a pica response that increased over the four day period for both the vehicle/exendin-4 group and for the exendin-(9-39)/exendin-4 group; however, the magnitude of the exendin-4-induced pica response was reduced with ICV co-administration of the GLP-1R antagonist exendin-(9-39) (Figure 4d). A significant main effect of IP drug group [F(1,19) = 11.61, p < 0.01] and a nearly significant main effect of ICV drug group were found [F(1,19) = 3.85, p = 0.065]. The ICV x IP drug group interaction was significant [F(1,19) = 4.12, p < 0.05], an effect that did not differ by day (non-significant ICV group x IP group x day interaction). Planned comparisons between the two exendin-4 treated groups on each day demonstrated that kaolin intake was significantly lower for the exendin-(9-39)/exendin-4 compared to the vehicle/exendin-4 group on day 3 [F(1,9) = 5.19, p < 0.05].
3.5. Experiment 5
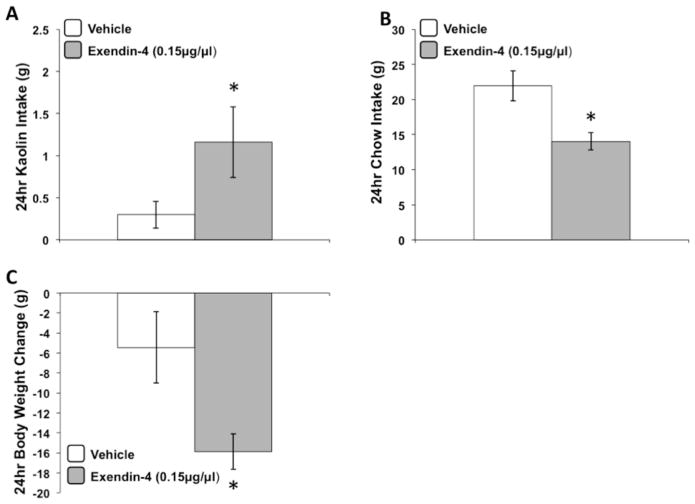
Exendin-4 delivered to the 3rd ventricle produced a significant increase in 24h kaolin intake and a significant decrease in 24h food intake and body weight relative to vehicle treatment [Fs(1,16) > 7.64, p < 0.05] (Figures 5a–5c). The magnitude of this response was comparable to that observed on day 1 following b.i.d. IP exendin-4 treatment in chronic studies.
Figure 5.
24hr kaolin intake (A), chow intake (B), and body weight change (C) following an acute 3rd icv administration of exendin-4 (0.15μg/1μl) or vehicle. Data are mean ± SEM, * = p < 0.05 vs. vehicle.
3.6. Experiment 6a and 6b
Experiment 6a
Parenchymal exendin-4 administration to the mNTS (Figures 7a–7c), but not the CeA (Figures 6a–6c) produced a significant increase in kaolin intake and a significant decrease in food intake and body weight relative to vehicle treatment [Fs(1,15) > 4.73, ps < 0.05].
Figure 6.
24hr kaolin intake (A), chow intake (B), and body weight change (C) following an acute intraparenchymal CeA administration of exendin-4 (0.05μg/100nl) or vehicle. In a two-bottle CTA learning paradigm, CeA administration of exendin-4 produced a CTA when compared to vehicle injections (D). Data are mean ± SEM, * = p < 0.05 vs. vehicle.
Experiment 6b
Exendin-4 delivered to the CeA (Figure 6d), but not the mNTS (Figure 7d) produced a CTA relative to vehicle, indicated by significantly lower intake of the drug-paired relative to the vehicle-paired flavor [F(1,6) = 6.53, p < 0.05]. For the mNTS rats overall fluid intake (and vehicle-paired flavor intake) during the two-bottle test was substantially lower than that observed from the CeA rats [F(1,15) = 13.30, p < 0.01] and from all CTA groups in Experiment 1. Data from a separate group of rats indicates that this effect is based on a competing CTA produced from direct mNTS vehicle (aCSF) injections, as mNTS vehicle injections produced a CTA relative to the no injection condition [F(1,6) = 5.97, p = 0.05] (Figure 7e). For this second mNTS group of rats, overall fluid intake during the two-bottle test did not significantly differ from the CeA group.
4. Discussion
GLP-1R agonists are currently used as a T2DM treatment and may also prove effective for the treatment of human obesity. Given that the current FDA-approved GLP-1R agonists, exendin-4 and liraglutide, produce nausea in up to half of subjects in clinical trials [e.g. (Astrup et al., 2009; Bergenstal et al., 2010; Buse et al., 2009)], future GLP-1-based pharmacotherapies would benefit greatly from a better understanding of the neurophysiological mechanisms mediating the anorectic effects and the nausea produced from GLP-1R agonists. Using two rodent models of nausea/malaise, present data inform about neuronal processes underlying GLP-1-related nausea/malaise and suggest that the food intake and body weight suppressive effects of long-acting GLP-1R agonists may require nausea.
Dose response analyses showed that all IP doses of exendin-4 that reduce food intake also produced a CTA, whereas one dose of liraglutide reduced 24h intake without producing a CTA. This difference in the CTA/food intake dose-response profile between these two GLP-1R ligands could be interpreted to indicate that compared to exendin-4, liraglutide is more efficacious in reducing food intake without producing nausea. Indeed, a human trial comparing liraglutide to exenatide (synthetic version of exendin-4) reported that the nausea incidence with liraglutide is less frequent and more transient than exenatide despite comparable body weight loss between the two drugs (Buse et al., 2009). Alternatively, this outcome may be based on differences in the delay period between the flavor presentation and the interoceptive drug state produced from liraglutide compared to exendin-4 given that the latency for food intake suppression following IP administration differs between the two drugs (Hayes et al., 2011a) and that CTA learning is sensitive to the flavor-drug delay duration [see (Welzl et al., 2001) for review]. Another limitation of the CTA paradigm for assessing drug-induced nausea is that CTA can also be established by rewarding drugs (e.g., amphetamine, cocaine, nicotine), even at doses that are self-administered (Mayer and Parker, 1993; Parker, 1984; Parker and Carvell, 1986). Accordingly, it has been argued by Parker and colleagues that rodents learn to avoid any food or flavor that predicts a change in affective homeostatic state, a change that does not necessarily require a nausea component (Parker et al., 2008). These limitations of the CTA paradigm are noteworthy with respect to GLP-1, given that previous reports examining the CNS mechanisms of GLP-1-related nausea have almost exclusively used the CTA paradigm for behavioral assessment of nausea/malaise (Kinzig et al., 2002b; Thiele et al., 1997).
Using pica as an alternative model for nausea assessment, we showed that peripheral twice-daily exendin-4 and once-daily liraglutide treatment (mimicking human administration) produces a comparable increase in kaolin consumption on the first day of administration. For exendin-4, the magnitude of the pica response and body weight loss relative to the control group progressively increased for approximately 10 days with chronic daily treatment, whereas the magnitude of food intake suppression remained constant. For liraglutide, however, the pica, food intake reduction, and magnitude of body weight loss relative to control rats were substantially reduced after the 3rd day in the treatment regimen. Thus, it appears that nausea (as measured by pica) largely occurs concomitant with intake suppression for both drugs; however, whether this is also true in diet-induced obese rodents is unknown. Further, it is not clear if the apparent increased tachyphylaxis that develops with chronic liraglutide vs. exenatide treatment is based on liraglutide’s longer half-life (Abu-Hamdah et al., 2009; Agerso et al., 2002), differences in antibody formation (Buse et al., 2011), or some other pharmacodynamic difference (e.g., PK profile) between these two GLP-1R ligands at the cellular/receptor level. Indeed, the PK profile of liraglutide and exendin-4 are quite different. In humans, levels of liraglutide are steadily maintained for ~24hr after injection, whereas exenatide levels rapidly peak and decline 10–12hr after injection, thus yielding more variation throughout the day (Meece, 2009). Regardless of these differences between the two drugs, it is the case that in most instances food intake suppression by the GLP-1R agonists does not occur without concomitant pica.
Our findings are similar to those reported in human clinical trials reporting that for liraglutide [e.g., (Buse et al., 2009; Garber et al., 2009; Garber et al., 2011)] both nausea incidence and the slope of body weight reduction occur more dramatically early in treatment, whereas for exenatide (Blonde et al., 2006b; Buse et al., 2007; Buse et al., 2009), body weight loss is more progressive and nausea incidence is stable or slightly reduced as treatment continues. One difference, however, between present findings and those reported in humans is that our data show that the pica response produced by exendin-4 treatment progressively increased with chronic daily administration. A follow-up control study showed that this effect is more likely based on increased nausea as opposed to being based on the rats learning across days about the potential reinforcing (i.e., anti-noxious) effects of kaolin consumption. Whether increased nausea by chronic exenatide treatment also occurs in human populations and is obscured by the poor validity of nausea self-reporting measures (Brearley et al., 2008; Wood et al., 2011) and/or due to the lack of long-term data analyses that include patients who have withdrawn from clinical trials due to adverse side effects (e.g., nausea), is unknown.
Present findings show that peripheral exendin-4 produces nausea, in part, via vagal-independent activation of CNS GLP-1Rs. The incretin and food intake suppressive effects of endogenous GLP-1 have been shown to require vagal afferent communication [e.g., (Holst, 2007; Ruttimann et al., 2009)], however, the extent that the nausea produced from GLP-1 or from longer-acting GLP-1R agonists requires the vagus nerve was previously unknown. Here we show that subdiaphragmatic vagotomy, which eliminates all vagal sensory and motor communication below the diaphragm, does not reduce the magnitude of the pica response produced by chronic peripheral exendin-4 administration in rats. Given that exendin-4 has been shown to readily cross the blood-brain barrier (BBB) (Kastin and Akerstrom, 2003) and that food intake suppression by liraglutide and exendin-4 does not require vagal afferent signaling (Kanoski et al., 2011), our data suggest that part of the nausea response accompanying peripheral administration of longer-acting GLP-1R agonists is mediated by BBB penetrance and subsequent CNS GLP-1R activation. This notion is further supported by present results showing that the pica response observed across four days of peripheral (IP) exendin-4 administration is attenuated with daily ICV co-administration of the GLP-1R antagonist exendin-(9-39).
Given that the nausea response accompanying IP exendin-4 treatment appears to involve brain GLP-1R activation, we assessed the CNS mechanisms mediating this effect. Results showed that acute 3rd ICV administration of exendin-4 produced a pica response of comparable magnitude to that observed after day 1 of IP exendin-4 treatment. This CNS-mediated pica response may be based, in part, on GLP-1R activation in the mNTS as parenchymal exendin-4 delivered to the mNTS produced a pica response and a suppression of food intake, an outcome that is consistent with findings showing that neurons in the NTS are activated by various emetic agents, including cisplatin (De Jonghe and Horn, 2009; Horn, 2009), phosphodiesterase-4 inhibitors (Bureau et al., 2006), and X-ray irradiation (Yamada et al., 2000). However, our findings are inconsistent with some features of previous work. Using the CTA model, previous findings show that direct CeA administration of GLP-1 produces a CTA in rats (Kinzig et al., 2002a). As current data also establish a CTA with CeA exendin-4 administration, yet an absence of pica response when the same dose was applied to the CeA, it may be that GLP-1R signaling in the CeA is critical in the formation of learned associations between flavors and agents that induce visceral malaise, but is not directly involved with viscerosensory processing of the nausea that accompanies GLP-1R activation. Kinzig et al. (Kinzig et al., 2002a) also found that hindbrain GLP-1 administration, which presumably provides access to mNTS GLP-1Rs, did not produce a CTA, whereas we observed a pica response and potent food intake suppression following mNTS delivery of exendin-4. One possibility to account for this discrepancy is that previous reports examined the effects of GLP-1(7-36), whereas current findings represent responses to a longer-acting pharmacological GLP-1R agonist. Another noteworthy difference is that we injected a GLP-1R agonist directly to a relevant site of viscerosensory processing (e.g, mNTS), as opposed to ventricular administration (4th ICV). Regardless, our data suggest that the anorectic response following mNTS GLP-1R activation may involve nausea, which is contrary to the notion that hindbrain GLP-1R signaling represents an anorectic pathway that is independent of the nausea associated with GLP-1R activation.
Interestingly, our data show that the same dose of exendin-4 that produced pica when administered to the mNTS did not produce a CTA relative to vehicle. A control experiment revealed that the presence of pica, yet the absence of CTA following mNTS exendin-4 administration is likely based on a competing CTA produced from mNTS vehicle injections (relative to no injection). This finding provides further evidence of the limitations of exclusively using the CTA as an index of drug-induced nausea. Given that mNTS vehicle injections did not produce a pica response, it is likely that the CTA following mNTS vehicle injections was not based on nausea, but rather due to some other, yet undefined change in homeostatic state. Further, given that intake of the flavor paired with CeA vehicle injections was not reduced as was observed for the mNTS, the mNTS vehicle injection-induced CTA does not appear to be based on a more general effect produced from parenchymal injections. Of course, this is only speculative and the mechanisms mediating CTA produced by mNTS vehicle injections require further investigation. We also acknowledge that while our data show CTA yet no pica response following CeA exendin-4 injections, it is unknown whether administration of native GLP-1 to the CeA produces pica as previous work only employed the CTA paradigm (Kinzig et al., 2002a).
4.1. Conclusions
In summary, present data demonstrate that food intake suppression by the FDA-approved GLP-1R agonists, exendin-4 and liraglutide, is accompanied by nausea. For exendin-4, all peripheral doses that reduce food intake also produce a CTA, and the magnitude of nausea produced by exendin-4 progressively increases with continual daily administration. Liraglutide also produces CTA, however, the nausea and food intake suppression by liraglutide were more transient than was observed for exendin-4, and food intake-suppressive doses of liraglutide can be obtained that do not appear to produce CTA. The nauseating effects accompanying peripheral exendin-4 are mediated by a vagal-independent pathway that appears to involve BBB penetrance and subsequent GLP-1R activation in the CNS. The GLP-1R expressed within the mNTS and potentially in other, yet undefined GLP-1R brain nuclei are likely key contributors to both the food intake suppressive and nauseating effects of these long-acting GLP-1R agonists. Collectively, these findings are informative about the neurophysiological mechanisms mediating the nausea produced by the two FDA-approved GLP-1R agonists, and are informative towards the development of improved GLP-1-based pharmacotherapies for T2DM and obesity.
Highlights.
Peripheral exendin-4 produces conditioned taste avoidance at all doses that suppress food intake
One dose of liraglutide reduced food intake without conditioned taste avoidance
Chronic peripheral exendin-4 produced pica via vagal-independent direct CNS action
Chronic peripheral liraglutide produced transient food intake suppression and pica
NTS but not the CeA exendin-4 injection produced pica and food intake suppression
Acknowledgments
The authors thank Dr. Harvey J. Grill for his invaluable guidance and support. The following individuals are also thanked for notable contributions to this work: Diana Olivos, Amber Alhadeff, Jeffrey Chen, Line Stensland, and Jennifer Gilbert. This work was supported by developmental funds from the Translational Neuroscience Program, in the Department of Psychiatry, Perelman School of Medicine at the University of Pennsylvania, as well as NIH grants DK089752 (SEK) and DK085435 (MRH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Overweight and Obesity: Data and Statistics. Center for Disease Control and Prevention; [Google Scholar]
- 2.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. The Journal of clinical endocrinology and metabolism. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 4.Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Autonomic neuroscience: basic & clinical. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 6.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 7.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006a;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 8.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes, obesity & metabolism. 2006b;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 9.Brearley SG, Clements CV, Molassiotis A. A review of patient self-report tools for chemotherapy-induced nausea and vomiting. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2008;16:1213–1229. doi: 10.1007/s00520-008-0428-y. [DOI] [PubMed] [Google Scholar]
- 10.Bureau Y, Handa M, Zhu Y, Laliberte F, Moore CS, Liu S, Huang Z, MacDonald D, Xu DG, Robertson GS. Neuroanatomical and pharmacological assessment of Fos expression induced in the rat brain by the phosphodiesterase-4 inhibitor 6-(4-pyridylmethyl)-8-(3-nitrophenyl) quinoline. Neuropharmacology. 2006;51:974–985. doi: 10.1016/j.neuropharm.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Garber A, Rosenstock J, Schmidt WE, Brett JH, Videbaek N, Holst J, Nauck M. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. The Journal of clinical endocrinology and metabolism. 2011;96:1695–1702. doi: 10.1210/jc.2010-2822. [DOI] [PubMed] [Google Scholar]
- 12.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 13.Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Maggs DG, Wintle ME. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clinical therapeutics. 2007;29:139–153. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 15.De Jonghe BC, Horn CC. Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R756–765. doi: 10.1152/ajpregu.00820.2007. [DOI] [PubMed] [Google Scholar]
- 16.De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus) American journal of physiology Regulatory, integrative and comparative physiology. 2009;296:R902–911. doi: 10.1152/ajpregu.90952.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Jonghe BC, Lawler MP, Horn CC, Tordoff MG. Pica as an adaptive response: Kaolin consumption helps rats recover from chemotherapy-induced illness. Physiology & behavior. 2009;97:87–90. doi: 10.1016/j.physbeh.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 19.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 20.Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2011;13:348–356. doi: 10.1111/j.1463-1326.2010.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- 22.Gutierrez LV, Kocak N, Cox AG. Alimentary transit and supersensitivity after vagotomy in the rat. Gut. 1971;12:625–628. doi: 10.1136/gut.12.8.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiology & behavior. 2010;100:503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative Effects of the Long-Acting GLP-1 Receptor Ligands, Liraglutide and Exendin-4, on Food Intake and Body Weight Suppression in Rats. Obesity. 2011a doi: 10.1038/oby.2011.50. [DOI] [PubMed] [Google Scholar]
- 25.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell metabolism. 2011b;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 27.Holst JJ, Seino Y. GLP-1 receptor agonists: targeting both hyperglycaemia and disease processes in diabetes. Diabetes Res Clin Pract. 2009;85:1–3. doi: 10.1016/j.diabres.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Horn CC. Brain Fos expression induced by the chemotherapy agent cisplatin in the rat is partially dependent on an intact abdominal vagus. Autonomic neuroscience: basic & clinical. 2009;148:76–82. doi: 10.1016/j.autneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John LE, Kane MP, Busch RS, Hamilton RA. Expanded Use of Exenatide in the Management of Type 2 Diabetes. Diabetes Spectrum. 2007;20:59–63. [Google Scholar]
- 30.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and Central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1 Receptor Agonists, Liraglutide and Exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 32.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 33.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002a;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002b;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudsen LB. Liraglutide: the therapeutic promise from animal models. International journal of clinical practice. 2010;(Supplement 64):4–11. doi: 10.1111/j.1742-1241.2010.02499.x. [DOI] [PubMed] [Google Scholar]
- 36.Lo CM, Xu M, Yang Q, Zheng S, Carey KM, Tubb MR, Davidson WS, Liu M, Woods SC, Tso P. Effect of intraperitoneal and intravenous administration of cholecystokinin-8 and apolipoprotein AIV on intestinal lymphatic CCK-8 and apo AIV concentration. American journal of physiology Regulatory, integrative and comparative physiology. 2009;296:R43–50. doi: 10.1152/ajpregu.90410.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas F, Sclafani A. Capsaicin attenuates feeding suppression but not reinforcement by intestinal nutrients. American Journal of Physiology. 1996;270:R1059–1064. doi: 10.1152/ajpregu.1996.270.5.R1059. [DOI] [PubMed] [Google Scholar]
- 38.Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. The Journal of pharmacology and experimental therapeutics. 1971;178:562–564. [PubMed] [Google Scholar]
- 39.Mayer LA, Parker LA. Rewarding and aversive properties of IP and SC cocaine: assessment by place and taste conditioning. Psychopharmacology. 1993;112:189–194. doi: 10.1007/BF02244909. [DOI] [PubMed] [Google Scholar]
- 40.Meece J. Pharmacokinetics and pharmacodynamics of liraglutide, a long-acting, potent glucagon-like peptide-1 analog. Pharmacotherapy. 2009;29:33S–42S. doi: 10.1592/phco.29.pt2.33S. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiology & behavior. 1976;17:691–697. doi: 10.1016/0031-9384(76)90171-2. [DOI] [PubMed] [Google Scholar]
- 42.Montanya E, Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clinical therapeutics. 2009;31:2472–2488. doi: 10.1016/j.clinthera.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 43.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 44.Parker LA. Behavioral conditioned responses across multiple conditioning/testing trials elicited by lithium- and amphetamine-paired flavors. Behavioral and neural biology. 1984;41:190–199. doi: 10.1016/s0163-1047(84)90569-7. [DOI] [PubMed] [Google Scholar]
- 45.Parker LA, Carvell T. Orofacial and somatic responses elicited by lithium-, nicotine- and amphetamine-paired sucrose solution. Pharmacology, biochemistry, and behavior. 1986;24:883–887. doi: 10.1016/0091-3057(86)90431-4. [DOI] [PubMed] [Google Scholar]
- 46.Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Canadian journal of experimental psychology = Revue canadienne de psychologie experimentale. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- 47.Pinkney J, Fox T, Ranganath L. Selecting GLP-1 agonists in the management of type 2 diabetes: differential pharmacology and therapeutic benefits of liraglutide and exenatide. Ther Clin Risk Manag. 2010;6:401–411. doi: 10.2147/tcrm.s7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. The American journal of physiology. 1999;277:R1537–1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- 49.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: Location in the hindbrain. Science. 1981;213:451–453. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 50.Russell-Jones D. The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int J Clin Pract. 2010;64:1402–1414. doi: 10.1111/j.1742-1241.2010.02465.x. [DOI] [PubMed] [Google Scholar]
- 51.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 53.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. The American journal of physiology. 1997;272:R726–730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- 54.Tinker J, Kocak N, Jones T, Glass HI, Cox AG. Supersensitivity and gastric emptying after vagotomy. Gut. 1970;11:502–505. doi: 10.1136/gut.11.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welzl H, D’Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behavioural brain research. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 56.Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- 57.Wood JM, Chapman K, Eilers J. Tools for assessing nausea, vomiting, and retching. Cancer nursing. 2011;34:E14–24. doi: 10.1097/ncc.0b013e3181e2cd79. [DOI] [PubMed] [Google Scholar]
- 58.Yamada Y, Tsukamoto G, Kobashi M, Sasaki A, Matsumura T. Abdominal vagi mediate c-Fos expression induced by X-ray irradiation in the nucleus tractus solitarii of the rat. Autonomic neuroscience: basic & clinical. 2000;83:29–36. doi: 10.1016/S0165-1838(00)00105-3. [DOI] [PubMed] [Google Scholar]