Abstract
The N-terminal fragment of prohormone brain natriuretic peptide (NT-proBNP) is a commonly used biomarker for the diagnosis of congestive heart failure, although its biological function is not well known. NT-proBNP exhibits heavy O-linked glycosylation, and it is quite difficult to develop an antibody that exhibits glycosylation-independent binding. We developed an antibody that binds to the recombinant NT-proBNP protein and its deglycosylated form with similar affinities in an enzyme immunoassay. The epitope was defined as Gly63–Lys68 based on mimetic peptide screening, site-directed mutagenesis and a competition assay with a peptide mimotope. The nearest O-glycosylation residues are Thr58 and Thr71; therefore, four amino acid residues intervene between the epitope and those residues in both directions. In conclusion, we report that an antibody reactive to Gly63–Lys68 of NT-proBNP exhibits O-glycosylation-independent binding.
Introduction
Natriuretic peptides (NPs) are recognized as useful biomarkers in the diagnosis of congestive heart failure. Although NP levels depend on the age and gender of the patient, these levels can drastically change based on the severity of congestive heart failure.1, 2
The NP family includes atrial natriuretic peptide, brain (or B-type) natriuretic peptide (BNP) and C-type natriuretic peptide. All NPs are important regulators of diuresis, natriuresis and vasolidation.3 Because the plasma concentration of BNP is most closely related to heart failure, this is most frequently used as a biomarker among the NP family.4
BNP is synthesized within cardiomyocytes as a pre-proBNP (134 amino acids). Pre-proBNP is released from cardiomyocytes into the blood when the ventricular wall is stretched. In the blood, proBNP is cleaved to yield mature functional BNP (32 amino acids) and the N-terminal fragment of proBNP (NT-proBNP, 76 amino acids).2 The biological function of NT-proBNP is unknown; however, NT-proBNP levels in blood are higher than those of BNP.5 Therefore, the measurement of NT-proBNP level is preferentially used over that of BNP in the diagnosis of congestive heart failure.
NT-proBNP circulating in human blood retains O-linked glycosylation.6 The physiological reason for the glycosylation of NT-proBNP is not fully understood; however, it is likely to increase the stability of the peptide.7 Approximately 10% of the amino acid residues of NT-proBNP are reported to be glycosylated.6, 7, 8, 9 The residues potentially glycosylated in NT-proBNP are Thr36, Ser37, Ser44, Thr48, Ser53, Thr58 and Thr71. All these residues are complete glycosylation sites except Thr36 and Thr58.6 In the development of antibodies for the diagnostic assay, antibodies binding to NT-proBNP irrespective of its glycosylation patterns are preferred. In addition, the N- and C-termini of NT-proBNP are cleaved in the blood by physiological proteolysis. Proteolysis frequently occurs between the residue pairs Pro2–Leu3, Leu3–Gly4, Pro6–Gly7 and Pro75–Arg76 of NT-proBNP.10 Therefore, the detection of N- and C- cleaved fragments of NT-proBNP is another hurdle in the development of an immunoassay.
In this context, it is quite difficult to develop an antibody pair that is reactive to epitopes not influenced by glycosylation or N- and C-terminal cleavage. In this study, we aimed to develop an antibody reactive to an epitope that is relatively less influenced by glycosylation and N- and C-terminal cleavage. First, we established a mammalian expression system for NT-proBNP, and confirmed that the purified recombinant protein is glycosylated by digestion with O-glycosidase and neuraminidase in gel electrophoresis. Next, using phage display of a combinatorial rabbit antibody library, we generated an antibody with reactivity to NT-proBNP independent of glycosylation. Furthermore, we defined the epitope using phage display of a random peptide library that turned out to be Gly63–Lys68. Finally, in a sandwich enzyme immunoassay, the reactivity of this antibody to both recombinant and deglycosylated NT-proBNP was confirmed to be quite similar, which claims its O-glycosylation-independent binding.
Materials and methods
Preparation of recombinant NT-proBNP-Fc fusion protein
Total RNA was prepared from HEK (human embryonic kidney) cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Subsequently, complementary DNA was synthesized using the Superscript III first-strand synthesis supermix with Oligo (dT) priming (Invitrogen). The human NT-proBNP gene was amplified using the forward primer 5′-GGCCCAGGCGGCCCACCCGCTGGGCAGCC-3′ and the reverse primer 5′-GGCCCCACCGGCCCCTCGTGGTGCCCGCAGGGT-3′. The PCR conditions were as follows: preliminary denaturation at 95 °C for 5 min, followed by 30 cycles of 30 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C. The reaction was ended in 5 min at 72 °C. The gene was subcloned into the modified pCEP4 vector that has two SfiI sites, as described previously.11 Subsequently, the vectors were used to transfect HEK293F cells (1.0 × 106 cells per ml) (Invitrogen) using polyethylenimine (Polysciences, Warrington, PA, USA), and the transfected cells were cultured in FreeStyle 293 expression medium (Invitrogen) in a 37 °C CO2 incubator as described previously.12 The recombinant NT-proBNP-Fc fusion protein was purified by affinity chromatography using protein A sepharose (Repligen, Waltham, MA, USA) column, as described previously.11
Generation of anti-NT-proBNP antibodies
Two New Zealand white rabbits were immunized with the purified recombinant NT-proBNP-Fc fusion protein. A phage display library of single-chain variable fragments (scFvs) was constructed by acquiring total RNA isolated from the harvested spleen and bone marrow of the immunized rabbits, as described previously.13 To select specific binders from the library, a total of five rounds of biopanning were performed as described previously.14 Briefly, 3 μg of NT-proBNP prepared from Escherichia coli (Scipac, Kent, UK) was conjugated to 5.0 × 106 paramagnetic beads (DynaBeads, Invitrogen) following the guidelines provided by the supplier. After overnight incubation at 37 °C on a rotator, the beads were washed four times with 500 μl of 0.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and blocked with 100 μl of 3% BSA in PBS (w/v). The phage library (100 μl) was added to the beads, followed by incubation for 2 h at 37 °C. The beads were washed once with 500 μl of 0.05% Tween-20 in PBS (v/v, PBST), and the number of washes was increased in subsequent rounds: three times for the second and third rounds and five times for the last round, respectively.11 For each round, 50 μl of 0.1 M glycine-HCl (pH 2.2) was added to the beads and the beads were incubated for 10 min at 37 °C for the elution of phages from the beads. The eluate was neutralized by adding 4 μl of 2 M Tris-Cl (pH 9.1). Afterwards, the eluted phages were used to infect 2 ml of E. coli ER2738 (New England Biolabs, Ipswich, MA, USA) culture, and the scFv-displaying phages were rescued by adding helper phages. Individual phage clones were selected from the output titration plate from the last round of biopanning, and scFv-displaying phages were prepared for the phage enzyme immunoassay, as described previously.15 The microtiter plates (Corning Inc., Tewkesbury, MA, USA) were coated with recombinant NT-proBNP-Fc fusion protein (10 μg ml−1 in PBS, 25 μl) overnight at 4 °C. The wells were blocked with 150 μl of 3% BSA in PBS (w/v) for 1 h at 37 °C. The plate was incubated with the phage-containing culture supernatant for 2 h at 37 °C and washed three times with 150 μl of 0.05% PBST. Then, 50 μl of horseradish peroxidase (HRP)-conjugated anti-M13 antibody (GE Healthcare, Piscataway, NJ, USA) diluted in blocking buffer (1:5000) was added to each well, and the plate was incubated for 1 h at 37 °C. After washing three times with 150 μl of 0.05% PBST, 50 μl of 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) substrate solution (Pierce, Rockford, IL, USA) was added to each well followed by incubation for 30 min at 37 °C. Subsequently, the absorbance of each well was measured at 405 nm using a plate reader (Labsystems S.L., Barcelona, Spain).
Affinity maturation of scFv clones
Using degenerate primers, each of the residues in the complementarity determining region (CDR) of the antibody was randomized in the construction of the secondary library. For the secondary library, three or four consecutive residues in the CDR were randomized with an NNK nucleotide sequence as described previously.16 For LCDR1, LCDR2 and HCDR1, two secondary libraries were generated for each CDR; for LCDR3, HCDR2 and HCDR3, three secondary libraries were constructed. To enrich higher affinity binders, these secondary libraries were subjected to four rounds of biopanning, and scFv-displaying phages were prepared as described above. To select clones with higher affinity, a phage enzyme immunoassay was performed as described above with a little modification, as follows. The microtiter plates were coated with the recombinant NT-proBNP-Fc fusion protein (4 μg ml−1 in PBS, 25 μl) overnight at 4 °C. The wells were blocked with 150 μl of 3% BSA in PBS (w/v) for 1 h at 37 °C. The plate was incubated with 1:10, 1:50 and 1:100 diluted phage-containing culture supernatant for 2 h at 37 °C. Then, the plates were washed three times with 150 μl of 0.05% PBST, and incubated with 50 μl of HRP-conjugated anti-M13 antibody (GE Healthcare) diluted in blocking buffer (1:5000) for 1 h at 37 °C. After washing three times with 150 μl of 0.05% PBST, 50 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (GenDEPOT, Houston, TX, USA) was added to each well followed by incubation at 37 °C. Finally, the optical density (OD) was measured at 450 nm (Labsystems S.L.). Using the variable region genes of the selected clones, an IgG1 antibody was prepared as described previously.17
Epitope mapping of the antibody
Epitope mapping was performed using Ph.D.-7 Phage Display Peptide Libraries (New England Biolabs) through a total of three rounds of biopanning on NPBR9 IgG1 or NPBC20 IgG1, immobilized on microtiter plates (Corning), as described previously.18 After the third round of biopanning, peptide-displaying phage clones were prepared and subjected to a phage enzyme immunoassay using NPBR9 IgG1 or NPBC20 IgG1-coated microtiter plates, as described previously.11 The nucleotide sequences of positive clones were determined by Sanger nucleotide sequencing using a sequencing primer (5′-CCCTCATAGTTAGCGTAACG-3′, NEB).
Site-directed mutagenesis of NT-proBNP and determination of its reactivity
The genes encoding the mutant NT-proBNP-Fc fusion protein were chemically synthesized and cloned into the modified pCEP4 vector as described above. The vectors encoding mutant proteins were transfected into HEK293F cells, and the recombinant proteins were purified by affinity chromatography as described above. Then, the reactivity of the mutant NT-proBNP-Fc fusion protein toward NPBR9 IgG1 or NPBC20 IgG1 was determined using a phage enzyme immunoassay performed as described previously.15 The microtiter plates (Corning) were coated with each of the purified mutant NT-proBNP-Fc fusion proteins (4 μg ml−1 in PBS, 25 μl) overnight at 4 °C. Then, the wells were blocked with 150 μl of 3% BSA in PBS (w/v) for 1 h at 37 °C. The plates were washed with 150 μl of 0.05% PBST and incubated with each IgG1 at 37 °C for 90 min. Then, the plates were washed three times with 150 μl of 0.05% PBST. Subsequently, 50 μl of HRP-conjugated goat anti-human Fab antibody (Pierce) diluted in blocking buffer (1:5000) was added to each well, followed by incubation for 1 h at 37 °C. The plates were further washed three times with 150 μl of 0.05% PBST, and 50 μl of ABTS substrate solution (Pierce) was added to each well, followed by incubation for 30 min at 37 °C. Finally, the OD was measured at 405 nm (Labsystems S.L.).
Overexpression and purification of a bispecific scFv-Cκ fusion protein
A gene sequence encoding the KpnI restriction site, the leader sequence of the human Ig κ-chain, followed by two SfiI restriction sites, a sequence for a linker (Gly-Gly-Gly-Ser)3,19 the human Ig κ-chain C region (Cκ), a sequence for another linker (Gly-Gly-Gly-Ser)3 and the restriction sites for AgeI (NEB), NotI (NEB) and BamHI were chemically synthesized (Genscript, Piscataway, NJ, USA) and cloned into the pCEP4 vector after digestion with the restriction enzymes KpnI and BamHI. Then, the anti-NT-proBNP scFv gene and anti-cotinine scFv gene11 were cloned into the expression vector after being digested with either SfiI or with AgeI and NotI, respectively, as described previously.19 The vector was transfected into HEK293F cells and the recombinant protein was purified by affinity chromatography using the KappaSelect resin (GE Healthcare) following the manufacturer's instructions.
Real-time interaction analysis
The peptides (pep 9–10; DVSSHRKGGGSC, pep 9–12; DIVGHRKGGGSC) were chemically synthesized and conjugated to BSA (Peptron Inc., Daejeon, Republic of Korea). Either the BSA–peptide conjugates or the recombinant NT-proBNP-Fc fusion protein was immobilized to a CM5 sensor chip (GE Healthcare) in 10 mM sodium acetate buffer (pH 5.0) using an amine coupling kit (GE Healthcare) at a flow rate of 5 μl min−1. The NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein was dissolved in HBS-EP buffer (GE Healthcare) at concentrations ranging from 6.25 to 100 nM and injected over the chip for 3 min at a flow rate of 30 μl min−1. After each binding assay, the chip was regenerated by washing with 10 mM glycine-HCl (pH 2.0) for 2 min at a flow rate of 30 μl min−1. All the experiments were performed using the Biacore T200 instrument (GE Healthcare) at 25 °C.
Competition enzyme immunoassay using BSA–peptide conjugates
The microtiter plates (Corning) were coated with BSA–peptide conjugates (0.2 μg ml−1 in PBS, 25 μl) or the recombinant NT-proBNP-Fc fusion protein (4 μg ml−1 in PBS, 25 μl) overnight at 4 °C. The wells were blocked with 150 μl of 3% BSA in PBS (w/v) for 1 h at 37 °C, and the plates were washed with 150 μl of 0.05% PBST. The recombinant NT-proBNP-Fc fusion protein was serially diluted fivefold in 3% BSA/PBS and preincubated with 0.1 μg ml−1 solution of NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein in 3% BSA/PBS for 15 min at room temperature. The mixture was added to each well and incubated for 2 h at 37 °C. After washing three times with 150 μl of 0.05% PBST, HRP-conjugated cotinine (1:1000) was added to each well. The plates were incubated for 1 h at 37 °C followed by washing three times with 150 μl of 0.05% PBST. Then, 50 μl of TMB substrate solution (GenDEPOT) was added to each well. After incubation, 50 μl of 2 M H2SO4 was added to stop the reaction. Subsequently, the OD was measured at 450 nm (Labsystems S.L.).
Real-time competition analysis using BSA–peptide conjugates
BSA–peptide conjugates and the recombinant NT-proBNP-Fc fusion protein were immobilized to a CM5 sensor chip (GE Healthcare) as described above. The recombinant NT-proBNP-Fc fusion protein was serially diluted fivefold in HBS-EP buffer (GE Healthcare) at concentration ranging from 0.08 to 50 nM and injected over the chip as described above. Each of these solutions was preincubated with 50 nM of NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein in HBS-EP buffer (GE Healthcare) at room temperature and injected over the chip for 3 min at a flow rate of 30 μl min−1. After each competition analysis, the chip was regenerated as described above.
Sandwich enzyme immunoassay
The microtiter plates (Corning) were coated with monoclonal mouse anti-human NT-proBNP 15F1113–27 (Hytest, Turku, Finland; 8 μg ml−1 in PBS, 25 μl) overnight at 4 °C. The wells were blocked with 150 μl of 3% BSA in PBS (w/v) for 1 h at 37 °C. The plates were washed with 150 μl of 0.05% PBST and incubated with glycosylated or deglycosylated recombinant NT-proBNP-Fc fusion protein serially diluted threefold in 3% BSA/PBS for 1 h at 37 °C. The plates were washed repeatedly as described above; next, a 2 μg ml−1 solution of each anti-NT-proBNP × anti-cotinine bispecific scFv-Cκ fusion protein was added to each well, and the plates were incubated for 2 h at 37 °C. After washing three times with 150 μl of 0.05% PBST, HRP-conjugated cotinine (1:250) was added to each well. The plates were incubated for 1 h at 37 °C and washed three times with 150 μl of 0.05% PBST. Following this, 50 μl of TMB substrate was added to each well followed by incubation, and 50 μl of 2 M H2SO4 was added to stop the reaction. The OD was the measured at 450 nm (Labsystems S.L.).
In a parallel experiment, the same procedure was performed up to the blocking step. Subsequently, the plates were incubated with 2 μg ml−1 of NPBR9 IgG1 or HRP-conjugated 24E1161–76 (Hytest). The plates were washed three times with 150 μl of 0.05% PBST, and HRP-conjugated anti-human Fc antibody (1:5000) was added to each well. After washing the plates, each plate was incubated with 50 μl of ABTS substrate for 30 min at 37 °C. The OD was then measured at 405 nm (Labsystems S.L.).
Results
Overexpression of glycosylated NT-proBNP as a Fc fusion protein
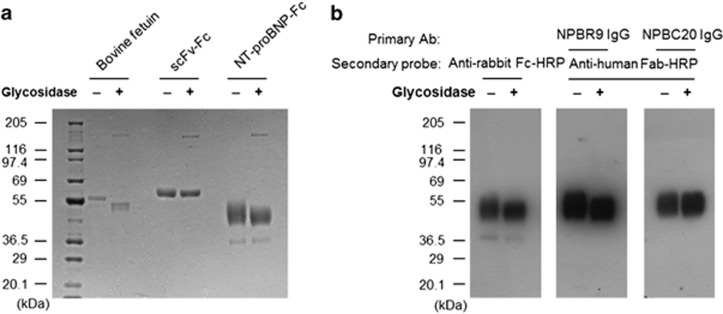
To develop the human NT-proBNP-Fc fusion protein, the expression vector encoding the human NT-proBNP-Fc gene was transfected into HEK293F cells. The recombinant NT-proBNP-Fc fusion protein was purified from the culture supernatant by protein A column chromatography. A broad protein band with a molecular weight of 50 kDa was observed upon SDS-polyacrylamide gel electrophoresis after Coomassie staining (Figure 1a). The expected molecular weight of the fusion protein was 39 kDa, and we confirmed its identity in an immunoblot assay using the anti-rabbit Fc antibody, which reacted well with the protein band (Figure 1b). To further confirm its identity as NT-proBNP, the protein was eluted from the gel and analyzed using mass spectrometry. The mass spectrometry data confirmed that the band represents the recombinant NT-proBNP-Fc fusion protein (data not shown).
Figure 1.
Expression and deglycosylation of the recombinant NT-proBNP-Fc fusion protein. (a) The recombinant NT-proBNP-Fc was expressed in HEK293F cells and purified by protein A column chromatography. The purified protein, bovine fetuin (O-glycosylated protein control), and an scFv-Fc protein (non O-glycosylated protein control) were treated with glycosidase and subjected to SDS-polyacrylamide gel electrophoresis and Coomassie staining along with the nontreated protein. (b) The untreated or treated recombinant NT-proBNP-Fc fusion proteins separated on the gel were transferred to a nitrocellulose membrane and probed with the NPBR9 antibody or the NPBC20 antibody, followed by an anti-human Fc antibody conjugated with horseradish peroxidase (HRP). The membrane was also probed with anti-rabbit Fc antibody conjugated with HRP to visualize the recombinant NT-proBNP-Fc fusion protein. NT-proBNP, N-terminal fragment of prohormone brain natriuretic peptide; scFv, single-chain variable fragment.
Because it has previously been reported that NT-proBNP is heavily O-glycosylated, we determined whether the higher molecular weight and the heterogeneity of the band are due to glycosylation. The purified recombinant NT-proBNP-Fc fusion protein was treated with O-glycosidase and neuraminidase to remove O-linked carbohydrate moieties, and then subjected to SDS-polyacrylamide gel electrophoresis and immunoblot analysis. As shown in Figure 1a, the heterogeneity and average molecular weight of the recombinant NT-proBNP-Fc fusion protein was decreased by the treatment; the molecular weight of the scFv-Fc fusion protein, which has only one N-glycosylation site in the Fc region, was used as a negative control. Bovine fetuin was used as a positive control and showed decreased molecular weight after treatment. These results indicate that the recombinant NT-proBNP-Fc fusion protein is heavily O-glycosylated.
Selection of antibodies to NT-proBNP
After immunization of rabbits with the recombinant NT-proBNP-Fc fusion protein, a phage display library of combinatorial scFvs was constructed, with a complexity of 2.2 × 109. A naive chicken scFv library, with a complexity of 1.3 × 109, was also subjected to the biopanning process for the selection of binders to NT-proBNP.
For biopanning, recombinant NT-proBNP overexpressed in E. coli was conjugated to paramagnetic beads using the epoxy groups on the beads. After biopanning, the phage clones from the output titer plates in the last round were rescued and subjected to a phage enzyme immunoassay using microtiter plates coated with the recombinant NT-proBNP-Fc fusion protein prepared in either prokaryotic or mammalian expression systems. Fourteen antibody clones from the rabbit immune library and two antibody clones from the chicken naive library showed reactivity to both antigens and were selected for further study.
Reactivity of antibodies to glycosylated recombinant NT-proBNP-Fc fusion protein
To select an antibody clone reactive to glycosylated NT-proBNP, we performed immunoblot assays using both glycosylated and deglycosylated recombinant NT-proBNP-Fc fusion protein. Those antibody clones whose reactivities were hindered by glycosylation, such as NPBC20, showed similar immunoblot bands in the lanes loaded with either untreated or deglycosylated recombinant NT-proBNP-Fc fusion protein (Figure 1b, right). In addition, antibody clones whose reactivities were not affected by glycosylation also showed reactivity to the heavily glycosylated and higher molecular weight recombinant NT-proBNP-Fc fusion protein in the lane loaded with untreated recombinant NT-proBNP-Fc fusion protein (Figure 1b, left). An anti-human Fc antibody, a monoclonal antibody not affected by O-glycosylation, was used as a positive control. In this analysis, the NPBR1 scFv-Fc fusion protein, a rabbit antibody clone, showed an immunoblot pattern similar to that of anti-Fc antibody, suggesting that its reactivity is not affected by O-glycosylation. The NPBR1 antibody clone was subjected to a process of affinity maturation. A higher-affinity, O-glycosylation-independent daughter antibody clone, NBBR9 (Figure 1b, middle), was selected for further study. NPBC20 was selected as a control antibody that was affected by O-glycosylation.
Determination of epitopes
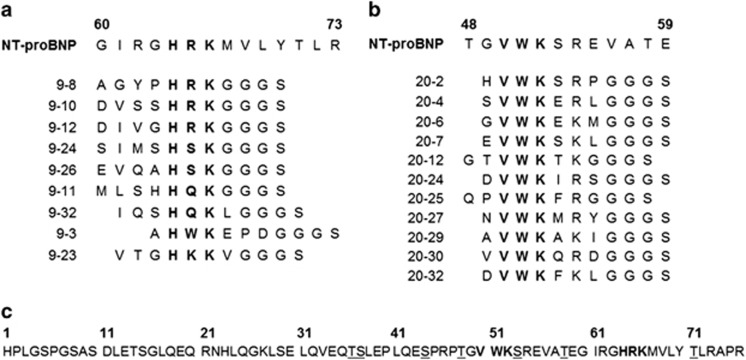
To define the epitopes of NPBR9 IgG1 and NPBC20 IgG1, we employed a phage-displayed random heptamer peptide library. Three rounds of biopanning were performed using microtiter plates coated with NPBR9 IgG1 and NPBC20 IgG1. The phage clones were rescued from the output titer plate of the third round and subjected to a phage enzyme immunoassay. Phagemid DNA was prepared from 9 clones reactive to NPBR9 IgG1 and 11 clones reactive to NPBC20 IgG1, and subjected to nucleotide sequence analysis. Among the NPBR9 clones, four, two, two, one and one clones had HRK, HSK, HQK, HWK and HKK sequences, respectively; these sequences were identical or very much homologous to H64R65K66 in NT-proBNP (Figure 2a). All the NPBC20 clones had VWK sequences that were identical to V50W51K52 in NT-proBNP (Figure 2b).
Figure 2.
Peptide sequences of phage clones reactive to (a) NPBR9 antibody and (b) NPBC20 antibody and (c) the peptide sequence of NT-proBNP. Consensus sequences are written in bold and O-linked glycosylation sites are marked by underline. NT-proBNP, N-terminal fragment of prohormone brain natriuretic peptide.
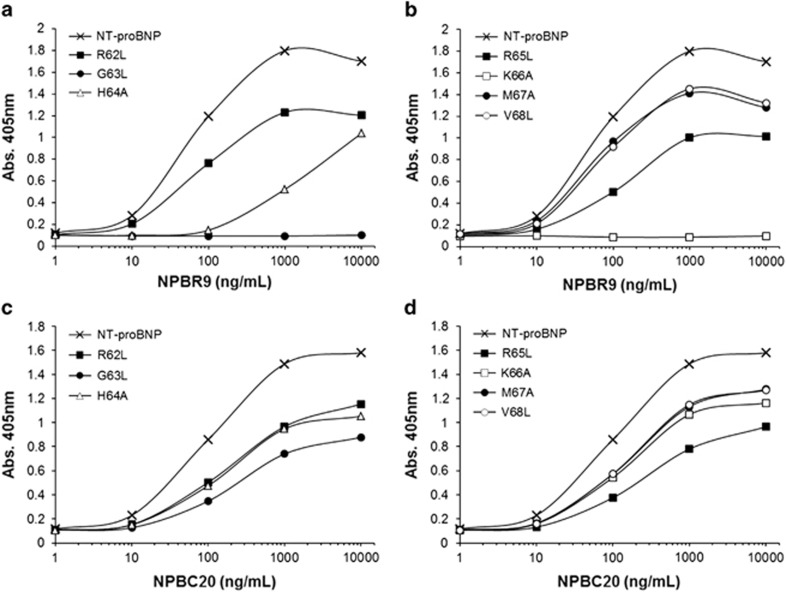
To further confirm the epitopes, recombinant NT-proBNP-Fc fusion protein mutants, bearing alanine or leucine substitutions at single amino acid residues in the HRK or VWK motifs or their flanking residues, were prepared and subjected to enzyme immunoassay (Figure 3). The recombinant NT-proBNP-Fc fusion protein mutants were individually coated onto microtiter plates by the addition of solution at the same concentration. After blocking, NPBR9 IgG1 or NPBC20 IgG1 was added to each well. The amount of IgG1 bound to the plate was determined using anti-human Fab antibody conjugated with HRP and ABTS substrate. In the case of NPBR9 IgG1, substitution of G63 or K66 in NT-proBNP completely abolished its reactivity to the antibody, whereas in the case of NPBC20 IgG1, these mutants showed similar reactivity to that of the wild type. Substitution of H64 reduced its reactivity to NPBR9 IgG1 compared with NPBC20 IgG1, but the effect was minimal. The substitution of R62, R65, M67 and V68 in NT-proBNP did not affect the reactivity of NBPR9 IgG1 significantly. From this observation, we conclude that the epitope of NPBR9 IgG1 includes G63, H64, R65 and K66.
Figure 3.
Site-directed mutagenesis of NT-proBNP-Fc fusion protein to determine the epitope of NPBR9. Single-residue mutants of NT-proBNP-Fc fusion protein were expressed in HEK293F cells. The mutant proteins were coated onto the microtiter plate. After blocking, (a, b) NPBR9 or (c, d) NPBC20 antibodies were incubated as the primary antibody. The amount of bound antibody was determined using anti-human Fc antibody conjugated to horseradish peroxidase (HRP). NT-proBNP, N-terminal fragment of prohormone brain natriuretic peptide.
Real-time interaction analysis of NPBR9 × anti-cotinine bispecific scFv-Cκ
To determine the affinity between NBPR9 antibody and peptide mimotopes, 9–10 and 9–12 peptides were chemically synthesized with the GGGSC linker at the C-terminal end and conjugated to BSA (Figure 2a). In real-time interaction analysis, the BSA–peptide conjugates or the recombinant NT-proBNP-Fc fusion protein were chemically cross-linked to a CM5 sensor chip. The kon, koff and Kd constants of NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein to BSA–peptide conjugates and the fusion protein was successfully determined and are summarized in Table 1.
Table 1. Real-time interaction analysis of NPBR9 antibody.
| Analyte | Ligand | ka (1/Ms) | kd (1/s) | KD (M) |
|---|---|---|---|---|
| NPBR9 bispecific scFv-Cκ | Pep 9–10/BSA | 1.935E+5 | 2.486E−4 | 1.287E−9 |
| Pep 9–12/BSA | 1.232E+4 | 3.548E−5 | 2.881E−9 | |
| NT-proBNP-Fc | 2.524E+4 | 4.272E−4 | 1.692E−8 |
Abbreviations: BSA, bovine serum albumin; NT-proBNP, N-terminal fragment of prohormone brain natriuretic peptide; scFv, single-chain variable fragment.
Competition assays with peptide mimotopes
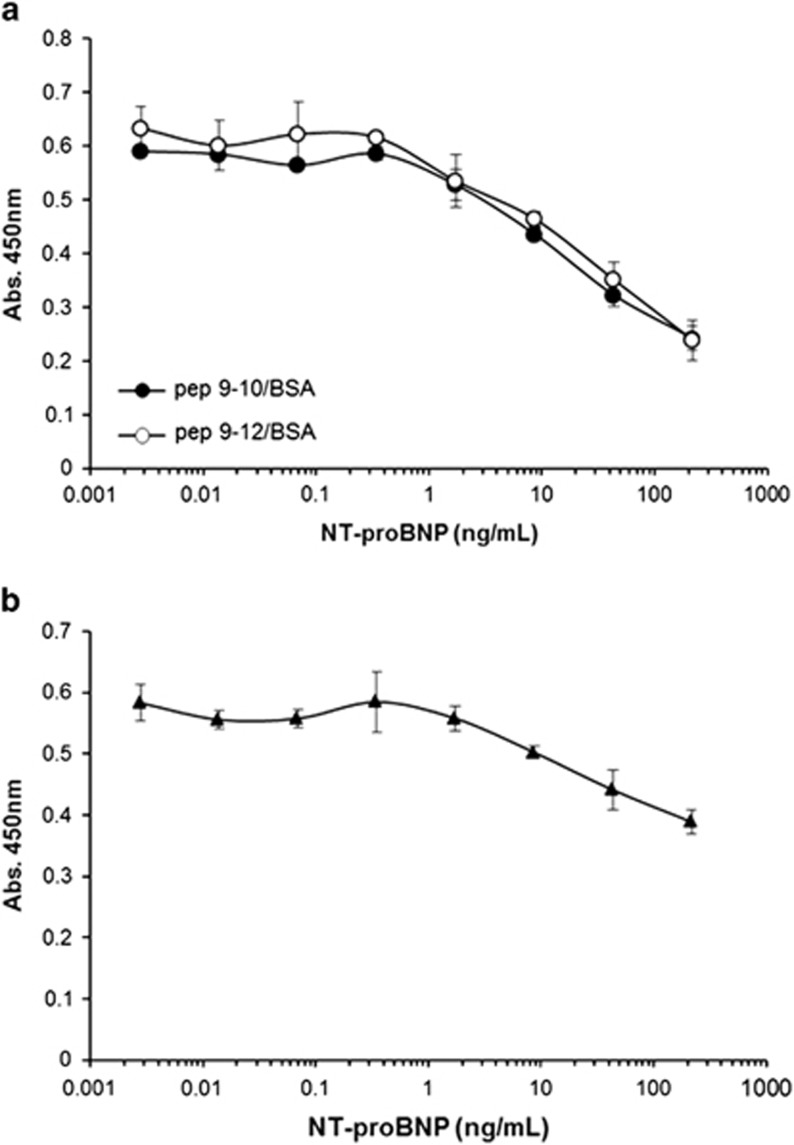
To further confirm that the peptides represent the epitope, a competition assay was developed. BSA–peptide conjugates were coated onto a microtiter plate. The recombinant NT-proBNP-Fc fusion protein was serially diluted, preincubated with NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein and added to each well. Next, HRP-conjugated cotinine and TMB substrate were sequentially added. In this competition assay the binding of the antibody to peptides was decreased at concentrations of 0.3 ng ml−1 or higher in a dose-dependent manner (Figure 4a). In a parallel experiment using the recombinant NT-proBNP-Fc fusion protein-coated plate, the addition of the recombinant NT-proBNP-Fc fusion protein to the soluble protein decreased antibody binding to the plate in a similar dose-dependent manner (Figure 4b). On the other hand, the addition of irrelevant Fc fusion protein did not affect the binding of the NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein either to BSA–peptide conjugates or the recombinant NT-proBNP-Fc fusion protein coated on the plate (data not shown). The competition assay was also performed using the surface plasmon resonance format. BSA–peptide conjugates were immobilized using amine coupling chemistry to a CM5 sensor chip. The binding of the antibody to the chip was decreased by the addition of a fusion protein in a dose-dependent manner at a concentration of ⩾0.4 nM (Supplementary Figure S1). A similar pattern of decrease in binding was observed in a parallel experiment using a fusion protein-conjugated chip.
Figure 4.
Competition enzyme immunoassay using peptide mimotopes. The microtiter plate was coated with either (a) bovine serum albumin (BSA)–peptide conjugates or (b) recombinant NT-proBNP-Fc fusion protein. Serially diluted NT-proBNP-Fc fusion protein was preincubated with NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein, and the mixture was added into each well. The amount of bound NPBR9 antibody was determined using horseradish peroxidase (HRP)-conjugated cotinine. NT-proBNP, N-terminal fragment of prohormone brain natriuretic peptide; scFv, single-chain variable fragment.
Sandwich enzyme immunoassay using NPBR9 antibody
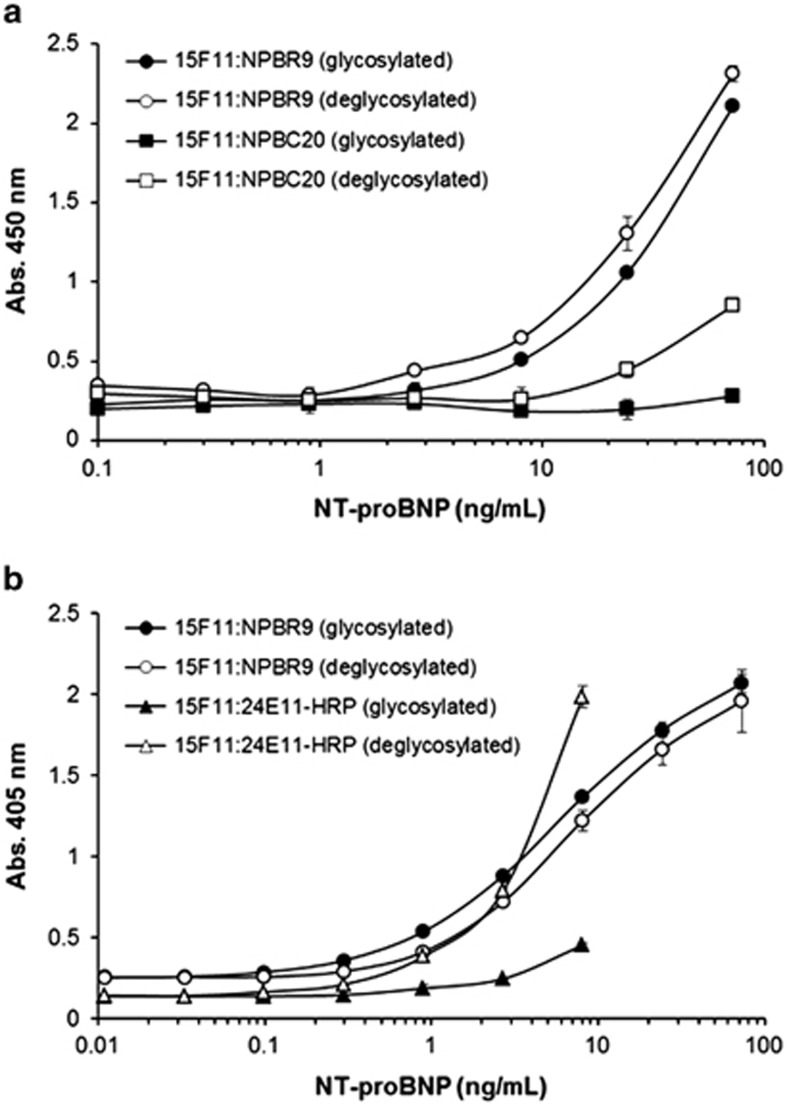
To further confirm the binding of NPBR9 was not affected by the O-glycosylation of NT-proBNP, we performed a sandwich enzyme immunoassay. The 15F1113–27 antibody, reactive to an epitope encompassing the 13th to 27th residue of NT-proBNP, was coated onto the plate. Subsequently, either untreated or deglycosylated recombinant NT-proBNP-Fc fusion protein was serially diluted threefold and added to each well. Following this, the binding of the NPBR9 or NPBC20 × anti-cotinine bispecific scFv-Cκ fusion proteins was determined using HRP-conjugated cotinine. The NPBR9 antibody exhibited similar binding to untreated as well as deglycosylated recombinant NT-proBNP-Fc fusion protein, whereas the binding of NPBC20 × anti-cotinine bispecific scFv-Cκ fusion protein was significantly increased upon deglycosylation (Figure 5a).
Figure 5.
Effect of deglycosylating NT-proBNP on its binding to the NPBR9, NPBC20 and 24E11 antibodies. The 15F1113–27 antibody reactive to residues 13–27 of NT-proBNP was coated onto a microtiter plate. Glycosidase-treated (○, □) or untreated (●, ▪) recombinant NT-proBNP-Fc fusion protein was serially diluted and added to each well. (a) Next, wells were incubated with NPBR9 × anti-cotinine bispecific scFv-Cκ fusion protein or NPBC20 × anti-cotinine bispecific scFv-Cκ fusion protein and subsequently probed with horseradish peroxidase (HRP)-conjugated cotinine, followed by HRP substrate. (b) Alternatively, the plate was incubated with either NPBR9 IgG1 and anti-human Fc antibody conjugated to HRP in succession, or an anti-NT-proBNP 24E1161–76 antibody conjugated to HRP, followed by HRP substrate. NT-proBNP, N-terminal fragment of prohormone brain natriuretic peptide; scFv, single-chain variable fragment.
We compared the binding characteristics of NPBR9 IgG1 with the 23E1161–76 antibody reactive to an epitope encompassing residues 61–76, including the glycosylated Thr71 residue. As expected, the binding of NPBR9 IgG1 was not significantly affected by the O-glycosylation status of the recombinant NT-proBNP-Fc fusion protein, whereas the binding of the 23E1161–76 antibody was dramatically increased on deglycosylation (Figure 5b).
Discussion
Antibody recognition of NT-proBNP is influenced by both the glycosylation pattern7 and its fragmentation in blood. NT-proBNP in human blood is reported to be O-glycosylated at seven residues (Thr36, Ser37, Ser44, Thr48, Ser53, Thr58 and Thr71).6 Five of these residues are found to be always glycosylated in all NT-proBNPs and two residues (Thr36 and Thr58) are glycosylated only in a fraction of NT-proBNPs.6 This glycosylation pattern affects the recognition of the NT-proBNP molecule by antibodies.8 When antibodies are raised by immunization with nonglycosylated recombinant NT-proBNP, antibodies reactive to epitopes consisting of residues 28–45, 31–39, 34–39 and 46–56 showed drastically reduced reactivity to glycosylated NT-proBNP extracted from pooled plasma of heart failure patients compared with the deglycosylated form.
Recently, it was reported that immunoreactive NT-proBNP is present as multiple N- and C- terminally truncated fragments, based on a study in which NT-proBNP molecules were immunoprecipitated from the pooled plasma of four heart failure patients using an antibody reactive to epitope 13–20, digested with trypsin and subjected to liquid chromatography–electrospray ionization–tandem mass spectrometry.10 In the spectrum, several peptides were identified; these were semi-tryptic peptides, in which one end of the peptide was not the result of cleavage at consensus trypsin recognition sites. The physiological proteolysis sites were expected to be between the Pro2–Leu3, Leu3–Gly4, Pro6–Gly7 and Pro75–Arg76 residue pairs. Consistent with these results, AlphaLISA immunoassays (PerkinElmer, Waltham, MA, USA) demonstrated that antibodies targeting the extreme N- or C- termini measured a low apparent concentration of circulating NT-proBNP.10 The apparent circulating NT-proBNP concentration was higher when antibodies targeting nonglycosylated and nonterminal epitopes were used in the immunoassay. The major limitation of this study was that NT-proBNP molecules physiologically fragmented beyond the 30th residue could not be enriched by the immune-precipitation process.
Based on the observation that circulating NT-proBNP is heavily and heterogeneously O-glycosylated and fragmented extensively from both ends, it is clear that the antibody binding site is important in the development of the enzyme immunoassay. There are several clinically available sandwich immunoassays. One assay employs polyclonal antibodies against epitopes of NT-proBNP1–21 and NT-proBNP39–50.20 Another polyclonal antibody pair that recognizes NT-proBNP8–29 and NT-proBNP31–57 is used in another assay kit.21 Monoclonal antibody pairs recognizing NT-proBNP13–27 and NT-proBNP61–76 or NT-proBNP1–21 and NT-proBNP61–76 are also employed.8 The reactivity of antibodies directed against NT-proBNP39–50 and NT-proBNP31–57 could be affected by the glycosylation pattern, those directed against NT-proBNP1–21 and NT-proBNP8–29 by physiological fragmentation and those against NT-proBNP13–27 and NT-proBNP61–76 by both modifications. Compared with these monoclonal antibodies, the epitope of NPBR9 was more precisely defined to be G63H64R65K66 by mimetic peptide screening (Figure 2), site-directed mutagenesis (Figure 3) and competition assay with peptide mimotope (Figure 4a). As the nearest O-glycosylation residues are Thr58 and Thr71, four amino acid residues intervene between the epitope and those residues in both directions. As there are only six, three and four amino acid residue gaps between the two O-glycosylation residues in Ser37–Ser44, Ser44–Thr48 and Thr48–Ser53, respectively, this epitope provides the longest gaps between the two O-glycosylation residues. Furthermore, only four amino acid residues are present between O-glycosylated Thr71 and Pro75 after possible physiological proteolysis. Based on these facts, the epitope G63H64R65K66 has the longest distances from the O-glycosylation residues. There has been no report that two antibodies for a sandwich immunoassay can bind to the 28-amino acid residue-long region between Gly7 and O-glycosylated Thr36 in physiologically digested NT-proBNP and, therefore, one antibody reactive to this 28-amino acid residue-long region and the other antibody binding to G63H64R65K66 is an ideal pair for an sandwich immunoassay detecting the greatest amount of O-glycosylated and physiologically digested NT-proBNP.
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government Ministry of Science, ICT and Future Planning (2013K000230).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
Supplementary Material
References
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- Palazzuoli A, Gallotta M, Quatrini I, Nuti R. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc Health Risk Manag. 2010;6:411–418. doi: 10.2147/vhrm.s5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerico A, Vittorini S, Passino C. Measurement of the pro-hormone of brain type natriuretic peptide (proBNP): methodological considerations and pathophysiological relevance. Clin Chem Lab Med. 2011;49:1949–1954. doi: 10.1515/CCLM.2011.686. [DOI] [PubMed] [Google Scholar]
- Cowie MR, Mendez GF. BNP and congestive heart failure. Prog Cardiovasc Dis. 2002;44:293–321. doi: 10.1053/pcad.2002.24599. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A, Santalo-Bel M, Zapico-Muniz E, Lopez L, Cotes C, Bellido J, et al. N-terminal probrain natriuretic peptide (NT-proBNP) in the emergency diagnosis and in-hospital monitoring of patients with dyspnoea and ventricular dysfunction. Eur J Heart Fail. 2004;6:301–308. doi: 10.1016/j.ejheart.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Schellenberger U, O'Rear J, Guzzetta A, Jue RA, Protter AA, Pollitt NS. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys. 2006;451:160–166. doi: 10.1016/j.abb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Peng J, Jiang J, Wang W, Qi X, Sun XL, Wu Q. Glycosylation and processing of pro-B-type natriuretic peptide in cardiomyocytes. Biochem Biophys Res Commun. 2011;411:593–598. doi: 10.1016/j.bbrc.2011.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferian KR, Tamm NN, Semenov AG, Tolstaya AA, Koshkina EV, Krasnoselsky MI, et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin Chem. 2008;54:866–873. doi: 10.1373/clinchem.2007.100040. [DOI] [PubMed] [Google Scholar]
- Semenov AG, Postnikov AB, Tamm NN, Seferian KR, Karpova NS, Bloshchitsyna MN, et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem. 2009;55:489–498. doi: 10.1373/clinchem.2008.113373. [DOI] [PubMed] [Google Scholar]
- Foo JY, Wan Y, Schulz BL, Kostner K, Atherton J, Cooper-White J, et al. Circulating fragments of N-terminal pro-B-type natriuretic peptides in plasma of heart failure patients. Clin Chem. 2013;59:1523–1531. doi: 10.1373/clinchem.2012.200204. [DOI] [PubMed] [Google Scholar]
- Park S, Lee DH, Park JG, Lee YT, Chung J. A sensitive enzyme immunoassay for measuring cotinine in passive smokers. Clin Chim Acta. 2010;411:1238–1242. doi: 10.1016/j.cca.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Lee JC, Choi CY, Chung J. Production and characterization of monoclonal antibody to botulinum neurotoxin type B light chain by phage display. Hybridoma (Larchmt) 2008;27:18–24. doi: 10.1089/hyb.2007.0532. [DOI] [PubMed] [Google Scholar]
- Zhuang G, Katakura Y, Furuta T, Omasa T, Kishimoto M, Suga K. A kinetic model for a biopanning process considering antigen desorption and effective antigen concentration on a solid phase. J Biosci Bioeng. 2001;91:474–481. doi: 10.1263/jbb.91.474. [DOI] [PubMed] [Google Scholar]
- Barbas CF, III, Burton DR, Scott JK, Silverman GJ. Phage Display: A Laboratory Manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA; 2001. [Google Scholar]
- Fennell BJ, McDonnell B, Tam AS, Chang L, Steven J, Broadbent ID, et al. CDR-restricted engineering of native human scFvs creates highly stable and soluble bifunctional antibodies for subcutaneous delivery. MAbs. 2013;5:882–895. doi: 10.4161/mabs.26201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Hur Y, Ryu EK, Rhim JH, Choi CY, Baek CM, et al. A neutralizable epitope is induced on HGF upon its interaction with its receptor cMet. Biochem Biophys Res Commun. 2007;354:115–121. doi: 10.1016/j.bbrc.2006.12.164. [DOI] [PubMed] [Google Scholar]
- Chung J, Park S, Kim D, Rhim J, Kim I, Choi I, et al. Identification of antigenic peptide recognized by the anti-JL1 leukemia-specific monoclonal antibody from combinatorial peptide phage display libraries. J Cancer Res Clin Oncol. 2002;128:641–649. doi: 10.1007/s00432-002-0390-x. [DOI] [PubMed] [Google Scholar]
- Kim H, Park S, Lee HK, Chung J. Application of bispecific antibody against antigen and hapten for immunodetection and immunopurification. Exp Mol Med. 2013;45:e43. doi: 10.1038/emm.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson PO, Barnes SC, Gaze DC, Galasko G, Lahiri A, Senior R. Analytical performance of the N terminal pro B type natriuretic peptide (NT-proBNP) assay on the Elecsys 1010 and 2010 analysers. Eur J Heart Fail. 2004;6:365–368. doi: 10.1016/j.ejheart.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Biomedica Medizinprodukte Gmbh & Co KG. Method of determining NT-proBNP. US patent 20100136590A12010
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.