Abstract
Experience shapes brain function throughout life to varying degree. In a recent issue of Nature, Donato et al. identify reversible shifts in focal parvalbumin-cell state during adult learning, placing it on a mechanistic continuum with developmental critical periods. A disinhibitory microcircuit controls the plasticity switch to modulate memory formation.
The brain is plastic – a hallmark often attributed to detailed mechanisms of synaptic potentiation and depression at single excitatory connections. Yet, these processes alone cannot explain the declining capacity to adapt with age, or the full complexity of learning and memory behaviors. In a recent issue of Nature, Pico Caroni and colleagues (Donato et al, 2013) elegantly reconfirm that the broader context of excitatory-inhibitory circuit balance may in fact hold the key to adult brain plasticity.
The hippocampus, and in particular its CA3 sub-region, accounts for the rapid generation and contextualization of episodic memories. Experience can affect these processes; environmental enrichment enhances hippocampal learning and memory, such that mice housed with toys and tunnels more readily discriminate objects from a familiar pair they had seen the day before. Instead, Pavlovian fear conditioning restricted to a specific training context impairs novel object recognition even a few hours later (Ruediger et al, 2011).
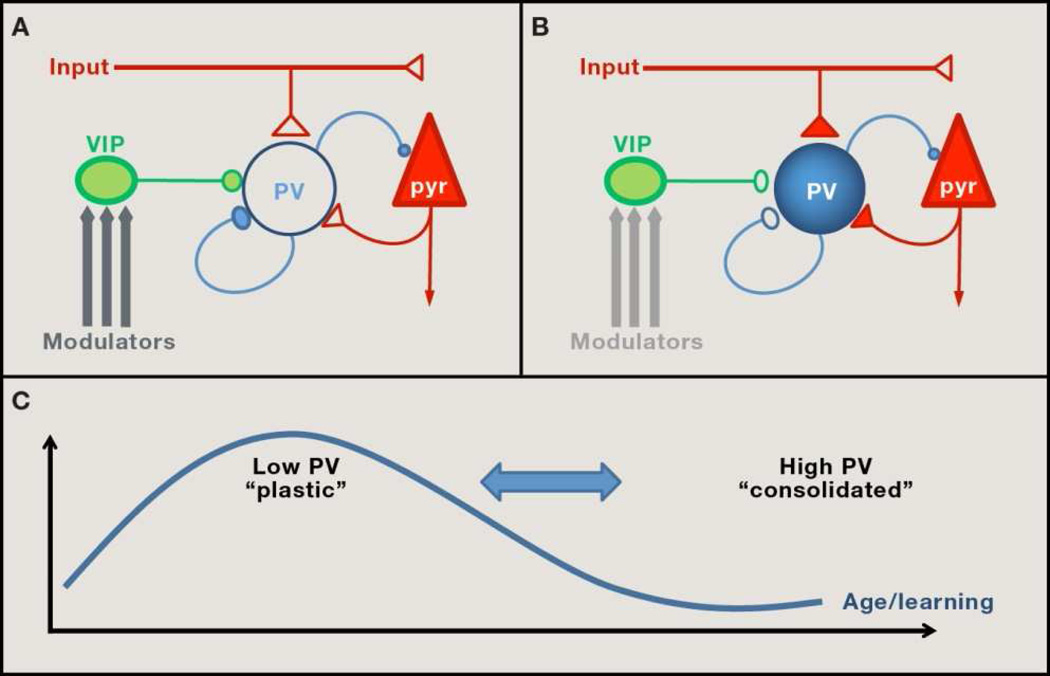
Donato et al. now find that a particular class of inhibitory neurons within the CA3, the parvalbumin (PV)-positive basket cells, exhibits a change in state under these conditions. Namely, PV expression is predominantly low after environmental enrichment, shifting to high PV content upon fear conditioning. This switch is likely to be functional, as PV-levels correlate with that of GAD67, the primary synthetic enzyme for the inhibitory neurotransmitter, GABA. Low- or high- PV states are, respectively, paralleled by an increase of GABAergic or excitatory synaptic inputs onto the PV-cells themselves (Figure 1A and B). These anatomical findings suggest that activation of PV-cells alone might causally promote a high-PV state and impede hippocampal plasticity. Direct stimulation of PV-cells by viral expression of light- or ligand-gated channels confirmed this prediction. Conversely, direct PV-neuron silencing was sufficient to induce a low-PV network configuration that enhanced novel object recognition. These manipulations also negated the plasticity benefits of environmental enrichment or the detrimental impact of conditioned fear.
Figure 1. Network configurations of PV-expressing neurons in brain plasticity.
(A) Network configurations favoring plasticity are biased toward ‘low-PV’ cells maintained by increased inhibitory input from VIP micro circuitry, which is readily engaged by neuromodulators under conditions of enriched environment or adult incremental training. (B) A ‘high-PV’ state, refractory to plasticity, is attained by enhanced excitatory drive from local pyramidal cells (pyr) or external input as memories are consolidated. (C) Focal PV-cell transitions during adult learning (Donato et al, 2013) mirror the prolonged trajectory of developmental critical periods. Notably, PV-cell state and plasticity are reversible throughout life (Takesian and Hensch, 2013).
Strikingly, the authors also found that the composition of PV-cells follows the trajectory of incremental trial-and-error learning. The hippocampus is essential for encoding spatial memories when mice learn to navigate, say in a tank of water in search of a submerged escape platform. Donato et al. observed that CA3 networks are biased toward low-PV cells during the learning phase of the task, shifting to high-PV as the memories become consolidated. Remarkably, this also predicted a sequential enhancement, then interference on a concurrent novel object recognition test. Moreover, the PV-cell transitions were specific to the hippocampus, and generalized to primary motor cortex (M1) during similar learning of a motor task.
Such a pivotal role for PV circuits in adult plasticity is satisfying for several reasons. First, these keen anatomical observations provide an understanding how performance on one memory task can be influenced by the learning of another one. Second, patchy PV staining has been reported across a number of transgenic mouse models (Canty et al, 2011; Gogolla et al, 2009). While potentially dismissible as labeling artifacts, the results of Donato et al. alternatively suggest that they are telling snapshots of regional in homogeneity in brain plasticity. Third, experiential changes in the PV-cell state are well known to be associated with developmental windows of robust plasticity, named “critical periods” (reviewed in Takesian and Hensch, 2013). Much of our adult brain function is powerfully shaped during these early critical periods when neural circuits are first adapting to their surrounding environment. Native language acquisition or the enduring loss of visual acuity and cortical connectivity upon discordant vision through the two eyes (“lazy eye”) are classic examples. Bidirectional plasticity of PV basket-cell inputs initiates this rewiring process both in cat and mouse visual cortex (Takesian and Hensch, 2013). Adult learning may then rely upon essentially the same local circuit mechanism, albeit on a finer scale or time-course (Figure 1C)
Which cellular factors may be regulating the change in PV state in adulthood? PV-cell maturation is maintained by a variety of molecules, including neurotrophins (BDNF, GDNF), homeoproteins (Otx2), neuronal pentraxins (NARP) and neuregulin-1 (NRG-1) signaling (Canty et al, 2011; Spatazza et al, 2013; Gu et al, 2013; Tamura et al, 2012). Curiously, these are all non-cell autonomous, activity-dependent factors, further supporting a hub-like role for PV-cells in monitoring the local circuit milieu. Moreover, they interact and are released from an extracellular glycosaminoglycan matrix surrounding PV-cells, known as the peri-neuronal net (PNN). Otx2 depletion or PNN removal in adulthood resets PV-cells to reopen a juvenile, plastic state (Takesian and Hensch, 2013; Spatazza et al, 2013), and Caroni and colleagues now validate that PNN removal also resets hippocampal PV-cells to a low-PV condition. They further confirm their previous report of robust, long-lasting and reversible increases in the number of mossy fiber filopodial synapses onto PV-cells during learning (Ruediger et al, 2011). This up-regulation of excitatory inputs onto high-PV cells suggests a common role for the pentraxin NARP, which is secreted at excitatory connections and obligatory for critical period plasticity (Gu et al, 2013). In limbic areas, proteolytic processing of NRG-1 by neurops in might further regulate PV-neurons to control neural plasticity (Tanaka et al, 2012). Importantly, too much or too little Otx2 or NRG-1 is not conducive to plasticity (Spatazza et al, 2013; Tanaka et al, 2012), suggesting an optimal efficacy range worthy of assessment in adult CA3.
Finally, Donato et al. identified a circuit mechanism regulating the shifts in PV state. Spatial memory training increased the density of synaptic boutons from a specific GABA neuron subtype containing the vasoactive intestinal peptide (VIP) on PV-cell dendrites(Figure 1A and B). Direct optogenetic stimulation of these inputs reduces PV expression, while VIP-neuron silencing shifts network configuration to a high-PV state, suppressing further learning. VIP cells are notably engaged by neuromodulators, such as acetylcholine or serotonin, released under enriched environments or moments of heightened arousal that enable plasticity (Letzkus et al, 2011). Critical periods of plasticity conversely close as these neuromodulatory systems are dampened with age (Takesian and Hensch, 2013).
In summary, regulation offeed-forward PV-inhibition may play an appropriately scaled role in plasticity throughout life (Figure 1C). Disinhibitory VIP microcircuits are a ubiquitous feature of cerebral cortex (Pi et al, 2013). Likewise, Otx2 broadcast globally from the choroid plexus, may have far-reaching impact on PV-cells across the adult brain (Spatazza et al, 2013). Compromised PV circuits, commonly seen in schizophrenia or autism spectrum disorders (see Takesian and Hensch, 2013; Gogolla et al, 2009; Tanaka et al, 2012), may then contribute mis-timed critical period trajectories or imprecise memory retrieval in these cognitive disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Canty AJ, Dietze J, Harvey M, Enomoto H, Milbrandt J, Ibáñez CF. J Neurosci. 2009;29:10695–10705. doi: 10.1523/JNEUROSCI.2658-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB, Caroni P. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. J Neurodev Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huang S, Chang MC, Worley P, Kirkwood A, Quinlan EM. Neuron. 2013;79:335–346. doi: 10.1016/j.neuron.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Lüthi A. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang JZ, Kepecs A. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruediger S, Vittori C, Bednarek E, Genoud C, Strata P, Sacchetti B, Caroni P. Nature. 2011;473:514–518. doi: 10.1038/nature09946. [DOI] [PubMed] [Google Scholar]
- Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, Prochiantz A. Cell Rep. 2013;3:1815–1823. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- Tamura H, Kawata M, Hamaguchi S, Ishikawa Y, Shiosaka S. J Neurosci. 2012;32:12657–12672. doi: 10.1523/JNEUROSCI.2542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]