Abstract
Purpose:
Obstructive sleep apnea hypopnea syndrome (OSAHS), a common sleep and breathing disorder, is independently associated with metabolic dysfunction, including impaired glucose tolerance and insulin resistance. Intermittent hypoxia (IH), a pathological component of OSAHS, increases oxidative stress damage to pancreatic β-cells in animal models resembling patients with OSAHS. However, the precise mechanisms of IH-induced pancreatic β-cell dysfunction are not fully understood. In the present study, we established a mice model to investigate the underlying mechanisms of oxidative stress in IH-induced pancreatic β-cell apoptosis through antioxidant N-acetylcysteine (NAC) pretreatment.
Methods:
Twenty-four Wistar rats were randomly divided into four experimental groups: normal control group, intermittent normoxia group, IH group and antioxidant intervention group. Pancreatic β-cell apoptosis rates were detected by terminal deoxynucleotidyl transferase-mediated dUTP-nick end-labeling; Bcl-2 and Bax protein expressions were detected by immunohistochemistry staining and western blotting.
Results:
In our study, we demonstrated that IH exposure causes an increased activation of pancreatic β-cell apoptosis compared with that in the normal control group and intermittent normoxia group, accompanied by the downregulation of Bcl-2 and upregulation of Bax (P<0.05). Furthermore, compared with the IH group, antioxidant (NAC) pretreatment significantly decreased IH-mediated β-cell apoptosis and reversed the ratio of Bcl-2/Bax expression (P<0.05).
Conclusion:
Taken together, these results demonstrate a critical role of oxidative stress in the regulation of apoptosis through Bcl-2 and Bax signaling. The antioxidant NAC has a protective effect against IH-induced pancreatic β-cell apoptosis.
Introduction
Multiple epidemiological studies have already shown an association between obstructive sleep apnea hypopnea syndrome (OSAHS) and type 2 diabetes mellitus.1, 2, 3 A recent study reported that OSAHS increased the rate of type 2 diabetes among a cohort of 4000 community residents even when controlled for obesity.4 Although, the presence of OSAHS is an independent risk factor for the emergence of insulin resistance, the underlying pathogenesis of glucose metabolism disorder in OSAHS patients remain less well understood.
OSAHS, a very common sleep and breathing disorder, is the repetitive collapse of the upper airway during sleep.5 Intermittent hypoxia (IH), a major pathological component of OSAHS, is characterized by repeated hypoxia and reoxygenation. During hypoxia, both cardiomyocytes and neurons produce large amounts of reactive oxygen species (ROS) that contribute to tissue injury.6,7 In addition, this condition can also induce various metabolic dysfunctions, including insulin resistance and impaired glucose tolerance.8 One of the major cellular death responses to IH-induced increased levels of ROS generation is apoptotic cell death. Cells undergoing apoptosis release a series of apoptotis-associated proteins from their resident places.9 Evidently, Bcl-2 family proteins are considered to have a critical role during the above-stated events.10 A common cell apoptosis characteristics after β-cell exposure to IH are Bax translocation to the mitochondria, cytochrome c release and caspase-9 initiation.11
Pancreatic β-cell is particularly susceptible to oxidative stress damage for the inadequacy of ROS-detoxifying systems.12 Compared with the muscle, liver and kidney, rat islets can be 25 times more sensitive to superoxide radicals.13 Furthermore, the quantity of superoxide dismutase in the islets is <30% of that measured in the liver.14 A previous study revealed that oxidative stress triggered glucose metabolism dysfunction and cell death in rats, whereas in MnSOD transgene rats antioxidant protein, MnSOD did not effect IH-induced β-cell proliferation but completely abrogated the IH-induced cell death.15 Therefore, the inhibition of ROS generation response to IH can be an important treatment principle to independently restore the normal functioning of the pancreas and control the progression of insulin resistance-induced type 2 diabetes.
N-acetylcysteine (NAC), a precursor of intracellular glutathione, is a powerful antioxidant in a cell. In some physiological or pathological states, NAC can intervene and clear endogenous oxygen radicals and delay the process of cell senescence eventually.16 At present, NAC has been applied as a treatment for systemic diseases, such as myocardial ischemia–reperfusion injury, azithromycin-induced cardiotoxicity, acute respiratory distress syndrome and so on.17
In this study, we establish an IH model mimicking the IH episodes of OSAHS to analyze the mechanism of cell death in pancreatic β-cell due to the exposure to IH. This is a study on how antioxidant NAC provides protection against IH-induced β-cell damage in the apoptotic pathway.
Materials and methods
Terminal deoxynucleotidyl transferase-mediated dUTP-nick end-labeling (TUNEL) apoptosis detection kit (Roche, Indianapolis, IN, USA), rabbit anti-Bcl-2 antibody, rabbit anti-Bax antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti-mouse β-actin primary antibody (Santa Cruz Biotechnology) and horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Boaosen, Beijing, China). Twenty-four adult male Wistar rats (body weight 280–350 g) were purchased from the Animal Laboratory Center of Tianjin Medical University (Tianjin, China).
Animals
Intermittent hypoxic conditions for animals
The male Wistar rats (280–350 g) were provided by the Experimental Animal Center of Tianjin Medical University (Tianjin, China). All the experiments were performed in accordance with the law for the protection of animals. Twenty-four Wistar rats were randomly divided into four experimental groups: normal control group (NC, n=6), intermittent normoxia group (NOX, n=6), IH group (IH, n=6) and antioxidant intervention group (AO, n=6). Briefly, the animals were housed in chambers with a 12-h light/dark cycle (0800–2000) for 6 weeks. IH profiles consisted of alternating normoxia (21% O2, 5% CO2 and balance N2) for 90 s and hypoxia (5% O2, 5% CO2 and balance N2) for 30 s.18 Oxygen concentration in the chamber was continuously measured by an O2 analyzer and was altered by an oxygen sensor linked to the computerized system controlling gas outlets. The normal control group was continuously exposed to room air (21% O2) at the same period of time and the intermittent normoxia group was kept under normoxia (21% O2) cyclically in similar chambers. The antioxidant intervention group received NAC administered subcutaneously in a dose of 300 mg kg−1 daily before hypoxic exposure and the rest of the rats received equal volume of physiological saline at the same time. All the experimental groups were maintained at 22 –25 °C and given normal food and water in the nonexperimental time (2000–0800). At the end of the experiment, the rats were dissected and the pancreas was removed. All the animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of China and the institutional guidelines for animal welfare and experimental conduct were followed.
Immunohistochemical staining for Bcl-2 and Bax protein expression
Rat pancreas tissue was fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 min. After antigen retrieval under high pressure, pancreas tissue was incubated with 3% H2O2 for 5 min to inactivate the endogenous peroxidase. Then the tissue slices were blocked with 5% goat serum (diluted by phosphate-buffered saline) and incubated with a primary antibody (1:50) at 4 °C overnight, then incubated with a secondary antibody for 30 min at room temperature. After 3,3'-diaminobenzidine staining, slides were observed under an optical microscope, five completed and non-overlapped high-magnification fields ( × 400) were selected. Average optical density values were regarded as the measured values.
Western blot for Bcl-2 and Bax protein expression
The Bcl-2 and Bax proteins from the pancreas tissue were extracted according to the instructions for the protein extraction. After the pancreas was centrifuged, the supernatant was extracted and centrifuged at 4 °C. Protein concentrations were measured by using a Bio-Rad Bradford assay (Bio-Rad Laboratories, Inc, Hercules, CA, USA). Before being transferred to a nitrocellulose membrane, Bcl-2 and Bax protein were separated on 12% SDS–polyacrylamide gel electrophoresis. Then the membranes were blocked with 5% non-fat milk for 2 h at room temperature, followed by incubation with primary antibody (anti-Bcl-2 at 1:500; anti-Bax at 1:800) at 4 °C overnight and secondary antibody conjugated with horseradish peroxidase for 1 h at room temperature. In the end, the membranes were treated with emitter-coupled logic and the signals were detected by exposure of the membranes to X-ray films, β-actin was used for normalization. The relative signal intensity was quantified by densitometry with Quantity One software (Bio-Rad Laboratories, Inc).
TUNEL staining for pancreatic β-cell apoptosis
Pancreatic β-cell apoptosis staining was done according to TUNEL apoptosis detection kit instructions. Animals were fixed by cardiac perfusion using 4% paraformaldehyde in 0.1 mol l−1 sodium cacodylate buffer. After being extracted and fixed, pancreatic tissue slices were embedded in paraffin and then deparaffinized and dehydrated. Then the slices were incubated with 100 μl proteinase K at 37 °C for 30 min and treated with 3% hydrogen peroxide for 10 min to inactivated endogenous peroxidase. Then the slices were permeabilized with 0.2% Triton X-100 and colored with terminal deoxynucleotidyl transferase buffer at 37 °C for 60 min. A negative control was prepared by omitting the terminal deoxynucleotidyl transferase enzyme to control for non-specific incorporation of nucleotides or binding of enzyme conjugate. TUNEL-positive cells were acquired and analyzed by counting the number of terminal deoxynucleotidyl transferase-positive nuclei in five high-magnification fields ( × 400).
Statistical analysis
Data were expressed as mean±s.d. Experimental data were analyzed by analysis of variance through SPSS version 13.0 statistical software (SPSS (IBM), Armonk, NY, USA). The Student–Newman–Keuls post hoc tests were used to compare the two groups. P-value <0.05 was considered statistically significant.
Results
Antioxidant intervention (NAC) prevents IH-induced pancreatic β-cell apoptosis
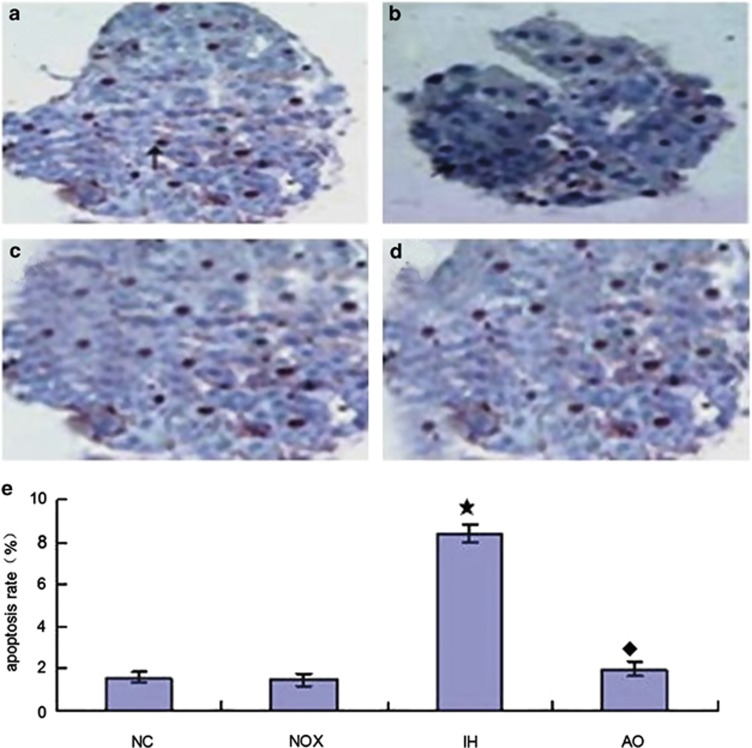
In this study, we established an adequate mice model to study the effects of IH on pancreatic β-cell death in vitro (Figure 1). The hypoxic and normoxic exposure duration to β-cell in each cycle is 90 min. After 6 weeks of IH exposure, we applied TUNEL staining to analyze IH-mediated β-cell apoptosis in the pancreas. As shown in Figures 1a–c and e, IH exposure resulted in more than fourfold increase in the number of apoptosis positively stained β-cells (mean±s.d.=8.380±0.938) compared with the intermittent normoxia group (mean±s.d.=1.247±0.082) and normal control group (mean±s.d.=1.583±0.349) (P<0.05). In contrast, mice treated with NAC had significantly decreased IH-mediated β-cell apoptosis (Figures 1d and e).
Figure 1.
Effect of NAC on IH-induced pancreatic β-cell apoptosis by TUNEL staining ( × 400) (a) Normal control group. (b) Intermittent normoxia group (arrow points: cytoplasm containing brown granular deposition). (c) Intermittent hypoxia group. (d) Antioxidant intervention group. (e) The statistical analyses of pancreatic β-cell apoptosis ratio in four groups. These results are expressed as x̄±s (n=6 per group, ‘★' indicates significance compared to the intermittent normoxia group, P<0.05; ‘♦' indicates significance compared with the intermittent hypoxia group, P<0.05).
Antioxidant intervention (NAC) reverses the imbalance of Bcl-2 and Bax protein expression induced by IH
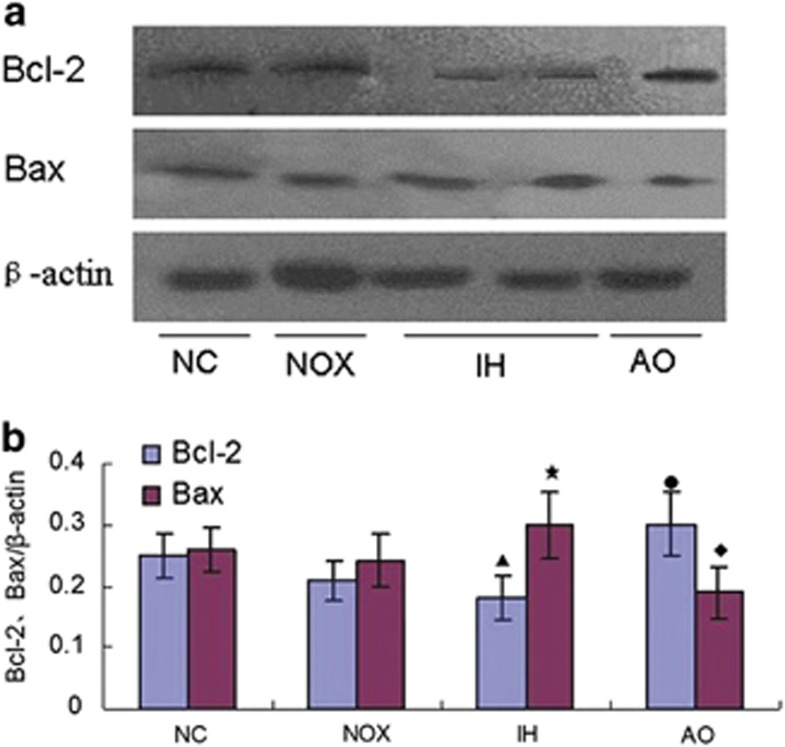
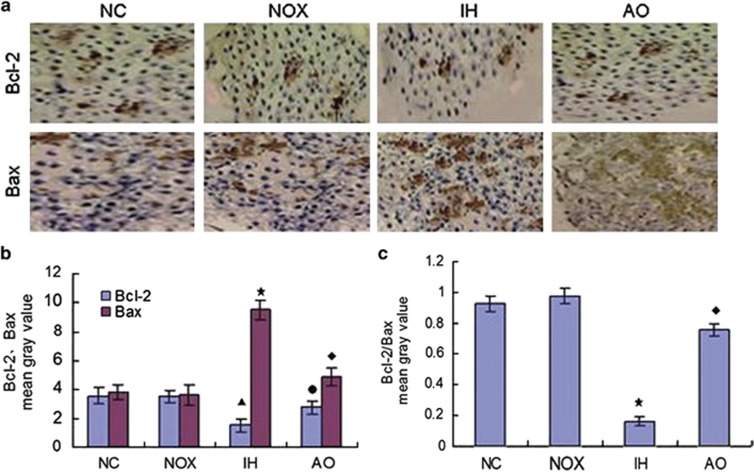
The level of Bcl-2 and Bax proteins expression were detected by western blot and immunohistochemical staining, respectively. Results showed that when compared with the intermittent normoxia group and normal control group, IH exposure decreased Bcl-2 expression and increased Bax expression simultaneously. On the contrary, antioxidant pretreatment reversed the ratio of Bcl-2/Bax expression in the pancreatic β-cells of IH-exposed mice (Figures 2 and 3).
Figure 2.
Effect of antioxidant intervention NAC on IH-induced Bcl-2 and Bax expression (western blotting). (a) A representation of western blotting analysis suggested antioxidant (NAC) intervention reversed IH-induced Bcl-2 and Bax expression. β-Actin was used as for normalization. (b) The statistical analyses of the Bcl-2 and Bax expression in the pancreatic β-cells of the four groups. These results are expressed as x̄±s (n=6 per group, ‘▴,★' indicate significance compared with the intermittent normoxia group, P<0.05; ‘●,♦' indicate significance compared with the intermittent hypoxia group, P<0.05).
Figure 3.
Effect of antioxidant intervention (NAC) on IH-induced Bcl-2 and Bax expression (immunohistochemical staining × 400). (a) A representation of immunohistochemical staining of Bcl-2 and Bax expression in the pancreatic β-cells of each group. (b) The statistical analyses of Bcl-2 and Bax expression in the pancreatic β-cells exposed to normal control group, intermittent normoxia group, intermittent hypoxia group and antioxidant intervention group. (n=6 per group, ‘▴,★' indicate significance compared with the intermittent normoxia group, P<0.05; ‘●,♦' indicate significance compared with the intermittent hypoxia group, P<0.05). (c) The statistical analyses of Bcl-2/Bax ratio in the four groups. (n=6 per group, ‘★' indicates significance compared with the intermittent normoxia group, P<0.05; ‘♦' indicates significance compared with the intermittent hypoxia group, P<0.05).
Discussion
Both epidemiologic and animal model reports have indicated that IH, a major component of OSAHS, leads to glucose metabolic dysfunctions.19 Nevertheless, the cellular and molecular mechanisms of IH-mediated deterioration in insulin sensitivity and insulin resistance remain unclear. Here, we use a mouse model mimicking OSAHS patients through IH exposure revealed an increased rate of β-cell apoptosis. Furthermore, the β-cell death in conjunction with the IH paradigms was reversed by the antioxidant NAC. NAC can reduce IH-mediated β-cell apoptosis and reverse the imbalance of apoptosis-related proteins Bcl-2/Bax ratio.
During sleep, the repeated episodes of hypoxia/ischemia and reoxygenation in the OSA patients may increase the level of ROS generation.20 Recent studies show important links between the OSAHS-related free radicals with oxidative stress and cardiovascular disease in OSAHS patients.21 In our present studies, we established IH paradigms in the mouse model, permitting delineation of the oxygenation profiles that imitate those of the patients with OSAHS.18 In addition, a previous study has found that exposure of the mice to chronic IH significantly aggravates pancreatic β-cell dysfunction, due to a high level of oxidative stress markers.22 These findings are extremely similar to the clinical observations in patients with OSAHS.
Although studies have attributed the effect of pancreatic β-cell death upon IH, the underlying mechanism of β-cell death is still unclear. To explore whether IH induces pancreatic β-cell apoptosis, we exposed the mice to 5% O2 hypoxia. Our results demonstrate that 6 weeks of IH treatment increased β-cell apoptosis, accompanied by the disturbance of Bcl-2 protein family expression (Figures 1, 2, 3). There is much support in literature for the idea that apoptosis is a consequence of IH. In vivo study has shown that IH treatment for 2 weeks have increased β-cell apoptosis due to oxidative stress.23 In Min6 cells, an increased activation of caspase-3 was detected after hypoxia treatment, suggesting that Min6 cell apoptosis occurs after a short period of hypoxia exposure.24 However, work from Ota et al.25 has detected that 24 h of IH treatment (1% O2) stimulates β-cells to induce IL-6 gene expression accompanied by a high expression of Reg family genes as well as HGF gene, consequently stimulating β-cell proliferation and inhibiting β-cell apoptosis. Intriguingly, Gozal and colleagues used a mouse model, mimicking OSAHS patients through IH (5.7% O2) exposure for 24 h, which revealed an increase in β-cell proliferation and death.15 Taken together, we preliminary speculate that IH has important roles in regulating pancreatic β-cell proliferation; however, prolonged IH can induce activation of cell apoptosis. Thus, the causal relationships between IH and β-cell death and the underlying molecular mechanisms need further discussion.
Apoptosis, known as programmed cell death, is regulated by the Bcl-2 family of proteins.26 In addition, a report shows that under hydrogen peroxide treatment Bcl-2 proteins cooperatively function in response to oxidative stress-induced apoptosis.27 Previous studies have demonstrated that the pancreatic β-cells are vulnerable to hypoxia-mediated oxidative stress because of the weak antioxidative defense mechanisms.28 Although oxygen radicals have important roles in regulating signal transduction for normal cellular physiological functions, overproduction of them can damage proteins and DNA, thus accelerating the process of cellular apoptosis or necrosis. More importantly, the increased oxidative stress damage is associated with a decline in the pancreatic β-cell function.22
Antioxidants were shown to improve basal insulin secretion and resistance.29 NAC is an antioxidant that decreases the level of intracellular hydrogen peroxide when β-cells are exposed to free fatty acids.30 It shows that chronic exposure to oleic acid impairs β-cell function through oxidative stress. NAC, at least in part, weakens the ROS-induced effects of glucotoxicity in β-cells, but the intrinsic mechanisms involved are unclear.30 In this study, we observed an improved cell survival rate by reducing apoptosis, when NAC was administered to mice before intermittent hypoxia (Figures 1d and e). Furthermore, downregulated β-cell apoptosis rate led to an increased expression in Bcl-2, which in turn cooperated with the decreased Bax levels (Figures 2 and 3). Thus, antioxidant treatment makes β-cells become susceptible to oxidative stress-induced apoptosis.
Conclusion
In conclusion, the current study here demonstrates that oxidative stress constitutes a critically important role in IH-induced pancreatic β-cell apoptosis. An important regulatory mechanism of apoptosis activation by oxidative stress is the imbalance of Bcl-2 and Bax expression. Furthermore, pretreatment with NAC significantly reduced IH-mediated β-cell deaths and reversed the imbalance of Bcl-2/Bax expression. These findings identify antioxidant NAC as a potential target for the therapy of type 2 diabetes mellitus in OSAHS patients.
Acknowledgments
This work was supported by the National Natural Foundation of China (Grant No.: 81370183), Tianjin Natural Science Foundation (Grant No.: 14JCYBJC27800) and National Clinical Key Subject Construction Project of NHFPC Fund.
The authors declare no conflict of interest.
References
- Lecomte P, Criniere L, Fagot-Campagna A, Druet C, Fuhrman C. Under diagnosis of obstructive sleep apnoea syndrome in patients with type 2 diabetes in France: ENTRED 2007. Diabetes Metab. 2013;39:139–147. doi: 10.1016/j.diabet.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Rozenfeld Y, Kai M, Stephens EA, Brown LK. Prevalence of diagnosed sleep apnea among patients with type 2 diabetes in primary care. Chest. 2012;141:1414–1421. doi: 10.1378/chest.11-1945. [DOI] [PubMed] [Google Scholar]
- Fredheim JM, Rollheim J, Omland T, Hofsø D, Røislien J, Vegsgaard K, et al. Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross-sectional study. Cardiovasc Diabetol. 2011;10:84. doi: 10.1186/1475-2840-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- Paiva T, Attarian H. Obstructive sleep apnea and other sleep-related syndromes. Handb Clin Neurol. 2014;119:251–271. doi: 10.1016/B978-0-7020-4086-3.00018-7. [DOI] [PubMed] [Google Scholar]
- Oktay B, Akbal E, Firat H, Ardic S, Akdemir R, Kizilgun M. Evaluation of the relationship between heart type fatty acid binding protein levels and the risk of cardiac damage in patients with obstructive sleep apnea syndrome. Sleep Breath. 2008;12:223–228. doi: 10.1007/s11325-007-0167-1. [DOI] [PubMed] [Google Scholar]
- Smith SM, Friedle SA, Watters JJ. Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS One. 2013;8:e81584. doi: 10.1371/journal.pone.0081584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarenbach CF, West SD, Kohler M. Is obstructive sleep apnea a risk factor for diabetes. Discov Med. 2011;12:17–24. [PubMed] [Google Scholar]
- Cosentino K, García-Sáez AJ. Mitochondrial alterations in apoptosis. Chem Phys Lipids. 2014;181C:62–75. doi: 10.1016/j.chemphyslip.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Shroff EH, Snyder C, Chandel NS. Role of Bcl-2 family members in anoxia induced cell death. Cell Cycle. 2007;6:807–809. doi: 10.4161/cc.6.7.4044. [DOI] [PubMed] [Google Scholar]
- Gurzov EN, Eizirik DL. Bcl-2 proteins in diabetes: mitochondrial pathways of β-cell death and dysfunction. Trends Cell Biol. 2011;21:424–431. doi: 10.1016/j.tcb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Wolf G, Aumann N, Michalska M, Bast A, Sonnemann J, Beck JF, et al. Peroxiredoxin III protects pancreatic ß cells from apoptosis. J Endocrinol. 2010;207:163–175. doi: 10.1677/JOE-09-0455. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- Grankvist K, Marklund SL, Täljedal IB. CuZn-superoxide dismutase, Mnsuperoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46:783–790. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Jin HM, Zhou DC, Gu HF, Qiao QY, Fu SK, Liu XL, et al. Antioxidant N-acetylcysteine protects pancreatic β-cells against aldosterone-induced oxidative stress and apoptosis in female db/db mice and insulin-producing MIN6 cells. Endocrinology. 2013;154:4068–4077. doi: 10.1210/en.2013-1115. [DOI] [PubMed] [Google Scholar]
- Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- He Q, Yang QC, Zhou Q, Zhu H, Niu WY, Feng J, et al. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One. 2014;9:e86326. doi: 10.1371/journal.pone.0086326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J, Shimoda LA, Drager LF, Undem C, McHugh H, Polotsky VY, et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep. 2013;36:1483–1490. doi: 10.5665/sleep.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang IG, Jung JH, Kim ST. The effect of obstructive sleep apnea on DNA damage and oxidative stress. Clin Exp Otorhinolaryngol. 2013;6:68–72. doi: 10.3342/ceo.2013.6.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran M, Ayas N, Laher I.Cardiovascular complications of sleep apnea: role of oxidative stress Oxid Med Cell Longev 20142014: 985258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic β-cell function by chronic intermittent hypoxia. Exp Physiol. 2013;98:1376–1385. doi: 10.1113/expphysiol.2013.072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2971–H2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Zheng X, Wang X, Ma Z, Gupta Sunkari V, Botusan I, et al. Acute hypoxia induces apoptosis of pancreatic β-cell by activation of the unfolded protein response and upregulation of CHOP. Cell Death Dis. 2012;3:e322. doi: 10.1038/cddis.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Miyaoka T, Fujimura T, et al. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 2013;93:664–672. doi: 10.1016/j.lfs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Hardwick JM, Youle RJ. SnapShot: BCL-2 proteins. Cell. 2009;138:404. doi: 10.1016/j.cell.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eno CO, Zhao G, Olberding KE, Li C. The Bcl-2 proteins Noxa and Bcl-xL co-ordinately regulate oxidative stress-induced apoptosis. Biochem J. 2012;444:69–78. doi: 10.1042/BJ20112023. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- Wang X, Li H, De Leo D, Guo W, Koshkin V, Fantus IG, et al. Gene and protein kinase expression profiling of reactive oxygen species-associated lipotoxicity in the pancreatic β-cell line MIN6. Diabetes. 2004;53:129–140. doi: 10.2337/diabetes.53.1.129. [DOI] [PubMed] [Google Scholar]