Abstract
Because histones bind DNA very tightly, the location and occupancy of nucleosomes can profoundly affect accessibility of non-histone proteins to chromatin, affecting virtually all DNA-dependent processes, such as transcription, DNA repair, DNA replication and recombination. Therefore, it is often necessary to determine positions and occupancy of nucleosomes to understand how DNA-dependent processes are regulated. Recent technological advancement made such analyses feasible in a genome-wide scale at a high resolution. In addition, we have recently developed a method to measure nuclease accessibility of nucleosomes in a global scale. Here we describe methods to map nucleosome positions, to determine nucleosome density, and to determine nuclease accessibility of nucleosomes using deep sequencing.
Keywords: nucleosome positioning, nucleosome occupancy, chromatin accessibility, deep sequencing, genome-wide analysis
Introduction
This unit describes methods for global yeast chromatin analyses. The first protocol describes a method for precisely identifying genomic nucleosome positions using micrococcal nuclease (MNase), an enzyme that cleaves between neighboring nucleosomes. This protocol is ideal for identifying, with high resolution, changes in nucleosome positions associated with different cellular conditions or investigating proteins involved in establishing chromatin architecture in vivo. The second protocol describes a method for determining genome-wide histone occupancy. Such information is critical to evaluate changes in density of nucleosomes at genomic regions, particularly regulatory elements, in response to environmental cues or chromatin remodeling events. The third protocol describes a procedure to evaluate the accessibility of specific nucleosomes across the genome using a combination of nucleosome positioning data and histone density (from protocols 1 and 2). Accessibility data provides information about how easily non-histone proteins can interact with local DNA in a chromatin environment or how “open” chromatin structure is for DNA-dependent processes such as replication or transcription.
Strategic Planning
It is important to select the protocol that will produce the information most relevant to your specific project. For example, nucleosome mapping by MNase-Seq (basic protocol 1) provides excellent high resolution information about nucleosome positions. However, MNase-Seq cannot provide accurate nucleosome occupancy information because the amount of nucleosomal DNA recovery is significantly affected by the accessibility of individual nucleosomes to nuclease (ie highly accessible nucleosomes will be preferentially recovered in a limited MNase digestion and preferentially lost in an extensive MNase digestion) (Weiner et al 2010). Occupancy information can readily be obtained by histone ChIP-Seq (basic protocol 2), but nucleosome position information is significantly limited compared to MNase-Seq. NCAM (basic protocol 3), a composite of nucleosome positions and density, can maximize information about individual nucleosomes, and should be employed if specific information regarding nucleosome accessibility is desired.
Basic Protocol 1: Determining Nucleosome Positions Using Micrococcal Nuclease Digestion and High Throughput Sequencing (MNase-Seq)
In this protocol, S. cerevisiae formaldehyde-crosslinked mononucleosomes are generated by membrane permeabilization with zymolyase followed by micrococcal nuclease (MNase) digestion of intact chromatin. Protected DNA is then isolated and prepared for high-throughput sequencing. The described library generation protocol is based on the TruSeq Sample Kit for Illumina-based sequencing, but the prepared mononucleosomal DNA is compatible with manufacturer protocols for other sequencing platforms. The ultimate MNase-Seq libraries will allow for high-resolution identification of global nucleosome positions. The entire protocol can be completed in a single week – two days are required for isolation of mononucleosomal DNA and 2–3 days are required for sequencing library prep. While MNase-Seq permits precise determination of nucleosome positions, it is important to remember that the extent of MNase digestion can change the measured signal strength of nucleosomes at different genomic regions. Limited digestions enrich for the most accessible nucleosomes while strong digestions may prevent recovery of the most labile nucleosomes. For this reason, histone occupancy and nucleosome accessibility are best evaluated using histone ChIP and NCAM (see protocols 2 and 3). When comparing nucleosome positions in different strains or conditions it is critical to match the digestion levels for each sample analyzed (Rizzo et al, 2012).
Materials
Overnight saturated culture of S. cerevisiae strain
37% Formaldehyde
2.5 M Glycine
Spheroblast solution (see recipe)
β-mercaptoethanol (14.3 M)
100T zymolyase (AMSBIO)
Micrococcal Nuclease (Worthington): Stored −80°C, 20U/μL in 10 mM Tris pH 7.4
Exonuclease III (NEB)
MNase Digestion Buffer (See Recipe)
MNase Stop Buffer (See Recipe)
Proteinase K (20 mg/ml)
37°C incubator/water bath
65°C incubator
Phenol:Chloroform:Isoamyl Alcohol (25:24:1)
Ethanol (100%)
3M Sodium Acetate pH 5.2
Glycogen (20 mg/ml)
NEB Buffer 2
RNase A (DNase-Free, 10 mg/ml)
PCR Clean-up Kit (Qiagen)
Alkaline Phosphatase (NEB)
NEB Buffer 3
Low-melt Agarose (GeneMate)
Gel Extraction Kit (Qiagen)
TruSeq Sample Prep Kit (Illumina) [or other desired library preparation kit]
MinElute PCR Purification Kit (Qiagen)
Thermal Cycler for PCR Amplification
Grow and fix cells
-
1
Dilute overnight culture to OD600 = 0.1 in 250 ml fresh YPD media. Grow cells at 30°C in shaking incubator until OD600 = 0.4–0.6.
Cells can be grown to higher optical densities, but stationary yeast cells will be require more extensive zymolyase treatment later in the protocol. -
2
Add formaldehyde to a final concentration of 1% and continue incubation at 30°C for 20 min.
Formaldehyde crosslinking helps prevent reorganization of nucleosome positions throughout the remainder of the protocol. -
3
Add glycine to a final concentration of 125 mM.
-
4
Pellet cells by centrifugation at ~4000 × g for 10 min.
-
5
Wash cells twice with 50 ml H2O.
Permeabilize Cell Walls (make spheroblasts)
-
6
Resuspend cells in 20 ml spheroblast solution in a culture flask
-
7
Add 500 μl zymolyase (10 mg/ml in spheroblast solution)
-
8
Incubate cells at 30°C for ~10–15 min until all cells appear to be spheroblasts
Spheroblasts are yeast with permeabilized cell walls. They can be identified microscopically as nonrefractive shadow-like spheres. Alternatively, they will readily lyse in water. The duration of this step may depend on strain or stage of cell cycle. -
9
Centrifuge spheroblasts at ~4000 × g for 20 min at 4°C (or 7000 × g for 10 min)
-
10
Carefully aspirate supernatant taking care not to disrupt fragile spheroblast pellet
Try to remove the majority of supernatant. This may require aspirating a small fraction of spheroblasts.
Digest Chromatin
-
11
Resuspend spheroblasts in 2 ml of MNase Digestion Buffer
Cells may be clumpy. Gently pipet cells to homogenize without vortexing. -
12
Add 30 units of Exonuclease III and 10, 20, or 40 units of Micrococcal Nuclease to three empty 1.5 ml tubes.
Exonuclease III is used to help eliminate the A/T cleavage bias of MNase and has been shown to reduce nucleosome footprints to the crystallographic footprint (Nikitina et al 2013). If scaling the protocol for a higher starting cell number, chromatin may be digested with higher MNase concentration or for a longer time and will likely require optimization. -
13
Add 600 μl of resuspended spheroblasts to each tube. Immediately mix by inverting tubes and incubate at 37°C for 10 min. Any remaining spheroblasts can be added to a no-digestion control tube.
-
14
During digestion, prepare MNase Stop Buffer
-
15
After 10 min incubation, add 150 μl of MNase Stop Buffer to each digestion and invert tubes.
-
16
Add 10 μl of Proteinase K and incubate overnight at 65°C to digest proteins and reverse crosslinks.
Isolate, Analyze, and Phosphatase-Treat Nucleosomal DNA
-
17
Add 600 μl phenol/chloroform/isoamyl alcohol to digested DNA and vortex for 10 seconds
-
18
Centrifuge sample in microcentrifuge at full speed (~16,000 × g) for 5 minutes to separate layers
-
19
Pipet aqueous layer into new eppendorf tube and add 0.1 volumes of 3M NaOAc pH 5.2, 0.01 volumes of glycogen (20 mg/ml), and 3 volumes of 100% ice cold ethanol.
You may need to split sample into two separate microcentrifuge tubes (300 μl sample per tube) to accommodate precipitation mixture. -
20
Precipitate sample at −80°C for >1 hour.
-
21
Centrifuge sample in microcentrifuge at full speed (~16,000 × g)
-
22
Remove supernatant and wash pellet with 500 μl of 70% EtOH.
-
23
Fully remove supernatant and let dry for ~10 minutes
-
24
Resuspend each sample in 60 μl of 1X NEB Buffer 2
-
25
Add 1 μl RNase A (10 mg/ml) and incubate for 1 hour at 37°C
-
26
Run small amount (1–5 μl) of each sample on a 2% agarose gel to identify properly digested sample(s); continue to digest remainder of sample with RNase A while running gel
Use Orange G as a loading dye to avoid co-migration with nucleosomal DNA band -
27
Purify DNA using PCR clean-up kit on properly-digested sample(s) and elute in 50 μl of 1X NEB Buffer 3.
Properly-digested sample should be approximately 80% mononucleosomes (~150 bp) with ~20% dinucleosomes and trace trinucleosomes visible. -
28
Add 10 units (1 μl) of calf intestine phosphatase to each sample and incubate at 37°C for 1 hour.
Phosphatase treatment eliminates 3′ phosphate groups that are remnants of MNase digestion. -
29
Purify DNA using PCR clean-up kit on phosphatase-treated samples and elute in 12 μl of buffer EB or 10 mM Tris pH 7.5.
-
30
Run entire sample (use Orange G loading dye) on 1.8% low-melt agarose gel.
-
31
Cut out entire mononucleosomal band and extract DNA using Gel Extraction Kit. Elute in 30 μl buffer EB.
Avoid prolonged exposure to UV irradiation during band excision and minimize excess agarose.
Construct Sequencing Libraries
Library construction generally involves preparing nucleosomal DNA for adapter ligation and amplification of ligated products. There are several commercial kits available for preparing libraries for different sequencing platforms. The described method uses the TruSeq kit from Illumina. Final DNA libraries will include genomic sequences that were previously protected by nucleosomes allowing for precise identification of nucleosome locations by DNA sequencing.
-
32
Use 150–200 ng of purified, phosphatase treated mononucleosomal DNA in 50 μl of buffer EB for library construction.
Note: other library preparation kits may be optimized for different amounts of starting material. -
33
Add 10 μl of resuspension buffer and 40 μl of End Repair Mix, mix by pipetting, and incubate at 30°C for 30 minutes.
End repair ensures blunt-ended DNA fragments with 5′ phosphates and 3′ hydroxyl groups for subsequent ligation steps. -
34
Purify DNA using PCR clean-up kit using a MinElute column and elute sample in 17 μl of buffer EB.
We find that the MinElute columns (Qiagen) are useful for maximizing yield of small quantities of DNA and RNA in our library preparations and allow for consistent library amplifications. -
35
Add 2.5 μl of resuspension buffer and 12.5 μl of A-tail mixture and incubate samples at 37°C for 30 minutes.
-
36
Add 2.5 μl of resuspension buffer, 2.5 μl of ligation mixture, and 2.5 μl of appropriate adapter index to samples, mix, and incubate at 30°C for 10 minutes.
-
37
Add 5 μl of stop ligation buffer and Purify DNA using PCR clean-up kit with MinElute columns (elute in 20 μl of buffer EB).
-
38
Combine 10 μl of sample, 2.5 μl of PCR primer cocktail, and 12.5 μl of PCR Master Mix.
Note: this is half of the suggested reaction volume than the manufacturer describes, but gives equivalent results using half of the material. The remaining 10 μl can be frozen at −20°C as a backup sample. -
39
Amplify DNA fragments using the following amplification scheme:
98°C for 30 sec
98°C for 10 sec
60°C for 30 sec
-
72°C for 5 min
Repeat steps b-d for a total of 16 cycles.
-
40
Purify DNA using PCR clean-up kit with MinElute columns and elute in 12 μl of buffer EB.
-
41
Load entire sample on 1.8% low melt agarose gel and excise the ligated mononucleosome band.
For the TruSeq kit, the mononucleosome band will be approximately 300 bp. If primer dimers are consistently a problem, an extra gel purification step can be performed to select for the 300 bp size range (product will not be visible) prior to PCR amplification. -
42
Purify DNA using Gel Extraction kit on excised gel slice and elute in appropriate volume/buffer for your desired high-throughput sequencing facility.
Basic protocol 2: chromatin immunoprecipitation of histone H3 from yeast cells
Here we obtain chromatin preparations from the budding yeast Saccharomyces cerevisiae suitable for the generation of high-resolution histone-DNA interaction maps using genomic approaches such as microarray hybridization and deep sequencing.
Materials
Yeast cells
YPD (see recipe)
Fix solution, 10× (see recipe)
2.5M Glycine
TBS solution (see recipe)
ChIP breaking buffer (see recipe)
FA buffer (see recipe)
Protease inhibitor cocktail, 100× (see recipe)
Protein G coupled Dynabeads (Invitrogen)
0.1 M sodium phosphate buffer pH=7 (see recipe)
Anti C terminal histone H3 antibody (Active Motif)
PBST buffer (see recipe)
FA-HS buffer (see recipe)
RIPA buffer (see recipe)
Stop buffer (see recipe)
10 mg/mL Proteinase K
10 mg/mL RNAse A
TBE, 10× (see recipe)
30°C incubator
Shaker
Spectrophotometer
50 ml polypropylene conical tube
Refrigerated swing-bucket centrifuge
0.5 mm glass beads acid washed (Sigma)
2 mL screw-cap flat bottom microcentrifuge tubes (Sarstedt catalog number 72.693.005)
Mini beadbeater 96 (Biospec)
20G needles
Lid-lock clips
15 mL polypropylene conical tubes
15 mL polystyrene conical tubes
Bioruptor sonication bath with cool bath and pump (Diagenode)
1.5 mL microcentrifuge tubes
1.5 mL siliconized microcentrifuge tubes
Refrigerated benchtop centrifuge
Magnetic particle concentrator
Anti-H3 antibody (Active Motif catalog number 39163)
Thermomixer (Eppendorf)
Microcentrifuge tube rotator
65°C incubator
MinElute PCR purification columns (Qiagen)
Crosslink histone-DNA interactions in yeast cells
-
1
Grow yeast cells in 70 ml of YPD to mid-log phase (OD660 ~0.7, which is ~1 × 107 cells/ml) shaking at 30°C.
-
2
Add 7 ml of freshly prepared 10× fix solution.
-
3
Incubate cells for 20 min gently shaking at room temperature.
-
4
Add 3.85 ml of 2.5 M glycine to quench crosslinking.
-
5
Incubate cells for 10 min, gently shaking at room temperature.
-
6
Evenly split cell volume and transfer to two 50 ml tubes.
-
7
Centrifuge cells 5 min at 4500 × g, 4°C. Decant medium from the cell pellet.
-
8
Wash cells twice by resuspending pellet in 10 ml of ice-cold TBS, centrifuge 5 min at 4500 × g, 4°C.
No need to use protease inhibitors at this step -
9
Freeze cell pellets and store at −80°C until use.
If chromatin is to be isolated immediately skip this step and proceed to step 11.
Chromatin isolation and fragmentation
-
10
Thaw the formaldehyde-crosslinked cell pellets on ice.
Keep samples on ice at all times for all the subsequent steps. -
11
Resuspend cell pellet in 300 μL breaking buffer.
Breaking buffer must contain freshly added protease inhibitors. -
12
Disrupt cells with 300 μl of glass beads in a 2 ml flat-bottom screw-cap tube in a mini beadbeater for 5 min.
Depending on the strain and specific experiment (i.e. asynchronous versus cell cycle synchronized cells) bead beating time will have to be adjusted to reach around 90% cell disruption. Cell disruption can be monitored under the microscope. -
13
Puncture one hole at the bottom first, then another one on the lid of the screw-cap tube with a 20G needle. Then partially unscrew the lid and place a lid-lock clip on the tube lip below the thread. Tightly screw back the lid and place the screw-cap tube with the clip inside an open 15 ml tube.
The lid-lock clip will hold the 2ml tube on top of the 15 ml tube. -
14
Drain supernatant by centrifuging the 2ml tube within the 15 ml tube for 2 min at 300 × g, 4°C.
The aqueous phase containing chromatin will be collected in the 15 ml tube. The beads will remain in the 2 ml screw-cap tube. -
15
Add 1 ml of ice-cold FA buffer and transfer to 1.5 ml microcentrifuge tube.
Add protease inhibitors to the FA buffer right before use. -
16
Centrifuge samples on a tabletop centrifuge for 1 min at 16000 × g, 4°C.
This step will partially purify chromatin by fractionation. Chromatin, which is insoluble at this step, will go to the pellet fraction, while the rest of soluble components will remain the supernatant. -
17
Remove supernatant with a pipette.
-
18
Add 1 ml of ice-cold FA buffer with protease inhibitors.
-
19
Thoroughly resuspend the insoluble pellet by gently pipetting up and down multiple times.
The chromatin-containing insoluble fraction is very sticky at this point.Make sure no sample is left behind stuck in the inner or outer walls of the pipette tip. -
20
Repeat steps 16 through 19 twice more.
A clearer supernatant will be obtained as soluble components are removed from the sample after each purification step. -
21
Transfer the sample into a 15 ml polystyrene tubes.
The harder plastic walls of polystyrene tubes better transmit the sonication waves within the sonication bath, thus ensuring a more efficient sonication. -
22
Sonicate samples in a Bioruptor sonicator bath. Sonication should be carried out using 30 seconds on, 30 seconds off cycles, for a total of 30 min, at maximum output setting. To keep the bath cold, the use of a circulating cold water bath is strongly advised. Alternatively, ice can be added directly to the sonication bath, although this may reduce the sonication power.
The above conditions have been optimized for the simultaneous sonication of three samples to achieve a desired fragment size ranging from 200 to 600 base pairs, with a maxima around 300 base pairs. Sonication conditions, especially the total amount of sonication time, will have to be tested for different strains and growth conditions. A cold water bath is required for maximal sonication efficiency, as well as to preserve sample integrity. The use of a circulating cold water bath is preferred, as it ensures constant temperature control and thus much better reproducibility. -
23
Transfer samples to 1.5 ml siliconized microcentrifuge tube.
-
24
Centrifuge samples for 30 min at 16000 × g, 4°C.
Solubilized chromatin after sonication will stay in the supernatant. -
25
Transfer supernatant to a new 1.5 ml siliconized tube.
-
26
Repeat steps 24 and 25 once more.
-
27
Snap freeze soluble chromatin preparations by immersing samples into liquid nitrogen. Store at −80°C until use.
Prepare anti-H3 antibody-coupled magnetic beads
-
28
Transfer 30 μl of Protein G-coupled magnetic bead suspension to a new 1.5 ml siliconized tube.
Numbers shown here correspond to the volumes required per sample. To prepare beads for a larger amount of samples simply multiply the volumes by the number of samples. -
29
Concentrate beads on a magnetic particle concentrator and remove the supernatant with a pipette.
-
30
Wash the beads twice by resuspending them in 0.5 ml of 0.1M Sodium Phosphate pH 7.0.
pH 7.0 is optimal for protein G-antibody binding. Use pH 8.0 if protein A beads are to be used. -
31
Resuspend beads in 30 μL 0.1M Sodium Phosphate pH 7.0.
-
32
Add 5 μl of anti H3 antibody and mix well by gently pipetting up and down.
-
33
Incubate the antibody-beads mix vigorously shaking on a Thermomixer set at 1400 rpm at room temperature for 30 min.
-
34
Resuspend and concentrate beads twice in 0.5 ml 0.1M Sodium Phosphate pH 7.0, 0.01% Tween20.
The presence of low concentration of Tween20, a detergent, will remove non-specific interactions between the antibody and the beads and/or protein G. -
35
Wash the beads in 1 mL PBST.
-
36
Concentrate beads and remove supernatant with a pipette.
-
37
Repeat steps 35 and 36 for a total of three PBST washes.
-
38
Suspend the antibody-coupled beads in 30 μL PBST.
The beads are ready to be used in a ChIP reaction.
Chromatin immunoprecipitation of histone H3
Before using chromatin for the first time, the size range of the sonicated chromatin should be determined. For best resolution in the subsequent genomic analysis, size range should be between 200 and 600 base pairs.
-
39
Thaw chromatin samples on ice.
-
40
Transfer 150 μl of chromatin sample to a new 1.5 ml siliconized tube.
The total amount of sample to be used in the ChIP reaction step will depend on the abundance of the epitope and the efficiency of the antibody. -
41
Set up ChIP reaction by adding 30 μl of antibody-coupled beads to 150 μl of chromatin.
-
42
Incubate the ChIP reaction for 90 min gently rotating at room temperature.
Rotation will ensure magnetic beads remain in suspension. -
43
Concentrate beads on the magnetic concentrator.
-
44
Wash beads in 1 ml of room temperature FA buffer by gently rotating for 5 min. Concentrate beads and remove supernatant. Repeat this step for a total of three FA buffer washes.
-
45
Wash beads in 1 ml of room temperature FA-HS buffer. Concentrate beads and remove supernatant. Repeat this step for a total of three FA-HS buffer washes.
-
46
Wash beads in 1 ml of room temperature RIPA buffer. Concentrate beads and remove supernatant.
Make sure all RIPA buffer is removed at this step. -
47
Elute beads by adding 50 μl of 2× stop buffer.
Elution buffer contains 1% SDS, which will disrupt the antibody-epitope interaction, thus solubilizing the ChIPed histone-DNA complexes. -
48
Incubate beads for 15 min at 65°C. Vortex samples every 5 min.
High temperature and vortexing will facilitate solubilization of the histone-DNA complexes. -
49
Concentrate beads on magnet.
-
50
Transfer supernatant to a new siliconized 1.5 ml tube.
The supernatant will contain the histone-DNA complexes. -
51
Repeat steps 47 to 49 once more.
-
52
Collect supernatant and merge with the previous eluate for a total of a 100 μl of eluted ChIP sample.
-
53
Reverse crosslinks overnight in a 65°C incubator.
-
54
Prepare input DNA sample by transferring 50 μl of chromatin sample to a new 1.5 ml siliconized tube.
This is the non-immunoprecipitated control sample. -
55
Add 50 μl of stop buffer to the input sample.
-
56
Reverse crosslinks overnight in a 65°C incubator.
-
57
Next day, add 2 μl RNAse A to ChIP and input samples.
-
58
Incubate for one hour at 42°C.
-
59
Add 2 μl Proteinase K to both ChIP and input samples.
-
60
Incubate for 3 to 5 hours at 55°C.
-
61
Purify ChIP and input samples with the MinElute PCR purification kit. Elute samples in 10 μl EB buffer.
We recommend using the MinElute columns as they allow elution in very low volumes. This will facilitate the downstream library preparation steps for array hybridization or deep sequencing. -
62
Samples are ready for library preparation for either array hybridization or deep sequencing.
Basic protocol 3: Normalized chromatin accessibility to MNase (NCAM)
Most DNA-dependent processes rely on the ability of regulatory factors to gain access to DNA in the context of chromatin. Thus, in order to understand how DNA-dependent processes are regulated it is essential to identify what genomic regions are in an accessible chromatin conformation and which ones are not at any given point during a cell cycle. Analysis of chromatin accessibility in vivo has been typically performed using DNA restriction endonucleases such as DNaseI and micrococcal nuclease (MNase). The underlying idea is that chromatin that is accessible to these endonucleases is likely accessible to regulatory factors that have to access DNA in order to perform their functions. However, we and others have shown that MNase signal is dependent on both nucleosome occupancy and accessibility, and thus cannot be directly used as a measure of either. To overcome this limitation we have recently developed a novel method, named NCAM, to analyze chromatin accessibility to MNase that is normalized for nucleosome density independently measured by histone H3-ChIP (Rodriguez and Tsukiyama, 2013). NCAM is generated by subtracting H3-ChIP signal from MNase signal and although it was originally devised using high-density microarray data, NCAM can also be generated using next generation sequencing data as long as log2 ratio data files are obtained.
-
Generate MNase nucleosome positioning (Basic Protocol 1) and histone H3 ChIP data (Basic protocol 2).
MNase and H3-ChIP data should be obtained from the same batch of cell culture. -
Obtain the average signal and standard deviation (SD) for each MNase and H3-ChIP dataset. This is typically achieved by averaging signals at transcription start sites (TSSs). Average and SD are calculated across a 2 Kb region centered at the TSS.
A comprehensive list of TSS in yeast has been generated by Nagalakshmi and colleagues (Nagalakshmi et al., 2008). - Normalize MNase and H3-ChIP datasets by generating Z scores of the log2 ratio files (Cheadle et al., 2003) using the average and SD from all TSSs. For each MNase or H3-ChIP data point X, from 1 to i :
- Generate NCAM for each data point X, from 1 to i :
Reagents and solutions
Spheroblast solution
1M Sorbitol
50 mM Tris-HCl pH 7.5
10 mM β-mercaptoethanol*
MNase Digestion Buffer
MNase Stop Buffer*
5% SDS
50 mM EDTA
Breaking buffer
100mM Tris pH 8.0
20% glycerol
Store up to 1 year at room temperature
FA buffer
50mM Hepes pH 7.6
150mM NaCl
1mM EDTA
1% Triton X100
0.1% sodium deoxycholate
FA-HS buffer
50mM Hepes pH 7.6
500mM NaCl
1mM EDTA
1% Triton X100
0.1% sodium deoxycholate
Store for up to one year at room temperature
Fix solution, 10×
11% Formaldehyde
100 mM NaCl
1 mM EDTA
50 mM Hepes pH 7.6
Make fresh prior to use
PBST buffer
137 mM NaCl
2.7 mM KCl
12 mM Na2HPO4
12 mM KH2PO4
0.05 % Tween 20
Store up to 1 year at room temperature
Protease inhibitor cocktail, 100×
100 mM PMSF
200 μM Pepstatin
60 μM Leupeptin
200 mM Benzamidine
200 μg/ml Chymostatin A
Prepare in Methanol
Store up to 1 year at −20°C
RIPA buffer
10mM Tris HCl pH 8.0
250 mM LiCl
1mM EDTA
0.5% NP40
0.5% Sodium deoxycholate
Store up to 1 year at room temperature
Sodium phosphate buffer, 0.1M pH = 7
To obtain 10 ml mix:
1.95 ml 0.2 M sodium phosphate buffer
3.05 ml 0.2 M sodium phosphate buffer
5 ml distilled water
Stop Buffer, 2×
20mM Tris HCl pH 8.0
100mM NaCl
20mM EDTA
1% SDS
Store up to 1 year at room temperature
TBE, 10×
890 mM Tris base
890 mM boric acid
4 mM EDTA
Store up to 1 year at room temperature
TBS
20mM Tris pH 7.6
150mM NaCl
1mM PMSF freshly added before each use
Store up to 6 months at 4°C
YPD
10% yeast extract
20% peptone
20% dextrose
Store up to several months at room temperature
Commentary
Background Information
In eukaryotic organisms, all DNA-dependent processes are carried out in the presence of chromatin. It is therefore critical to consider the effects of nucleosomes in the regulation of DNA replication, transcription, and DNA repair. Since each nucleosome is known to occlude ~147 bp of DNA, there must be significant regulation of nucleosome positions and density to alter the exposure of underlying DNA elements to factors that function through binding to genomic DNA. Many factors contribute to the genomic organization of chromatin, so having a means to characterize nucleosome positions and occupancy on a global scale is highly beneficial.
High resolution mapping of nucleosome positions in yeast was first accomplished using densely-tiled microarrays (Yuan et al, 2005) (Lee et al, 2007), and was increased to single-nucleotide resolution when coupled to Illumnia-based high-throughput sequencing (Shivaswamy et al, 2008). Today, the high resolution data sets are acquired by paired-end high-throughput sequencing to precisely identify nucleosome midpoints with single bp resolution. Recent experiments have shown that the height of nucleosome signal from MNase-Seq data sets depends on the extent of MNase digestion and nucleosome occupancy, and therefore is not suitable to measure nucleosome occupancy (Weiner et al, 2010). Since DNA recovery from histone ChIP does not depend on a nuclease-sensitive digestion step for fragmentation, high resolution histone ChIP provides a better indication of true nucleosome occupancy. NCAM was developed to globally measure accessibility of nucleosomes to MNase in a way normalized to histone density by subtracting nucleosome signals from MNase-Seq experiments with density from histone ChIP experiments, essentially calculating the number of MNase digestion per amount of nucleosome (Rodriguez and Tsukiyama, 2013). Together, these are currently the most straightforward and accurate methods for determining nucleosome positions, occupancy, and accessibility in S. cerevisiae.
Critical Parameters and Troubleshooting
Zymolyase Treatment
Zymolyase treatment can affect DNA yield from MNase-digested chromatin. Incomplete zymolyase treatment will result in a high molecular weight genomic DNA band that will be visible on an agarose gel (Protocol 1, step 26). This will not affect ultimate nucleosome positioning data, so if enough properly-digested mononucleosome can be recovered, incomplete zymolyase is not a significant problem. However, if zymolyase treatment is not optimum, the extent of MNase digestion in subsequent steps will be variable, since the total number of MNase-permeable cells will not reflect the number of harvested cells. Over-digestion with zymolyase can result in low chromatin yield and should be avoided. The amount of zymolyase used and duration of treatment should be optimized for different mutant strains and specific experimental conditions.
Formaldehyde chrosslinking
Histones strongly interact with DNA in multiple positions within the nucleosome, which makes the histone-DNA interaction very stable. However, we strongly recommend crosslinking chromatin to avoid any potential histone loss during the extensive sonication step. The crosslinking conditions described here are suitable for performing H3 ChIP in wild type cells as well as in certain mutant strains. However, certain mutants may require the incorporation of changes in either the length of the crosslinking reaction, formaldehyde concentration or both to achieve optimal H3 ChIP.
Chromatin sonication
In order to precisely determine the locations of protein-DNA interactions, it is essential to achieve maximal resolution. Even though both microarray and deep sequencing platforms provide base pair resolution, the resolution achieved in genome-wide ChIP analysis is limited by the size and range of the sonicated fragments, where chromatin that is homogeneously sonicated to small fragments will result in better resolution. Therefore, controlling sonication size and range is crucial to precisely map the regions of histone-DNA interactions. In our experience, a sonication size around 300 base pairs, ranging from 100 to 500 base pairs, is sufficient to generate high-resolution H3 density maps in yeast. The sonication conditions described here are suitable to generate chromatin fragments around 300 base pairs from wild type yeast cells grown under normal conditions in YPD at 30°C. However, the number and duration of the sonication cycles will have to be determined for each strain and growth conditions. We recommend performing a time course sonication, where samples are drawn at fixed intervals, to determine how many cycles are necessary for each strain background to generate the desired fragment size. We strongly recommend routinely monitoring the size of the DNA fragments generated by sonication by running the purified DNA on an agarose gel.
ChIP
The antibody to beads ratio used in this protocol has been optimized for H3 antibodies. If using a different antibody, the optimal ratio should be determined.
NCAM
It is critical that the level of MNase digestion is matched between samples that have to be directly compared. This will be further facilitated by performing Z score normalization of the data. MNase digests for NCAM should not be either too mild or too extensive. We typically isolate mononucleosomes from chromatin that has been digested down to 60–80% mononucleosomes. If digestion is too mild, the isolated mononucleosomes will be the most accessible, typically those nucleosomes around nucleosome depleted regions (NDRs). If, on the contrary, mononucleosomes are isolated from heavily MNase-digested samples, the isolated mononucleosomes will be depleted for the most accessible nucleosomes, again those around NDRs. Thus, to avoid biases in MNase signal around NDRs it is best to isolate mononucleosomes from chromatin samples moderately digested.
Anticipated Results
Basic Protocol 1
The MNase-Seq protocol will provide more than enough mononucleosomal DNA libraries for the major high throughput sequencing platforms. If paired-end sequencing is employed, the precise location of nucleosome positions can be determined with base-pair resolution. By comparing nucleosome positioning maps from different genetic backgrounds or different experimental conditions, one can gain insights into the reorganization of chromatin by cellular factors or in response to environmental cues or different cell states.
Basic protocol 2
Histone proteins, specially histone H3, are found at virtually all nucleosomes in yeast and therefore small amounts of chromatin will suffice to retrieve enough DNA for subsequent array hybridization or deep sequencing. We estimate that, using the antibody-to-bead ratio described here, around 10 ng of DNA are recovered from a single ChIP reaction using 150 μl of starting chromatin. We strongly recommend routine monitoring of ChIP efficiency by western blot where the levels of histone H3 can be compared between the input, unbound and immunoprecipitated fractions. Ideally, the unbound fraction should contain lower H3 than the input fraction. The collection of immunoprecipitated DNA fragments should cover the entire yeast genome with maximal histone H3 density expected to occur at ORFs. Even though the ChIP method described here has been optimized for histone H3, it can also be performed to ascertain the binding locations of other DNA-binding proteins genome-wide. Critically, the amount of chromatin used for each immunoprecipitation will have to be adjusted for each antibody: proteins that bind fewer locations will require the use of more chromatin than proteins that bind more ubiquitously.
NCAM
Maximal NCAM signal should be expected at and around NDRs, with minimas corresponding to internal ORF regions.
Time Considerations
MNase-Seq libraries (Basic Protocol 1) can be generated and ready for submission to a sequencing facility in approximately one week. Chromatin digestion and isolation typically requires two consecutive days while library construction can be accomplished in 2–3 days. Library generation is well-suited for multiplexing, so a variety of samples can be processed in parallel without significantly affecting the timing of individual steps. Sequencing facilities have widely variable turnaround time, so it may take a couple months to receive sequencing results from prepared libraries.
Basic protocol 2 is typically performed over the course of two to three days. Chromatin crosslinking can be performed in less than one hour and is usually done immediately before chromatin isolation and sonication, which usually take between 1 to 2 hours. Allow about one hour for coupling the antibody to the protein G magnetic beads and 4 to 5 hours for ChIP. It is therefore feasible to perform all the steps from chromatin crosslinking to ChIP in one day. However, at least one overnight incubation step of the immunoprecipitated chromatin at 65°C is required to reverse crosslinks. On the next day, RNAse A and proteinase K incubations and sample purification require 5 to 6 hours.
Figure 1.

Time course of spheroblast production. No zymolyase (left), incomplete lysis (middle), and complete lysis by zymolyase as described in protocol. Complete lysis is determined by absence of highly refractive cells and appearance of dark, hollow spheres.
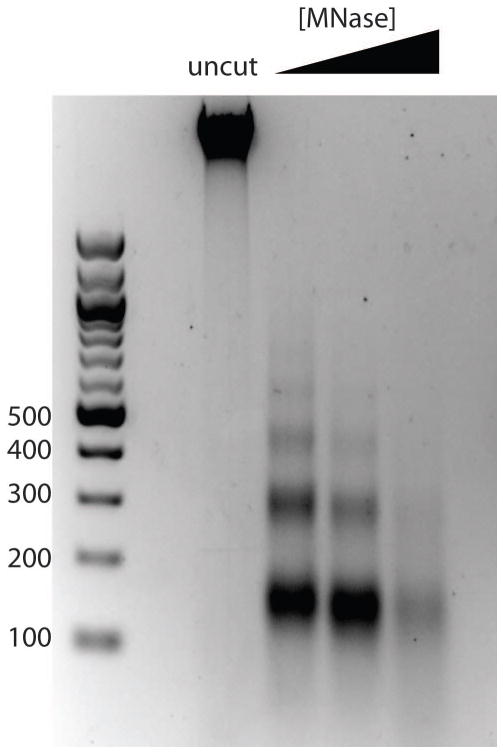
Figure 2.
Typical MNase digestion. Cells were treated with 0, 10, 20, or 40 units of MNase and 30 units of ExoIII for 10 minutes in digestion buffer as described in the protocol. Here, 20 units of MNase provided an ideal MNase ladder with roughly 80% mononucleosome band.
Acknowledgments
The authors acknowledge funding support from the National Institutes of Health (R01GM058465, R01GM078259, and LLS / 5078-14).
Footnotes
Add fresh before spheroblast protocol
Add fresh before digestion
Prepare during MNase digestion
Literature Cited
- Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. J Mol Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Tillo D, Bray N, Morse RH, Davis RW, Hughes TR, Nislow C. A high-resolution atlas of nucleosome occupancy in yeast. Nat Genet. 2007;39:1235–1244. doi: 10.1038/ng2117. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi u, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina T, Wang D, Gomberg M, Grigoryev SA, Zhurkin VB. Combined micrococcal nuclease and exonuclease III digestion reveals precise positions of the nucleosome core/linker junctions: implications for high-resolution nucleosome mapping. J Mol Biol. 2013;425:1946–1960. doi: 10.1016/j.jmb.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo JM, Bard JE, Buck MJ. Standardized collection of MNase-seq experiments enables unbiased dataset comparisons. BMC Mol Biol. 2012;13:15. doi: 10.1186/1471-2199-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Tsukiyama T. ATR-like kinase Mec1 facilitates both chromatin accessibility at DNA replication forks and replication fork progression during replication stress. Genes Dev. 2013;27:74–86. doi: 10.1101/gad.202978.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaswamy S, Bhinge A, Zhao Y, Jones S, Hirst M, Iyer VR. Dynamic remodeling of individual nucleosomes across a eukaryotic genome in response to transcriptional perturbation. PLOS Biology. 2008;6:e65. doi: 10.1371/journal.pbio.0060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Hughes A, Yassour M, Rando OJ, Friedman N. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Research. 2010;20:90–100. doi: 10.1101/gr.098509.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan GC, Liu YJ, Dion MF, Slack MD, Wu LF, Altschuler SJ, Rando OJ. Genome-scale identification of nucleosome positions in S. cerevisiae. Science. 2005;309:626–630. doi: 10.1126/science.1112178. [DOI] [PubMed] [Google Scholar]