Abstract
Nectins are Ca2+-independent immunoglobulin-like cell adhesion molecules that compose a family of four members that regulate several cellular activities such as movement, proliferation, survival, differentiation, polarization, and the entry of viruses. Nectin-4 has recently emerged as a metastatis-associated protein in several cancers. Here, we have evaluated the association between the expression of Nectin-4 and the clinical outcome of patients with node-negative, T1/T2 breast cancers.The study group consisted of 197 patients presenting with primary unilateral breast carcinoma (T1/T2), with no evidence of nodal involvement and distant metastases. Nectin-4 protein expression was assessed by immunohistochemistry on tissue microarrays, and the results correlated with the clinical data using Kaplan–Meier curves and multivariate Cox regression analysis. Thirty-four out of 197 tumors (17.3%) exhibited Nectin-4 expression on cell membrane (m-Nectin-4) and 122 out of the 163m-Nectin-4 negative tumors (74.8%) showed high cytoplasmic expression of Nectin-4 (c-Nectin-4High). At Kaplan–Meier analysis, m-Nectin-4 positivity was significantly associated with a lower disease-free survival (DFS) and distant relapse-free survival (DRFS) rate in patients with a luminal-A phenotype (P=0.030 and P=0.002, respectively). Multivariate analysis showed that in luminal-A tumors m-Nectin-4 positivity is an independent prognostic factor for DFS (P=0.018) and DRFS (P=0.004), but not for local relapse-free survival (LRFS). On the other hand, c-Nectin-4High was significantly associated with higher rates of DFS and LRFS, but not DRFS, in the whole population (P=0.008 and P=0.004, respectively) and in patients with luminal-A tumors (P=0.022 and P=0.018, respectively). In patients with luminal-A tumors, multivariate analysis showed that the prognostic value of c-Nectin-4Low/Negative is limited to DFS (P=0.012) and LRFS (P=0.022). We suggest that Nectin-4 represents a prognostic factor and a therapeutic target in luminal-A early stage breast cancer.
Introduction
Breast cancer is the most frequent malignancy in women, accounting for more than 200 000 new cases per year in the US and 350 000 in Europe.1, 2, 3, 4 Decline in mortality from breast cancer has been recently documented most likely as the result of the combined increase of public awareness, the implementation of screening programs and advances in the adjuvant treatments.1,3 Screening programs have indeed favored the detection of a greater number of early stage, node-negative tumors associated with a low risk of recurrence. Nevertheless, only about 70–80% of these patients are cured by local or regional treatment alone or combined with adjuvant systemic therapy, which significantly reduces the risk of recurrence.5 On the basis of the risk-group discrimination provided by established prognostic factors, such as tumor size and grade, hormone receptor status, age or menopausal status, 85–90% of patients without nodal involvement receive currently some kind of adjuvant treatment, although only about one-third will recur.6,7 A prerequisite for individualized therapy is the identification of node-negative patients at low or high risk of relapse or death. Although intuitive, sound selection criteria that could avoid unnecessary morbidity to low risk patients while providing benefits to the others are still far from being widely available. In this regard, a new generation of randomized clinical trials are exploring the possibility to tailor treatment on the basis of genetic portraits of tumors. This will steadily revolutionize the management of these malignancies which despite decreasing mortality, display increasing incidence.3
The Nectin family of the Ca++-independent immunoglobulin-like molecules consists of four members (Nectin-1, Nectin-2, Nectin-3 and Nectin-4), which play a role in cell–cell adhesion by homophilic and heterophilic interactions.8 Nectins are connected to the actin cytoskeleton through afadin, an F-actin-binding protein,9 and by a complex interplay with other cell adhesion molecules and signal transduction molecules, regulate several cellular activities ranging from movement to polarization, differentiation and entry of viruses.10 While Nectins 1, 2 and 3 are widely expressed in adult tissues, Nectin-4 is confined to the embryo and placenta11,12 and released in a soluble form into circulation. Recently, Nectin-4 overexpression has been documented in breast, lung and ovarian carcinomas,13, 14, 15, 16, 17 but the biological significance of this finding in human cancer progression as well as its potential role as a diagnostic and therapeutic biomarker in early breast cancer have not been established.
In the present paper, we examined by immunohistochemistry (IHC) the prognostic significance of the expression levels of Nectin-4 in the tumor tissue of 197 patients with completely resected (T1-T2, N0) tumors.
Results
Expression of Nectin-4 in non-neoplastic breast tissue
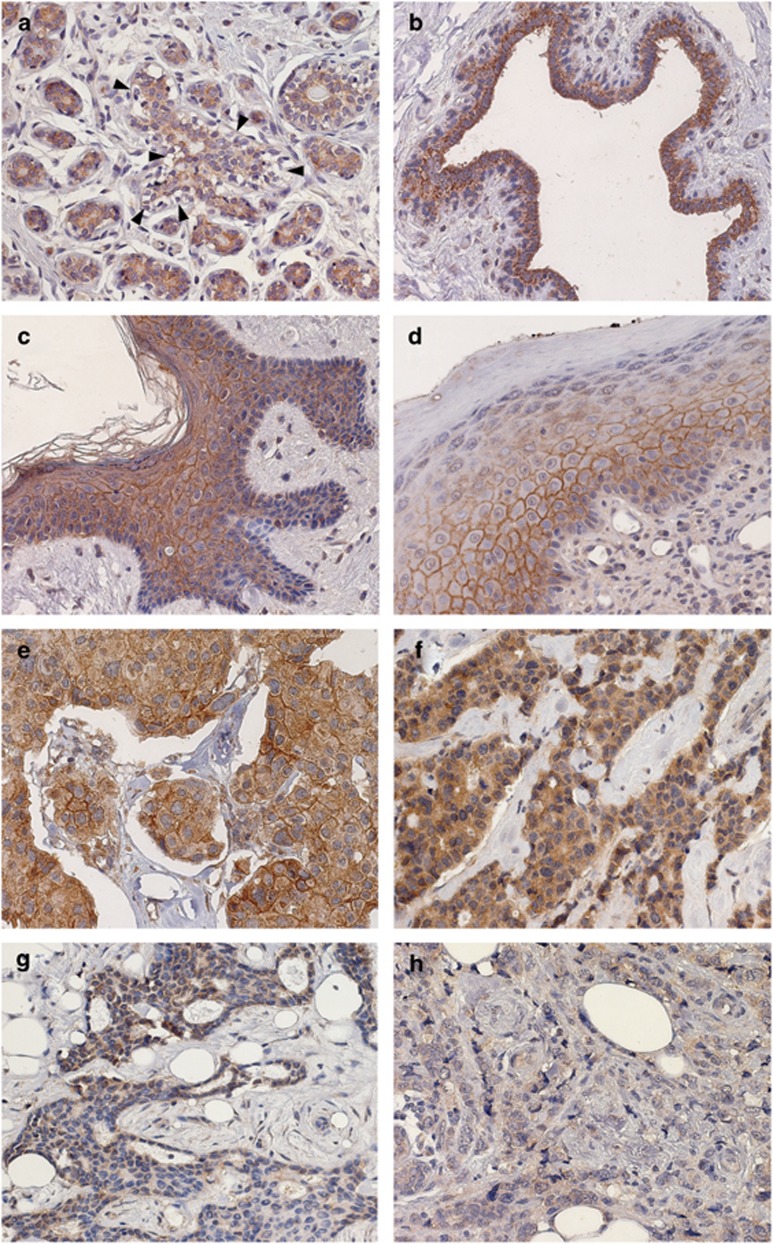
Whole sections of non-neoplastic breast tissues from 30 patients stained with the AF2659 antibody, revealed in all cases a specific Nectin-4 immunoreactivity, confined to the cytoplasm of luminal cells of terminal duct lobular units and of galactophorous ducts (Figures 1a and b). Myoepithelia were consistently negative. According to previous findings,18 immunohistochemical analysis on non-neoplastic skin, including the breast nipple, detected Nectin-4 both in the cytoplasm and on the cell membrane, at cell–cell junctions of keratinocytes. In particular, expression of Nectin-4 was observed in all the suprabasal layers of epidermis, from the spinous to the granular layer. With increasing keratinization, expression became lower (Figures 1c and d).
Figure 1.
Nectin-4 in non-neoplastic tissues. Specific immunoreactivity is confined to the cytoplasm of almost all luminal cells of terminal duct lobular units (a) and of galactophorous ducts (b). Myoepithelia are negative (A, arrow). In nipple (c) and in non-neoplastic skin (d), Nectin-4 is expressed both in the cytoplasm and on the cell membrane, at cell–cell junctions of keratinocytes. In particular. Nectin-4 can be observed in all the suprabasal layers from the spinous to granular layer. With increasing keratinization, the expression became lower. Nectin-4 in breast tumors. Membranous expression in (e). c-Nectin-4High tumor in (f) with cytoplasmic staining in almost all cancer cells. c-Nectin-4Low: tumor with <36% positively stained cells in (g), and tumor with barely detectable, if any, cytoplasmic Nectin-4 expression in (h). (Original magnification × 40).
Expression of Nectin-4 in breast tumor tissue
Thirty-four out of 197 (17.3%) cases expressed Nectin-4 on the cell membrane (m-Nectin-4) of tumor cells. The proportion of m-Nectin-4-positive cells was in the range of 5–65%. All these cases were considered m-Nectin-4 positive. One hundred fifty-one out of 163 (92.6%) m-Nectin-4-negative tumors showed specific cytoplasmic expression of Nectin-4 (c-Nectin-4). The percentages of c-Nectin-4-positive tumor cells ranged from 5 to 100%, with a mean±s.e. of 68.0±2.3. To dichotomize c-Nectin-4 expression, a cutoff of 36% positive cells was chosen, corresponding to the 25th percentile. Then, tumors with ⩾36% positive cells (n=122) were considered c-Nectin-4High and those with <36% positive cells (n=29) were c-Nectin-4Low. Twelve cases did not display any specific cytoplasmic staining for Nectin-4 (c-Nectin-4Negative tumors). For statistical analysis, c-Nectin-4Low and c-Nectin-4Negative cases were grouped (c-Nectin-4Low/Negative tumors, n=41) and compared with the c-Nectin-4High counterpart. (n=122). Examples of m-Nectin-4 and c-Nectin-4 expression in breast tumors, are shown in Figures 1e–h. m-Nectin-4 expression inversely correlated with progesterone receptor (PR) expression (P=0.045). c-Nectin-4 expression directly correlated with that of estrogen receptor (ER) (P=0.038) (Table 1).
Table 1. Nectin-4 status according to clinicopathological features of patients.
| Variable |
m-Nectin-4 |
c-Nectin-4 |
||||||
|---|---|---|---|---|---|---|---|---|
| Negative:n (%) | Positive:n (%) | P-value | R | Low/Negative:n (%) | High:n (%) | P-value | R | |
| Tumor size | ||||||||
| ⩽2 cm | 109 (85.8) | 18 (14.2) | 0.167 | 0.110 | 25 (22.9) | 84 (77.1) | 0.443 | −0.073 |
| >2 cm | 54 (77.1) | 16 (22.9) | 16 (29.6) | 38 (70.4) | ||||
| Tumor grade | ||||||||
| 1 | 20 (90.9) | 2 (9.1) | 0.379 | 0.077 | 8 (40.0) | 12 (60.0) | 0.166 | 0.128 |
| 2–3 | 143 (81.7) | 32 (18.3) | 33 (23.1) | 110 (76.9) | ||||
| ER | ||||||||
| Negative | 42 (75.0) | 14 (25.0) | 0.093 | −0.129 | 16 (38.1) | 26 (61.9) | 0.038a | 0.176 |
| Positive | 121 (85.8) | 20 (14.2) | 25 (20.7) | 96 (79.3) | ||||
| PR | ||||||||
| Negative | 50 (74.6) | 17 (25.4) | 0.045a | −0.154 | 15 (29.4) | 36 (70.6) | 0.438 | 0.066 |
| Positive | 113 (86.9) | 17 (13.1) | 19 (21.6) | 69 (78.4) | ||||
| Ki-67 | ||||||||
| Low | 89 (86.4) | 14 (13.6) | 0.187 | 0.102 | 22 (24.7) | 67 (75.3) | 1.000 | −0.011 |
| High | 74 (78.7) | 20 (21.3) | 19 (25.7) | 55 (74.3) | ||||
| HER-2 | ||||||||
| Negative | 143 (84.6) | 26 (15.4) | 0.105 | 0.122 | 39 (27.3) | 104 (72.7) | 0.108 | 0.131 |
| Positive | 20 (71.4) | 8 (28.6) | 2 ( 8.3) | 18 (90.0) | ||||
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Statistically significant.
m-Nectin-4 expression and disease-free survival
A disease relapse was observed in 35.3% (12/34) of patients with m-Nectin-4-positive and in 23.9% (39/163) of those with m-Nectin-4 negative tumors. Local recurrence rates were 8.8% and 10.4% for patients with positive and negative m-Nectin-4 tumors, respectively. Distant metastases occurred in 26.5% and 13.5% of patients with positive and negative m-Nectin-4 tumors, respectively.
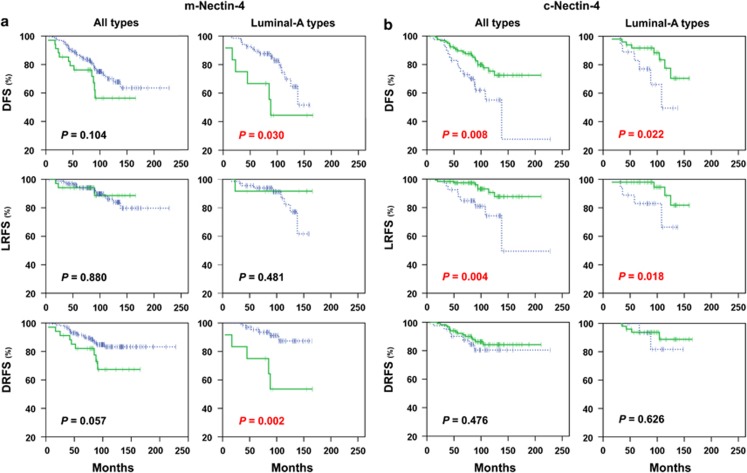
At Kaplan–Meier analysis, the expression of m-Nectin-4 was significantly associated with a lower disease-free survival (DFS) and distant relapse-free survival (DRFS) rate in patients with luminal-A phenotype (P=0.030 and P=0.002, respectively) but not in the whole population (Figure 2a) or in patients with other tumor subtypes.
Figure 2.
Kaplan–Meier estimates (DFS, LRFS and DRFS) in all patients and in patients with luminal-A tumors. (a) m-Nectin-4 plots: green solid lines and blue dashed lines indicate positive and negative m-Nectin-4 cases, respectively; (b) c-Nectin-4 plots: green solid lines and blue dashed lines indicate high and low/negative expression of c-Nectin-4 cases, respectively.
Multivariate analyses of DFS did not show an independent prognostic significance of the m-Nectin-4 expression in the whole population (Table 2), but only in luminal A tumors (hazards ratio (HR)=4.0: 95% confidence interval (CI), 1.5–10.8; P=0.007) (Table 3). In particular, high expression of m-Nectin-4 was an independent factor influencing DRFS (HR=6.0: 95% CI, 1.8–19.8; P=0.003), but not local relapse-free survival (LRFS; Table 3).
Table 2. Multivariate analysis of m-Nectin-4 and c-Nectin-4 expression in breast tumors.
| Variable | HR | 95% CI | P-value | Variable | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|
| Disease-free survival | Disease-free survival | ||||||
| Tumor size, cm (>2 vs⩽2) | 1.2 | 0.7–2.1 | 0.530 | Tumor size, cm (>2 vs ⩽2) | 1.3 | 0.7–2.5 | 0.476 |
| Tumor grade (2–3 vs 1) | 2.3 | 0.7–7.7 | 0.186 | Tumor grade (2–3 vs 1) | 1.6 | 0.5–5.5 | 0.461 |
| ER (negative vs positive) | 1.6 | 0.7–4.0 | 0.288 | ER (negative vs positive) | 1.1 | 0.4–2.8 | 0.911 |
| PR (negative vs positive) | 1.2 | 0.5–2.6 | 0.699 | PR (negative vs positive) | 1.6 | 0.7–3.9 | 0.273 |
| Ki-67 (high vs low) | 1.0 | 0.6–1.9 | 0.888 | Ki-67 (high vs low) | 1.3 | 0.7–2.6 | 0.409 |
| HER-2 (negative vs positive) | 1.2 | 0.5–2.8 | 0.622 | HER-2 (negative vs positive) | 1.3 | 0.4–3.7 | 0.658 |
| Hormonal therapy (no vs yes) | 1.3 | 0.6–3.0 | 0.530 | Hormonal therapy (no vs yes) | 1.5 | 0.5–4.1 | 0.443 |
| Chemotherapy (no vs yes) | 2.5 | 1.2–5.4 | 0.017a | Chemotherapy (no vs yes) | 1.6 | 0.7–3.7 | 0.233 |
| m-Nectin-4 (positive vs negative) | 1.8 | 0.9–3.6 | 0.107 | c-Nectin-4 (low/negative vs high) | 1.9 | 0.9–3.8 | 0.085 |
| Local relapse-free survival | Local relapse-free survival | ||||||
| Tumor size, cm (>2 vs⩽2) | 2.7 | 1.0–6.9 | 0.046a | Tumor size, cm (>2 vs⩽2) | 3.6 | 1.2–10.7 | 0.018a |
| Tumor grade (1 vs 2–3) | 1.7 | 0.4–6.8 | 0.431 | Tumor grade (1 vs 2–3) | 2.1 | 0.5–9.1 | 0.301 |
| ER (positive vs negative) | 1.2 | 0.3–4.9 | 0.762 | ER (positive vs negative) | 1.6 | 0.4–7.2 | 0.516 |
| PR (negative vs positive) | 2.3 | 0.7–7.5 | 0.182 | PR (negative vs positive) | 3.2 | 0.9–12.3 | 0.085 |
| Ki-67 (high vs low) | 1.2 | 0.5–3.3 | 0.653 | Ki-67 (high vs low) | 1.1 | 0.4–3.1 | 0.869 |
| HER-2 (negative vs positive) | 1.1 | 0.2–4.9 | 0.973 | HER-2 (negative vs positive) | 1.0 | 0.3–3.9 | 0.974 |
| Hormonal therapy (no vs yes) | 1.5 | 0.4–5.1 | 0.555 | Hormonal therapy (no vs yes) | 1.2 | 0.3–5.2 | 0.773 |
| Chemotherapy (no vs yes) | 2.7 | 0.9–8.3 | 0.087 | Chemotherapy (no vs yes) | 2.3 | 0.7–7.3 | 0.158 |
| m-Nectin-4 (negative vs positive) | 1.2 | 0.3–4.2 | 0.824 | c-Nectin-4 (low/negative vs high) | 2.9 | 1.0–8.0 | 0.043a |
| Distant relapse-free survival | Distant relapse-free survival | ||||||
| Tumor size, cm (⩽2 vs >2) | 1.4 | 0.7–3.0 | 0.369 | Tumor size, cm (⩽2 vs >2) | 1.8 | 0.7–4.7 | 0.231 |
| Tumor grade (2–3 vs 1) | 1.0 | 0.5–2.4 | 0.972 | Tumor grade (2–3 vs 1) | 1.0 | 0.3–3.3 | 0.975 |
| ER (negative vs positive) | 2.3 | 0.7–7.3 | 0.158 | ER (negative vs positive) | 1.5 | 0.4–5.3 | 0.502 |
| PR (positive vs negative) | 1.3 | 0.5–3.8 | 0.599 | PR (negative vs positive) | 1.0 | 0.3–3.2 | 0.978 |
| Ki-67 (high vs low) | 1.0 | 0.5–2.3 | 0.947 | Ki-67 (high vs low) | 1.7 | 0.7–4.3 | 0.228 |
| HER-2 (positive vs negative) | 1.4 | 0.6–3.3 | 0.461 | HER-2 (positive vs negative) | 1.5 | 0.5–4.5 | 0.511 |
| Hormonal therapy (no vs yes) | 1.2 | 0.4–3.7 | 0.799 | Hormonal therapy (no vs yes) | 1.4 | 0.3–6.1 | 0.697 |
| Chemotherapy (no vs yes) | 2.3 | 0.8–6.6 | 0.133 | Chemotherapy (no vs yes) | 1.3 | 0.4–4.2 | 0.700 |
| m-Nectin-4 (positive vs negative) | 2.2 | 0.9–5.1 | 0.068 | c-Nectin-4 (low/negative vs high) | 1.5 | 0.6–4.2 | 0.398 |
Abbreviations: CI, confidence interval; ER, estrogen receptor; HR, hazards ratio; PR, progesterone receptor.
Statistically significant.
Table 3. Multivariate analysis of m-Nectin-4 and c-Nectin-4 expression in luminal-A tumors.
| Variable | HR | 95% CI | P-value | Variable | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|
| Disease-free survival | Disease-free survival | ||||||
| Tumor size, cm (>2 vs⩽2) | 1.0 | 0.4–2.8 | 0.930 | Tumor size, cm (⩽2 vs >2) | 1.3 | 0.4–4.5 | 0.657 |
| Tumor grade (2–3 vs 1) | 7.2 | 0.9–56.0 | 0.058 | Tumor grade (2–3 vs 1) | 5.4 | 0.6–45.5 | 0.120 |
| Hormonal therapy (no vs yes) | 1.4 | 0.5–4.0 | 0.554 | Hormonal therapy (no vs yes) | 1.3 | 0.3–5.5 | 0.700 |
| Chemotherapy (no vs yes) | 3.2 | 0.9–10.3 | 0.052 | Chemotherapy (no vs yes) | 1.5 | 0.4–5.8 | 0.586 |
| m-Nectin-4 (positive vs negative) | 4.0 | 1.5–10.8 | 0.007a | c-Nectin-4 (low/negative vs high) | 3.5 | 1.1–11.6 | 0.038a |
| Local relapse-free survival | Local relapse-free survival | ||||||
| Tumor size, cm (>2 vs ⩽2) | 1.2 | 0.3–5.0 | 0.811 | Tumor size, cm (>2 vs⩽2) | 1.3 | 0.3–5.7 | 0.754 |
| Tumor grade (2–3 vs 1) | 2.9 | 0.3–26.0 | 0.333 | Tumor grade (2–3 vs 1) | 4.0 | 0.4–44.5 | 0.259 |
| Hormonal therapy (no vs yes) | 2.8 | 0.6–13.5 | 0.205 | Hormonal therapy (no vs yes) | 2.7 | 0.4–18.8 | 0.328 |
| Chemotherapy (no vs yes) | 3.3 | 0.7–15.4 | 0.132 | Chemotherapy (no vs yes) | 2.5 | 0.5–12.6 | 0.278 |
| m-Nectin-4 (negative vs positive) | 2.3 | 0.3–20.2 | 0.449 | c-Nectin-4 (low/negative vs high) | 3.9 | 0.9–17.6 | 0.076 |
| Distant relapse-free survival | Distant relapse-free survival | ||||||
| Tumor size, cm (⩽2 vs >2) | 1.1 | 0.3–4.5 | 0.865 | Tumor size, cm (⩽2 vs >2) | 2.6 | 0.3–24.2 | 0.392 |
| Tumor grade (2–3 vs 1) | 1.0 | 0.3–4.9 | 0.971 | Tumor grade (2–3 vs 1) | 1.1 | 0.1–18.5 | 0.985 |
| Hormonal therapy (yes vs no) | 1.3 | 0.3–6.6 | 0.730 | Hormonal therapy (yes vs no) | 1.9 | 0.1–25.2 | 0.614 |
| Chemotherapy (no vs yes) | 1.8 | 0.3–11.3 | 0.512 | Chemotherapy (yes vs no) | 1.3 | 0.1–18.3 | 0.842 |
| m-Nectin-4 (positive vs negative) | 6.0 | 1.8–19.8 | 0.003a | c-Nectin-4 (low/negative vs high) | 2.3 | 0.3–16.3 | 0.406 |
Abbreviations: CI, confidence interval; HR, hazards ratio.
Statistically significant.
c-Nectin-4 expression and disease-free survival
Twenty-three out of 122 patients (18.9%) harbouring c-Nectin-4High tumors and 16 out of 41 patients (39.0%) with c-Nectin-4Low/Negative tumors had a disease relapse. A local recurrence was observed in 8 out of 122 (6.6%) c-Nectin-4High and 9 out of 41 (22.0%) c-Nectin-4Low/Negative tumors. Distant metastases developed in 12.3% (15/122) and 17.1% (7/41) of patients with c-Nectin-4High and c-Nectin-4Low/Negative tumors, respectively.
At Kaplan–Meier analysis, c-Nectin-4High expression was significantly associated with higher LRFS, but not DRFS rate in all patients (P=0.004) and in those with luminal-A cancers (P=0.018) (Figure 2b).
Multivariate analyses of DFS adjusted for other prognostic factors revealed that low cytoplasmic expression of Nectin-4 was an independent prognostic factor influencing LRFS (HR=2.9: 95% CI, 1.0–8.0; P=0.043) in the whole population (Table 2). In patients with luminal-A cancers, c-Nectin-4 status was an independent predictor of DFS (HR =.3.5: 95% CI, 1.1–11.6; P=0.038), while it showed a trend toward statistical significance for LRFS (HR=3.9: 95% CI, 0.9–17.6; P=0.076) (Table 3).
Discussion
In the emerging era of targeted therapy, the identification of risk markers of recurrence and of sensitivity to treatments represents a major challenge. The achievement of this task is even more compelling in those malignancies that have benefited from the development of early diagnosis strategies, the identification of molecular and clinical subsets and more efficient adjuvant therapies. Breast cancer, which epitomizes such malignancies, is currently undergoing an intense molecular scrutiny to improve its molecular taxonomy and potentially its management. The clinical and biological implications of breast cancer heterogeneity has recently been revealed by gene expression profiling. Four main molecular classes have been identified (Luminal-A, Luminal-B, HER-2 positive and Triple Negative) that show significant differences in incidence, survival and response to therapy.19, 20, 21 As the genetic analysis improves, new tumor subtypes will be discovered requiring a more personalized care. Indeed, the use of integrated platforms22,23 is confirming and further documenting that the Luminal A/ER+ subtype is the most heterogeneous one in terms of gene expression, mutation spectrum, copy number changes and patients'outcomes.22 The exploitation of this new knowledge, which will result into further subclassifications, is likely to become more rapidly cost-effective by the stepwise addition of surrogate phenotypic markers to available clinical-pathological data (St Gallen, 2011).24
In the present study, we have investigated the expression of Nectin-4 in a retrospective cohort of 197 patients with node-negative early breast cancer (T1-T2, N0) to assess its prognostic significance in terms of disease-free survival. Unlike other Nectins, Nectin-4 was originally described as a carcinoembryogenic antigen because of its restricted expression in the embryo and placenta and its re-expression in breast and lung cancers.11,12,15, 16, 17 However, in normal human tissues, Nectin-4 mRNA, in addition to placenta, is also expressed in trachea, prostate, lung, stomach and skin.18 In the skin, immunohistochemical analysis detected membranous Nectin-4 at cell–cell junction of normal human keratinocytes, and Nectin-4 mutations have been casually linked to ectodermal dysplasia-syndactyly syndrome, which results in webbed hands and feet and in the loss of membranous Nectin-4 expression.18 Membrane Nectin-4 has been also identified as the receptor for measles virus, present on the surface of airway epithelial cells, and responsible for the virus infection in macaques and humans.25,26 In non-neoplastic breasts, our results demonstrated the expression of Nectin-4 in the cytoplasm of almost all luminal cells of terminal duct lobular units, in the absence of cell membrane staining, whereas a previous study16 was unable to find immunoreactivity for Nectin-4 in normal breast epithelium. This discrepancy could be becasue of the different antibodies employed. Fabre et al.11 after immunization with human recombinant soluble Nectin-4, obtained15 and selected for IHC staining procedures two anti-Nectin-4 monoclonal antibodies, N4.61 and N4.40, recognizing the IgV (Gly32-Arg144) and the IgC domains (Pro148-Asp331) of the protein, respectively. We used a commercially available, affinity purified polyclonal goat antibody (AF2659; R&D Systems), produced by immunization with recombinant human Nectin-4 from Gly27 to Val351, also including the extracellular domain and ending with the transmembrane one (http://www.uniprot.org/uniprot/Q96NY8). Immunoblot analysis of Nectin-4 performed with this antibody demonstrated a very strong reduction of protein expression in keratinocytes from patients with mutated PVRL4, when compared with control subjects,18 thus supporting antibody specificity. By flow-cytometry and/or IHC, Nectin-4 was detected at plasma membrane and/or cytoplasm in several cancer cell lines and primary breast, lung and ovarian cancers.14,16,17 A soluble form of Nectin-4, released into the blood after cleavage of its extracellular portion,15 has been also identified,14,16,17 and its serum levels correlated with the number of metastases and the therapeutic efficacy in breast and lung cancers.16,17 Thus, Nectin-4 is a potential serum/tissue marker as well as a therapeutic target in solid tumors. We extend these findings by showing the association between m-Nectin-4 expression and risk of tumor progression in early breast cancer patients. Indeed, at multivariate analysis, the presence of Nectin-4 on cell membrane was significantly associated with a lower metastasis-free survival rate in patients with luminal-A tumors only. These results confirm previous findings that Nectin-4 is mainly expressed in breast cancer cell lines with a luminal-like phenotype, and absent or weakly expressed in lines with a basal-like phenotype.27 Unexpectedly, we found that in luminal-A tumors with undetectable cell membrane Nectin-4, the absence or a marked reduction of the cytoplasmic form was associated with a higher risk of relapse. Pro-apoptotic effects have been attributed to Nectin-4 during epithelial morphogenesis.18 Small interfering RNAs against Nectin-4 have resulted in suppression of lung cancer cell growth.17 In addition, Nectin-4 expression increased lamellipodia formation and the cell invasive ability through activation of small GTPase Rac1.17 Altogether, these findings imply that Nectin-4 can modulate a spectrum of still incompletely defined biological activities depending on its levels and intracellular localization, both in normal and tumoral tissues. Whatever the mechanism(s) underlying its function, Nectin-4 might represent a prognostic marker in early breast cancers, and a potential target for antibody-mediated and measles-virus-based oncolytic therapies.25,26 An antibody-drug conjugate targeting Nectin-4 is currently in a phase I clinical trial in patients with solid tumors.28
Our findings suggest that evaluation of Nectin-4 expression may represent a novel reliable predictive marker of distant relapse in luminal-A early breast cancer, and a potential target for antibody-mediated and for measles-virus-based oncolytic therapies. Moreover, immunohistochemical detection of Nectin-4, alone and/or in addition to other techniques, could be useful in patients selection, but reagents, methods and positivity criteria are to be defined.
Materials and methods
Patients
Eligible patients were extracted from archival cases of invasive breast cancer diagnosed between 1988 and 1996 at the Regina Elena National Cancer Institute (Rome, Italy) and presenting with primary unilateral tumors. From the original series, only patients with no pathological evidence of nodal involvement (n=230) and among them, only those with T1/T2 tumors (n=219) were included into the study. pN0 cases underwent a second look by lymph node step-sectioning and anti-cytokeratin immunohistochemical analysis as reported.29 Lymphonodal micrometastases, defined as previously reported,29 were present in 15 cases, which were therefore excluded from further analysis. Because of lack of archival material in 7 cases, the final number of evaluable patients reduced to 197. Patients and tumor characteristics are summarized in Table 4. Radiotherapy was offered to all patients, 56 of them were treated exclusively with hormonal therapy and 116 received adjuvant chemotherapy followed or not by hormonal therapy. Patients with HER-2-positive tumors did not receive trastuzumab, because it was unavailable at the time patients were treated. The median follow-up was of 95 months (range 6–298 months). Follow-up data were obtained from institutional records or by the referring physician. During follow-up, local recurrences and distant metastases were observed in 20 (10.2%) and 31 (15.7%) patients, respectively. The study was reviewed and approved by the ethics committee of the Regina Elena National Cancer Institute, and written informed consent was obtained from all patients.
Table 4. Patients and tumor characteristics.
| Variable | Value (%) |
|---|---|
| Age at diagnosis (year) | |
| Median | 54.9 |
| <50 | 50 (25.4) |
| 50–65 | 82 (41.6) |
| >65 | 65 (33.0) |
| Menopausal status | |
| Pre/perimenopausal | 70 (35.5) |
| Postmenopausal | 127 (64.5) |
| Tumor size | |
| ⩽2 cm | 127 (64.5) |
| >2 cm | 70 (35.5) |
| Molecular subtypes | |
| Luminal-A | 80 (40.6) |
| Luminal-B/HER-2-negative | 53 (26.9) |
| Luminal-B/HER-2-positive | 17 ( 8.6) |
| HER-2 | 13 ( 6.6) |
| Triple negative | 34 (17.3) |
| Tumor grade | |
| 1 | 22 (11.2) |
| 2–3 | 175 (88.8) |
| ER | |
| Negative | 56 (28.4) |
| Positive | 141 (71.6) |
| PR | |
| Negative | 67 (34.0) |
| Positive | 130 (66.0) |
| Ki-67 | |
| Low | 103 (52.3) |
| High | 94 (47.7) |
| HER-2 | |
| Negative | 169 (85.8) |
| Positive | 28 (14.2) |
| m-Nectin-4 | |
| Negative | 163 (82.7) |
| Positive | 34 (17.3) |
| c-Nectin-4 | |
| Low/Negative | 41 (20.8) |
| High | 122 (61.9) |
| Patient outcome | |
| Without recurrence | 146 (74.1) |
| Local recurrence | 20 (10.2) |
| Distant recurrence | 31 (15.7) |
Immunohistochemistry
Tissue microarrays were constructed by extracting 2-mm diameter cores of histologically confirmed invasive breast carcinoma areas, as previously described.30 Five-micrometer tissue sections were cut and stained using the purified goat polyclonal antibody raised against the recombinant human Nectin-4 extracellular domain (1:60 dilution, 60 min, AF2659, R&D Systems Inc., Minneapolis, MN, USA). Whole sections of non-neoplastic breast tissues from 30 patients were also stained. As positive controls of Nectin-4 expression, skin and nipple sections were used.18 Antigen retrieval was performed by microwave treatment at 750 W for 10 min in 1 M urea buffer (pH 8.0). The LSAB kit (K0679, Dako, Glostrup, Denmark) was used for signal amplification. In control sections, the specific primary antibody was replaced with isotype-matched immunoglobulins. The following antibodies were used for the identification of tumor subtypes, as previously detailed:31 the anti-ER-α MoAb 6F11 (Novocastra, Menarini, Florence, Italy), the anti-PR MoAb 1A6 (Menarini), the anti-Ki67 MoAb MIB-1 (Dako) and the anti-HER-2 (Herceptest, Dako). Immunohistochemical analysis was done by two pathologists (MP, RL) by consensus without knowledge of the clinicopathological information.
Statistical methods
Pathologic tumor size and tumor grade, as well as ER, PR and Ki-67 expression were dichotomized according to the St Gallen criteria.24 HER-2 membranous staining was scored according to Herceptest (Dako) and classified as positive if the intensity was scored 3+, with more than 30% of cells showing complete membrane staining,32 or if the intensity was scored 2+ in presence of an amplification of the HER-2 gene as assessed by fluorescent in situ hybridization. On the basis of IHC of ER, PR, Ki-67 and HER-2, we also studied the Nectin-4 distribution in breast cancer molecular subtypes:24 Luminal-A (n=80), Luminal-B/HER-2-negative (n=53), Luminal-B/HER-2-positive (n=17), HER-2 (n=13) and Triple Negative (n=34). The relationships between Nectin-4 expression and clinicopathological parameters were assessed by Pearson's χ2 test. DFS was defined as the time from surgery to the first of the following events: tumor recurrence at local site or at distant sites. LRFS and DRFS were defined as the times from surgery to the occurrence of relapse at local and distant sites, respectively. Kaplan–Meier plots were used to illustrate the survival in specified cohorts and the log-rank test to test for equality of survival curves. The association of Nectin-4 expression with outcome, adjusted for other prognostic factors, was tested by Cox's proportional hazards model. The following covariates were included in the multivariate DFS models: tumor size and grade, and ER, PR, Ki-67, HER-2 and Nectin-4 status. Appropriateness of the proportional hazard assumption was assessed by plotting the log cumulative hazard functions over time and checking for parallelism. SPSS Version 15.0 (SPSS, Chicago, IL, USA) was used throughout.
Acknowledgments
This research was supported by Associazione Italiana Ricerca sul Cancro (AIRC) to Pier Giorgio Natali, Mauro Piantelli and Marcella Mottolese and by Italian Ministry of Education, University and Research (MIUR). SI, MM, PGN, MP: Senior Authors, in alphabetical order.
The authors declare no conflict of interest.
References
- Botha JL, Bray F, Sankila R, Parkin DM. Breast cancer incidence and mortality trends in 16 European countries. Eur J Cancer. 2003;39:1718–1729. doi: 10.1016/s0959-8049(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- Tyczynski JE, Bray F, Parkin DM. Breast cancer in Europe. ENCR Cancer Fact Sheets. 2002;2:1–4. [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ, Jr, Deshler A, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol. 2007;19:593–602. doi: 10.1016/j.ceb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, et al. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C'-C"-D beta-strands of the nectin1 V domain. J Biol Chem. 2002;277:27006–27013. doi: 10.1074/jbc.M203228200. [DOI] [PubMed] [Google Scholar]
- Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001;276:43205–43215. doi: 10.1074/jbc.M103810200. [DOI] [PubMed] [Google Scholar]
- Athanassiadou AM, Patsouris E, Tsipis A, Gonidi M, Athanassiadou P. The significance of Survivin and Nectin-4 expression in the prognosis of breast carcinoma. Folia Histochem Cytobiol. 2011;49:26–33. doi: 10.5603/fhc.2011.0005. [DOI] [PubMed] [Google Scholar]
- Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol. 2010;134:835–845. doi: 10.1309/AJCPGXK0FR4MHIHB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Lafay S, Garrido-Urbani S, Reymond N, Goncalves A, Dubreuil P, Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J Biol Chem. 2005;280:19543–19550. doi: 10.1074/jbc.M410943200. [DOI] [PubMed] [Google Scholar]
- Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestier C, Reymond N, et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer. 2007;7:73. doi: 10.1186/1471-2407-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69:6694–6703. doi: 10.1158/0008-5472.CAN-09-0016. [DOI] [PubMed] [Google Scholar]
- Brancati F, Fortugno P, Bottillo I, Lopez M, Josselin E, Boudghene-Stambouli O, et al. Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am J Hum Genet. 2010;87:265–273. doi: 10.1016/j.ajhg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale G. Integrating molecular profiling, histological type and other variables: defining the fingerprint of responsiveness to treatment. Breast. 2009;18 (Suppl 3:S32–S36. doi: 10.1016/S0960-9776(09)70269-3. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011. 22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012;20:429–439. doi: 10.1016/j.tim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adelaide J, Cervera N, et al. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25:2273–2284. doi: 10.1038/sj.onc.1209254. [DOI] [PubMed] [Google Scholar]
- Astellas Pharma Inc A Phase 1 Study of the safety and pharmacokinetics of escalating doses of AGS-22M6E or ASG-22CE given as monotherapy followed by expansion cohorts in subjects with malignant solid tumors that express nectin-4 Current Controlled Trials 2013 ; http://clinicaltrials.gov/ct2/show/NCT01409135/ .
- Querzoli P, Pedriali M, Rinaldi R, Lombardi AR, Biganzoli E, Boracchi P, et al. Axillary lymph node nanometastases are prognostic factors for disease-free survival and metastatic relapse in breast cancer patients. Clin Cancer Res. 2006;12:6696–6701. doi: 10.1158/1078-0432.CCR-06-0569. [DOI] [PubMed] [Google Scholar]
- Lattanzio R, Marchisio M, La Sorda R, Tinari N, Falasca M, Alberti S, et al. Overexpression of activated phospholipase Cgamma1 is a risk factor for distant metastases in T1-T2, N0 breast cancer patients undergoing adjuvant chemotherapy. Int J Cancer. 2013;132:1022–1031. doi: 10.1002/ijc.27751. [DOI] [PubMed] [Google Scholar]
- Novelli F, Milella M, Melucci E, Di Benedetto A, Sperduti I, Perrone-Donnorso R, et al. A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 2008;10:R74. doi: 10.1186/bcr2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]