Abstract
Biological soil crusts (BSC) are the dominant functional vegetation unit in some of the harshest habitats in the world. We assessed BSC response to stress through changes in biotic composition, CO2 gas exchange and carbon allocation in three lichen-dominated BSC from habitats with different stress levels, two more extreme sites in Antarctica and one moderate site in Germany. Maximal net photosynthesis (NP) was identical, whereas the water content to achieve maximal NP was substantially lower in the Antarctic sites, this apparently being achieved by changes in biomass allocation. Optimal NP temperatures reflected local climate. The Antarctic BSC allocated fixed carbon (tracked using 14CO2) mostly to the alcohol soluble pool (low-molecular weight sugars, sugar alcohols), which has an important role in desiccation and freezing resistance and antioxidant protection. In contrast, BSC at the moderate site showed greater carbon allocation into the polysaccharide pool, indicating a tendency towards growth. The results indicate that the BSC of the more stressed Antarctic sites emphasise survival rather than growth. Changes in BSC are adaptive and at multiple levels and we identify benefits and risks attached to changing life traits, as well as describing the ecophysiological mechanisms that underlie them.
Keywords: Antarctica, carbon allocation, gas exchange, lichens, life traits, net photosynthesis
Introduction
In semi-arid, arid, alpine and polar areas, where vascular plants are excluded or limited by water availability or temperature, the dominant vegetation units are biological soil crusts (BSC). BSCs are considered as ecosystem engineers (Pointing and Belnap, 2012) in terms of soil stabilisation (Belnap et al., 2003a), carbon fixation and nitrogen budgets (Elbert et al., 2012). At climatically extreme terrestrial sites, they may be the only photoautotrophic primary producers (Warren-Rhodes et al., 2006; Colesie et al., 2013). With predicted increases in extreme climatic conditions and desertification (Reynolds and Stafford-Smith, 2002), BSC are likely to have an even more important role in global carbon cycles. Lichens often form the major proportion and constitute a late successional stage of this complex community (Büdel et al., 2009). They aggregate soil particles in surface soil layers (Campbell, 1979), can form microhabitats for other organisms (Grube et al., 2009) and also serve as a food source (Baur et al., 1994). Studies on the physiological performance and acclimation potential in lichens and lichen-dominated BSC will be especially important to predict climate change scenarios in the above described habitats (Belnap et al., 2003b).
Lichens are known to colonise the harshest terrestrial environments (Kappen, 1973) and have been described from the hottest (Nash et al., 1977), the driest (Bewley and Krochko, 1982) and the coldest (Wynn-Williams et al., 2000) places on earth. According to the Competitor—Stress tolerator—Ruderal Triangle theory, they are stress tolerators, a grouping that is typically found in areas of high-intensity stress and low-intensity disturbance (Grime, 2002) such as alpine or arid habitats, deep shade, nutrient deficient soils and areas of extreme pH levels (Grime and Pierce, 2012). Species classified as stress tolerators generally have slow growth rates, high rates of nutrient retention and low-phenotypic plasticity (Grime and Pierce, 2012) and respond to environmental stresses through physiological variability. Lichens grow and survive under a wide range of stressful conditions and are known to show considerable plasticity. Domaschke et al. (2013) showed that across habitats from temperate to polar regions, the same lichen species can undergo large changes in maximal photosynthetic rate, temperature optimum for net photosynthesis (NP) and photobiont numbers. Rapid acclimation in order to allow optimal performance under different conditions is also known for lichens. The response of respiration to temperature can change throughout the year to the extent, that it meets the definition of full acclimation as defined by Körner (2003) for vascular plants (Lange and Green, 2005). Acclimation occurs due to ambient light also (Green et al., 1997). Even their water balance can acclimate to different conditions and, for soil crust lichens from the Namib Desert, it was not only shown that minimum thallus water content (WC) allowing positive NP can be extremely low (Lange et al., 1994), but also that photosynthesis is reactivated by water vapour uptake alone (Lange et al., 2006). Finally, Poorter et al. (2012) showed that carbon partitioning acclimates to different habitat conditions and patterns of assimilate allocation are known to serve as excellent indicators for growth performance in vascular plants. Palmquist et al. (2002) propose that lichens are able to optimise their resource investments between carbohydrate input and expenditure, suggesting that a carbon economy view might be a fruitful way to compare and understand the performance of different lichens. An acclimation of resource allocation was suggested to be related to environmental conditions (Palmquist et al., 2008). Studies on lichens revealed that photosynthetically produced sugars can be allocated to different pools within the thallus (Farrar, 1978), but studies of shifts between these pools are very rare. However, there are examples showing a rapid shift with decreasing carbon release and a low incorporation of 14C to simple carbohydrates like ribitol in green algal photobionts directly after being isolated from the lichen thallus (Green and Smith, 1974). Brown et al. (1981) showed that there were changes in respiration rate over 4 days depending on whether the hydrated lichen was kept in the dark or light indicating possible changes in respiratory pool size.
This study investigates how BSC respond to different habitat conditions. We chose three sites that differ considerably in their local environmental conditions and that support a natural BSC. The first, and climatically most extreme, ‘Site Darwin', is located at Diamond Hill in the Darwin Mountains of continental Antarctica (79°S), (Figures 1c and d). This site is considered to be one of the harshest coastal ice-free areas in the Ross Sea region, indicated by a very low diversity (Cannone et al., 2013; Colesie et al., 2013). The second site, ‘Site Garwood', is in the Garwood Valley (Figures 1a and b) and represents an area that is less extreme for poikilohydric organisms than Site Darwin. It is a part of the Dry Valley region of Antarctica, well-known for its cold desert climate (Cary et al., 2010) with annual precipitation around 50–100 mm rain equivalent per year (Sancho et al., 2007). The third site, ‘Site Homburg', has a temperate climate and is situated in the nature reserve 'Ruine Homburg' in Germany. In contrast to the other sites, Site Homburg is a calcareous low-nutrient xerothermic grassland (Figures 1e and f), where BSC are also well-established (Büdel, 2003). Accordingly, we have three habitats with different levels of severity in terms of temperature and water availability: the very extreme Site Darwin, the less extreme Site Garwood and moderate Site Homburg.
Figure 1.
Organisms and map of the study sites: close-up pictures of the organisms are on the left site with (a) a lichen-dominated BSC with the lichen species Lecanora expectans and Caloplaca darbishirei from Garwood Valley, (c) the lichen-dominated BSC from Diamond Hill with Acarospora gwynii and (e) the lichen-dominated BSC from the research site near Würzburg with Psora decipiens. At top right, a map of western Ross Sea coastline showing the locations of the two research sites, Site Garwood, McMurdo Dry Valleys (Garwood Valley) and Site Darwin, Diamond Hill; the inset, top right shows the location of the Ross Sea in Antarctica (both maps downloaded from the SCAR Antarctic Digital Database). Pictures of the two research areas are in the middle of the figure. (b) Garwood Valley, view towards Garwood Lake, Joyce Glacier (background) and Péwé Peak (centre left), (d) Diamond Hill, view towards the Brown Hills (Antarctica New Zealand Pictorial Collection, Shulamit Gordon K002 04/05).At the bottom, centre of the figure is a map of Germany showing the location of the research site near Würzburg and, on the right, a map showing the position of Germany in Europe. A picture of the site is in the middle, (f) Nature reserve ‘Ruine Homburg'.
Herms and Mattson (1992) summarised the growth differentiation balance in plants as a hypothesis, premised upon a physiological trade-off between growth and differentiation processes, and described the plants' dilemma as they must grow fast enough to maintain defence necessary to survive in the presence of pathogens. Our aim is to enlarge this established framework of the dilemma of plants: to grow or to defend, to lichens and BSC and suggest that in this case, the habitat stress level drives changes in life traits. Green (2008) discussed the environment filters for lichen species that can grow in polar and alpine habitats, and underlined that changes in major processes like photosynthesis do not explain their occurrence alone, but an emphasis on survival compounds combined with an overall change in life traits could be a reason for their success in harsh environments. We have looked at the adaptation of the BSC by determining the responses of various lichen traits to habitat severity in terms of temperature and water availability at multiple levels:
Level 1: species composition in terms of dominating organisms.
Level 2: carbon gain as changes in aspects of CO2 exchange such as NP, respiration, light adaptation, optimal temperature and thallus water relations.
Level 3: allocation of recently fixed carbon to internal metabolic pools.
We expect, by combining the responses of all traits at the three levels to extract information about overall BSC life traits, such as growth versus survival/resistance in response to the different severity of habitat conditions. We hypothesise that BSC of the more stressed Antarctic sites emphasise survival rather than growth.
Material and methods
Study site and organisms
The common lichen species and other photoautotrophs in the BSC were identified in order to describe the changes in species composition in terms of dominating organisms. Only BSC dominated by green algal photobiont lichens (chlorolichens) were studied. This was the only (both Antarctic sites) or the dominant (Site Homburg) crust type and therefore represents a suitable proxy for general statements about BSC for each site.
Site Darwin
Site Darwin lies close to Diamond Hill in the Brown Hills region in Antarctica (79°50'S, 159°19'E) (Figure 1d) and represents the ‘very extreme site'. The immediate vicinity is dominated by outcrops of granites, granodiorites and high-grade metamorphic rocks, dated as Cambrian and Ordovician (Carosi et al., 2007). Cosmogenic exposure ages of exposed surfaces in the vicinity range from 1000 to 1 million years, and the Diamond Hill site is estimated to have a surface age of about 400000 years (Storey et al., 2010). This location is characterised as a cold desert with a mean summer temperature of −5.9 °C, mean summer vapour pressure deficit is 0.2 kPA. The local climate differs from other sites along the western Ross Sea coast in being drier and colder. Biodiversity is very low with only one bryophyte species (Cannone et al., 2013), no collembola and only two cyanobacterial species living endolithically and none in BSC (Colesie et al., 2013). Mean BSC coverage in this area is 0.8% (Colesie et al., 2013). The area is described to act as a dispersal block along the Ross Ice Shelf coast line (Demetras et al., 2010).
Site Garwood
The second sampling area, the ‘extreme' site, is Garwood Valley (78°01'23.9'S; 163°53'24.2'E), situated within the Dry Valleys region of Antarctica (Figure 1b). The Garwood Valley site is dominated by outcrops of granites and gneisses, together with amphibolites, marble and dolomites. The valley floor is covered with glacial drift of several ages ranging from 22 000 to 35 000 years. This location is also characterised as a cold desert, but is more benign to life than Diamond Hill, and has a higher biodiversity with around four moss species and 17 lichen species. Mean BSC coverage is 3.3% and all functional groups of photoautotrophic cryptogams (lichens, mosses green algae and cyanobacteria) are present as components of the BSC.
Site Homburg
The third sampling site, the ‘moderate' site, is the nature reserve ‘Ruine Homburg' in Germany (50°01'N, 9°48'E) (Figure 1f). The bedrock is middle Triassic lacustrine limestone. It is an open landscape with bare rock and gravel spots covered by a thin vegetation layer dominated by cryptogams (Psora decipiens, Toninia sedifolia, Fulgensia fulgens, Collema tenax, Cladonia convoluta, Squamarina lentigera, Tortella tortuosa and Syntrichia ruralis). The vegetation is a relict steppe vegetation and is maintained by a grazing regime. The climate is warm temperate; mean air temperature is –0.3 °C in January and 18.3 °C in July (annual mean 9.2 °C). BSC cover about 58% of the area and are composed of mosses, lichens, algae and cyanobacteria (personal communication, L. Williams).
Sample collection
Sample collection for laboratory measurements was in January 2009 for Site Darwin, in December 2009 and January 2010 for Site Garwood and in August 2012 for Site Homburg. Sampling was carried out using a random generator adapted from a ‘blind man's bluff'-based sampling approach. A sampling grid (25 × 25 cm) was thrown with random distance, direction and angle from last sampling point. If there was a chlorolichen-dominated soil crust in the sampling grid, the sample was taken. A chlorolichen-dominated BSC was defined as having at least 5 × 5 cm surface area with clearly visible lichen cover, with the lichen growing directly on the soil or on gravel but not over mosses. Nine samples were taken from each sampling site. If the samples were not completely dry when collected, they were first allowed to dry at room temperature and then all samples were kept frozen at −20 °C until used for measurement. This cryopreservation is described to be a suitable mode of long-term storage of viable lichen thalli for experimental studies (Honegger, 2008).
CO2 exchange
In order to determine the changes in carbon gain, we conducted CO2 exchange measurements. Before measurement, the intact BSC samples underwent a reactivation procedure composed of 2 days dry storage at 4 °C in the dark and 24 h at 4 °C at 200 μmol photons m−2 s−1, before they were fixed in CO2-inert wire-mesh baskets and sprayed with distilled water to activate their metabolism. Immediately before measurements, full water saturation was achieved by spraying the samples three times until water droplets were visible on the surface of the BSC. Excessive water and droplets were carefully shaken from the basket before measurements were made.
CO2 gas exchange measurements were conducted under controlled laboratory conditions using a minicuvette system (CMS400 and GFS 3000, Walz Company, Effeltrich, Germany). The response of NP and dark respiration (DR) to WC was determined for three samples from each of the sites. Complete desiccation cycles (saturated thalli to air dry) were measured at 750 μmol photons m−2 s−1 (saturating light), ambient CO2 and at different temperatures. For the samples from Site Darwin and Site Garwood, the temperature steps were −2 °C, 0 °C, 2 °C, 5 °C, 7 °C, 10 °C and 15 °C and for the samples from Site Homburg, it was 0 °C, 2 °C, 5 °C, 7 °C, 15 °C, 17 °C, 21 °C, 25 °C and 32 °C. Samples were weighed between each measurement and WC was later calculated as mm precipitation equivalent after determination of dry weight following 5 days in a desiccator over silica gel. To obtain the NP response to light, fully hydrated samples (n=3 for each site) were exposed to stepwise increasing light from 0 to 1500 μmol photons m−2 s−1 at optimal temperature and ambient CO2 concentration. The light cycle (about 30 min duration) was repeated until the samples were completely dry (after 3–4 h). Light saturation was defined as the photosynthetic photon flux density at 90% of maximum NP. The CO2 exchange of the samples was related to soil crust surface. Differences between mean values of cardinal points for photosynthesis (maximum NP, respiration (at same WC and temperature for maximum NP), light compensation points, light saturation levels, optimal water content and water compensation points) were analysed using one way analysis of variance (Statistica 10, Stat soft) and a Tukey Posthoc test where there were significant differences.
Photosynthetic reactivation from water vapour alone was only tested on samples from Site Darwin, because of limited research material from the other sites. Dried samples were placed in the gas exchange cuvette and treated from the beginning with an air stream of 98% relative humidity. The required air humidity was achieved by saturating the gas stream with water, first by being passed through water at 20 °C and then through a Peltier-operated water-vapour trap that precisely regulated the dew point. The temperature of the cuvette was then set to a value that ensured 98% relative humidity in the cuvette. Great care was taken that water did not condense on the thallus of lichens at any time during this procedure. The samples were kept dark with light (500 μmol photons m−2 s−1) switched on for 15 min at an interval of 1 h to obtain a value for NP. The measurement took place under optimal temperature for NP and ambient CO2. After 44 h of humidity treatment, the enclosed soil crust were sprayed with water and the CO2 exchange of the initially saturated soil crusts was monitored as they dried at an air relative humidity of 80% with alternating light and dark phases to obtain values for DR and NP.
14CO2 uptake and allocation
To measure allocation of recently fixed carbon to internal metabolic pools in the BSC, samples were exposed to 14C-labeled gaseous CO2 in a gas-tight incubation chamber (156 cm3). Wet samples (n=3 each site) with the exact size of 4.7 cm2 were put into a closed chamber together with two small basins, one containing 300 μl 14C sodium-bicarbonate solution (≈ 75 μCi; Hartmann Analytic, Braunschweig, Germany) and the other, a piece of dry filter paper. The chamber was then closed, gaseous 14CO2 was generated by injecting acid (500 μl 0.1 M HCl) onto the bicarbonate solution and the sampling chamber was immediately placed under a light source (200 μmol photons m−2 s−1) for 12 h (complete drying out cycle). The complete experiment was done at two different temperatures, 5 °C and 17 °C. At the end of the incubation period, any remaining 14CO2 was first absorbed by injecting KOH solution onto the filter paper. Samples were then removed from the incubation chamber and extracted for 3 × 20 min in hot, 65 °C methanol, followed by 3 × 20 min extraction in boiling water (100 °C). The alcohol extraction both kills the lichen and extracts small-molecular weight sugars, which are the early products of photosynthesis (ribitol) or the first storage products in the fungi (mainly arabitol and mannitol). The boiled water extracts polysaccharides that are initial storage compounds. The remaining insoluble material is the end use fraction of photosynthetic products and can be interpreted as allocation to growth. Due to limited capacities in the isotope laboratory, the lipophyllic material could not be isolated separately as suggested from Cowan et al. (1979). Fixed 14C in the three fractions of each sample (the methanol-soluble, the hot water-soluble and the insoluble material) was counted using a TriCarb 2810TR Low Activity Liquid Scintillation Analyser (Perkin Elmer, Waltham, MA, USA). Differences between fixed 14C in samples from the different sites were analysed with a two way analysis of variance with both temperature and site as category predictors.
Results
Lichen traits
The three organisational levels, BSC community composition, CO2 gas exchange patterns and carbon allocation, the traits within them and the possible drivers of change in the traits are listed in Table 1.
Table 1. Three organisational levels of BSCs as a functional unit, their traits, units and possible drivers or anticipated drivers of change.
|
Functional unit: BSC | |||
|---|---|---|---|
| Level | Trait | Unit | Driver |
| BSC community members | Lichen species | Name | Genetic limits to phenotypic/metabolic plasticity |
| Number of species | Number | Overall metabolic fate: growth versus survival | |
| Functional group (mosses, lichens, cyanobacteria, green algae) | Occurrence/number | Succession state/habitat suitability | |
| Carbon gain | NP | μmol m−2 s−1 | Carbon gain |
| Dark respiration | μmol m−2 s−1 | Maintenance/growth | |
| Carbon gain efficiency | ratio | Overall gain efficiency | |
| Light level to saturate photosynthesis | μmol photons m−2 s−1 | Light use/Protection against excess light | |
| Light level to achieve NP compensation | μmol photons m−2 s−1 | Low light use efficiency | |
| Thallus water content | gH2O gDW−1 | Water availability | |
| Optimal temperature for NP | °C | Active temperature | |
| Carbon allocation | Small sugars | % Fixed carbon | Stress protection/Respiratory substrate |
| Polysaccharides | % Fixed carbon | Intermediate storage for growth support versus respiratory backup | |
| Insolubles | % Fixed carbon | Growth versus survival | |
Abbreviations: BSC, biological soil crust; NP, net photosynthesis.
Species composition
BSCs from the very extreme Site Darwin are dominated by the green algal lichen Acarospora gwynnii CW Dodge & ED Rudolph. Also present in this particular crust were green algae of the genera Diplosphaera, Heterococcus, and Trebouxia but no mosses or cyanobacteria. Samples from the extreme Site Garwood were dominated by two lichen species Lecanora expectans Darb. and Caloplaca darbishirei (Hoffm.) Th. Fr. together with several species of filamentous and unicellular species of cyanobacteria, several unicellular and immobile green algae and also protonema from the moss species Hennediella heimii (Hedw.) RH Zander. The dominant lichen species in the samples from Site Homburg is Psora decipiens (Hedw.) Hoffm. together with filamentous and unicellular species of cyanobacteria as well as unicellular and immobile green algae, but no mosses were present in the tested samples.
Photosynthetic performance
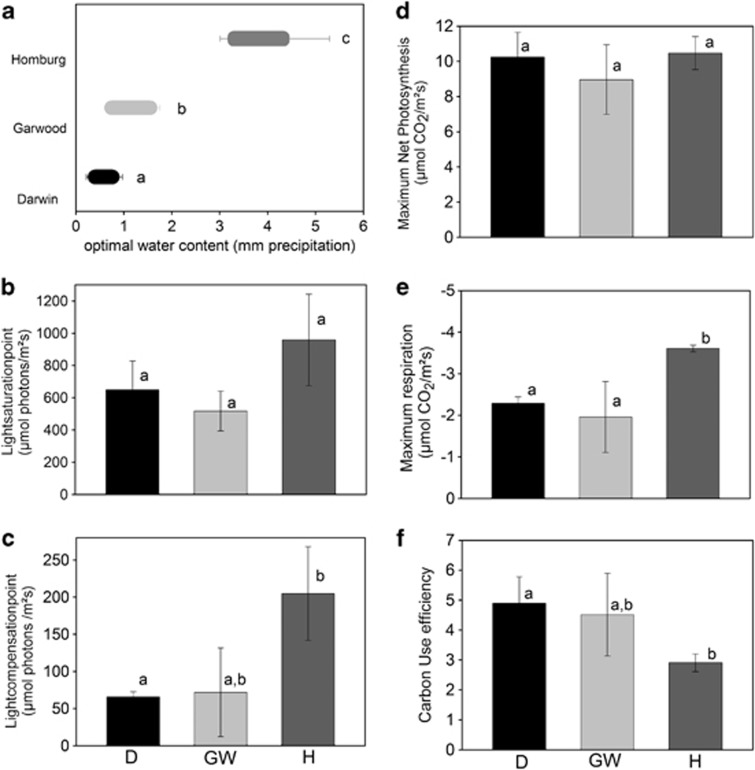
Optimal water content for NP was significantly different between all the three sites (F=28.575, P=0.000, Figure 2a). It was lowest for BSC from Site Darwin extending over a very narrow range of 0.39–0.75 mm precipitation equivalent, compared with 3.32 mm precipitation equivalent for BSC from Site Homburg and a much broader range that reached 4.29 mm precipitation at Site Homburg. Optimal temperature for NP of BSC was 5 °C at Site Darwin, 7 °C at Site Garwood and 17 °C at Site Homburg. Maximum NP was similar for the BSC from all three sites when measured at their individual optimal water content, optimal temperature and saturating light (F=0.875, P=0.463, Figure 2d). Respiration rate under these conditions were significantly different between the sites (F=8.975, P=0.0157). Respiration rates were similar for BSC from Site Darwin and Site Garwood (P=0.718) and both were significantly lower than DR for samples from Site Homburg (P=0.0168 Homburg vs Garwood, P=0.0424 Homburg vs Darwin) (Figure 2e). As a consequence, the carbon use efficiency (NP/DR at optimal conditions for NP) of BSC from Site Darwin is almost double that of BSC from Site Homburg (Figure 2f). The light saturation points for NP were between 400 and 900 μmol photons m−2 s−1 but were statistically not different for the three sites (F=3.609, P=0.0935; Figure 2b). The light compensation point was lowest for BSC from Site Darwin (F=7.356, P=0.024), but it was not significantly different to BSC from Site Garwood (P=0.87; Figure 2c).
Figure 2.
Cardinal points of photosynthesis of BSC from Site Darwin (D, black), Site Garwood (GW, light grey) and Site Homburg (H, dark grey); (a) range of optimal water content (mm precipitation), (b) mean light saturation points (μmol photons m−2 s−1; n=3), (c) mean light compensation point (μmol photons m−2 s−1; n=3), (d) mean maximum NP under optimal conditions (μmol CO2 m−2 s−1; n=3), (e) mean maximum respiration (DR; μmol CO2 m−2 s−1; n=3), (f) mean carbon use efficiency (NP/DR; n=3). In all cases, error bars are±s.d. Different letters indicate significant differences in the means between habitats (significance level P<0.05).
BSC from Site Darwin were not reactivated by water vapour alone so that, even after 44 h exposure to 98% relative humidity, they did not show any DR or NP activity. The crusts were only reactivated following spraying with liquid water. The ensuing NP depression caused by water oversaturation was only very brief and optimal NP was reached within 40 min drying.
14CO2 uptake and allocation
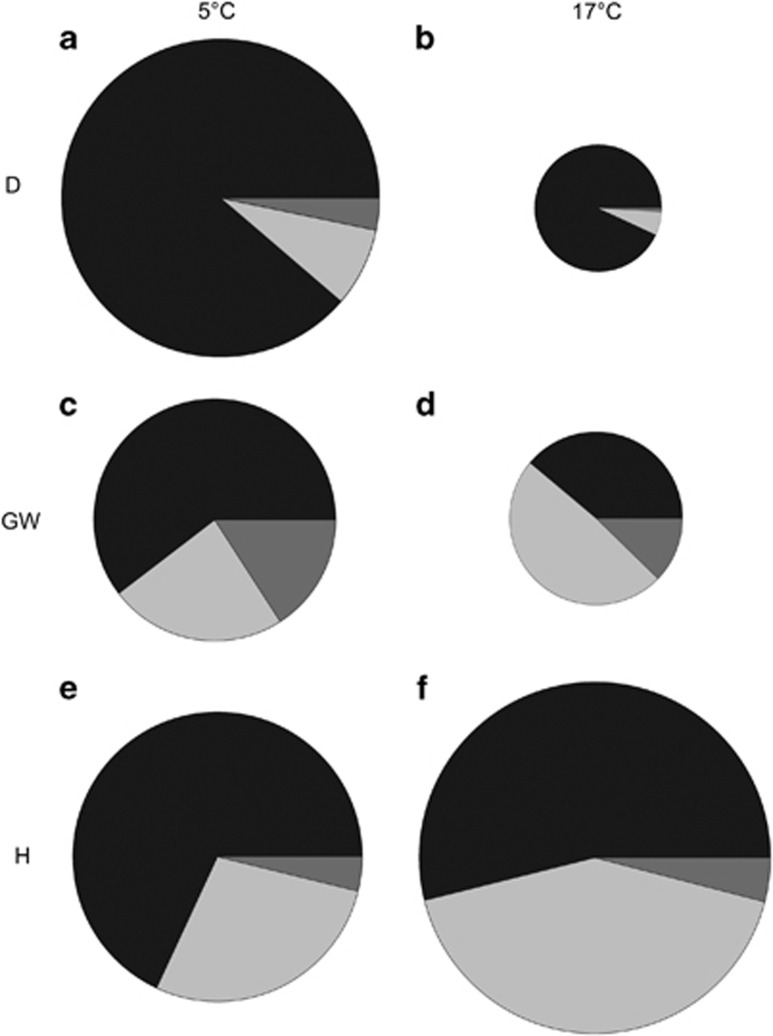
Total 14CO2 fixed at 5°C was similar for BSC from all three sites (F=15.51, P=0.924; Figures 3a, c and e). However, at 17°C 14CO2 fixation by BSC from Site Homburg was not only the highest (difference to both Site Garwood and Site Darwin P=0.000) but had also increased in relation to fixation at 5°C (Figures 3e and f). In contrast, BSC from the sites Garwood and Darwin at 17 °C had lower 14CO2 fixation than at 5°C, in line with their lower optimal temperatures for NP. Site Darwin had the lowest 14CO2 fixation. Allocation of fixed 14CO2 was also very different between sites and at the two experimental temperatures. At 5°C, the methanol fraction was largest in samples from Site Darwin (F=36.021; P=0.000), at almost 90% of the total fixed carbon (Figure 3a). 14CO2 fixed into the methanol fraction is one-third lower in the samples from Site Garwood and Site Homburg, which are both similar (P=0.062). 14CO2 fixed into the polysaccharide fraction (hot water extraction) ranges from 10% of total 14CO2 fixed at Site Darwin to 23% at Site Garwood to 30% at Site Homburg (F=34.30; P=0.000). 14CO2 fixed into the insoluble material is three times greater in samples from Site Garwood (Figure 3c) compared with the other sites (F=6.38; P=0.002) but the proportion is low at all sites being only about 3% of the total fixed carbon at Site Darwin and Site Homburg.
Figure 3.
14C carbon uptake and allocation. The area of the circle indicates the total uptake of 14C during the 12 h incubation time. Left column 5 °C, right column 17 °C; (a) and (b) Darwin (D); (c) and (d) Garwood (GW); (e) and (f) Homburg (H). Methanol fraction (short-chained sugars) is indicated in black, the hot water fraction (polysaccharides) is indicated in light grey, the insoluble fraction is indicated in dark grey.
The overall allocation pattern remain the same at 17°C for Sites Darwin and Homburg. Although samples from Site Garwood fix the same amount of 14CO2 under the different temperature treatments (P=0.889), there are significant differences in the allocation. The total amount of short-chained sugars decreased drastically (P=0.000) with an increase in temperature (Figure 3d), whereas the polysaccharide fraction increased from 25% to 35% of the total carbon uptake (P=0.014).
Discussion
In this study, we have treated samples from different sites as the same functional unit, a biological soil crust rather than targeting individual lichen species. The BSC responded at multiple levels with changes in species composition, photosynthetic performance and carbon allocation and several traits showed changes that can be related to the habitat conditions at the three sites, very extreme, extreme and moderate in terms of temperature and water availability. We suggest that the differences in metabolic performance also allow conclusions to be made about different general life traits of organisms that allow BSC to be successful even in most extreme terrestrial environments on earth.
Species composition
The functional unit chosen for this study was BSC dominated by green algal lichens and the dominating lichen species differed at the three sites. BSC from the very extreme Site Darwin were dominated by Acarospora gwynnii, which is an Antarctic endemic species described as occurring in fully exposed situations (Øvstedal and Smith, 2001). The lichen is composed of aggregated squamules, each 0.2–1 mm, attached by coarse rhizomorphs. The harshness of the environment is suggested by the unusual occurrence of this lichen on soil rather than on rock and by the lack of two other, normally important, functional groups, the bryophytes and the cyanobacteria, both of which show a strong link to better water availability than found at Site Darwin. The less extreme nature of Site Garwood is indicated by the presence in BSC of all other functional groups of cryptogams with a relatively high diversity (Colesie et al., 2013). The dominant lichen species were Lecanora expectans, an Antarctic endemic leprose lichen covered by 0.3–0.8 mm big apothecia and Caloplaca darbishirei, a vivid orange species with 0.5–1 mm wide squamules with sorediate margins, occurring in almost all ice-free areas of the Antarctic continent and also suspected to be more widespread, for example in Southern Patagonia (Søchting and Castello, 2012). At Site Homburg, the dominant lichen was Psora decipiens, with 3–4 mm wide squamules and a usually white, slightly upturned margin. This lichen has a cosmopolitan distribution and is known to be a key species in BSC from many different localities (Brodo et al., 2001; Büdel et al., 2009; Büdel et al., 2013). The occurrence of this species, together with the presence of all additional functional groups of cryptogams in BSC, underlines the moderate character of this site.
The occurrence of a species is related to dispersal, competitiveness and local climate, which is a general phenomenon in vegetation ecology and related to shifts in species composition of plant communities along different gradients. The advantage of our approach, which takes changes of the dominant lichen species into account as a response to the habitat conditions is that we can show such a shift occurring also in cryptogamic communities along a habitat stress gradient. This, taken together with the modulation of CO2 exchange and carbon allocation, provides robust support for BSC as excellent indicators of local climate and therefore is suitable for a comparison of life traits, such as growth versus survival, under different levels of stress.
Photosynthetic performance
Convergent traits in photosynthetic performance by the BSC at the three different sites underline the characteristic ecology of soil crusts communities. One similarity found here is the light level required to saturate positive NP, which differs little between the BSC from the three sites, despite large differences in other habitat conditions. Light is known to generate physiological variability in lichens (Heber and Lüttge, 2011), light saturation of the photosystems can be habitat specific (Pardow et al., 2010) and high light-saturation points classify lichen species as favoring high light conditions (Singh et al., 2013). The lack of difference in this parameter is, therefore, not surprising because an open habitat is typical of BSC environments. The sites in this study had no (Site Garwood and Site Darwin) or only a few (Site Homburg) vascular plants that might possibly shade the organisms while they are active. We consider that the general light regime of the three study sites is probably very similar and bright during the active periods of the organisms. More intriguing is the second general similarity: the nearly identical maximum photosynthetic rates. Although species composition of the BSC is different between the sites, the photosynthetic capacity is the same under their individual optimal conditions, which are likely to be site related. This shows that the optimal response to the environment of BSC photosynthesis can be regulated by several factors.
Lichens as stress tolerators respond to environmental stresses through physiological variability (Grime and Pierce, 2012) so that divergent traits in BSC performance between the sites should reflect the stress level. One such difference can be found in their optimal water contents for NP. BSC from the most extreme Site Darwin had an optimal WC for NP of only 0.57 mm precipitation equivalent, whereas the BSC from Site Garwood had 1.1 mm precipitation equivalent and the BSC from the moderate Site Homburg had the optimum at 3.32 mm precipitation equivalent (Figure 2a). By what means do BSCs acclimatise their optimal water content to the specific environmental water availability? We suggest that this is regulated by their biomass per unit area.
Lichens generally reach optimal photosynthetic performance at a WC of around 100%, so the presence of less biomass on an area basis would mean less water needed for optimal NP. This suggestion is strongly supported by the very low-respiration rates of the BSC at Site Darwin (Figure 2e), suggesting the presence of lower active biomass. A lower-respiration rate has several implications for the overall performance of the lichens. The similar maximal NP rates of the BSCs from the three sites implicate that one benefit of low-respiration rates are high-carbon use efficiencies (Figure 2f), found for the samples from Sites Darwin and Garwood, resulting in higher net carbon fixation.
Another effect resulting from low-respiration rates are differences in light compensation points. These are reached when photosynthesis is sufficient to balance respiration within the lichen thallus (Green et al., 2008) and lower-respiration rates result in compensation points being reached at lower-light intensities. A reduced biomass within the samples from site Darwin would therefore result in three major benefits for survival: low optimal water content for maximal NP, higher carbon use efficiency and positive NP is reached at lower-light intensities.
Another trait that aligns strongly with the individual environments is the optimal temperature for NP. This is lowest at 5 °C for site Darwin and slightly higher at 7 °C in Site Garwood. The small difference is not unexpected as recent studies suggest that temperatures during activity can be very similar in Antarctic lichens (Schroeter et al., 2010) and, although 2 ° latitude further south, the temperature at Site Darwin differs little from Site Garwood. The optimal temperature for NP at Site Homburg, 17 °C, is 10 °C higher, indicating BSC acclimation to temperature possibly through domination by other lichen species than in the BSCs from the other sites.
The type of water source for thallus hydration also influences BSC life traits. In addition to reactivation with liquid water, chlorolichens are known to be reactivated by water vapour only (Lange and Kilian, 1985; Lange et al., 1986) as has also been found for Psora decipiens (Büdel et al., 2013), the dominat lichen species in BSC of Site Homburg. The possibility of activation by water vapour alone is controversial for lichens in Antarctica. It has been experimentally demonstrated that some Antarctic lichens can reactivate from water vapour alone (Kappen, 1993) and Schroeter and Scheidegger (1995) showed the Antarctic lichen Umbilicaria aprina Nyl. being activated by water uptake in the gaseous phase from snow at subzero temperatures. Nevertheless, there is little evidence that activation by water vapour occurs naturally in Antarctica (Hovenden et al., 1994; Pannewitz et al., 2003). Here, we found that the BSC from Site Darwin dominated by the green algal lichen Acarospora gwynnii did not reactivate from water vapour alone. Although unusual for chlorolichens, this agrees with the results of Del Prado and Sancho (2007) who also found that Teloschistes lacunosus in the Tabernas desert (Spain) behaved similarly. We suggest that this is a long-term effect within soil crust lichens from very dry environments, resulting from the probable absence, or only brief occurrence of suitable high humidity conditions in the habitat. Lichens in very stable and dry climatic conditions can in some way hinder reactivation by water vapour so that hydration by water vapour is species-specific in chlorolichens.
Carbon allocation
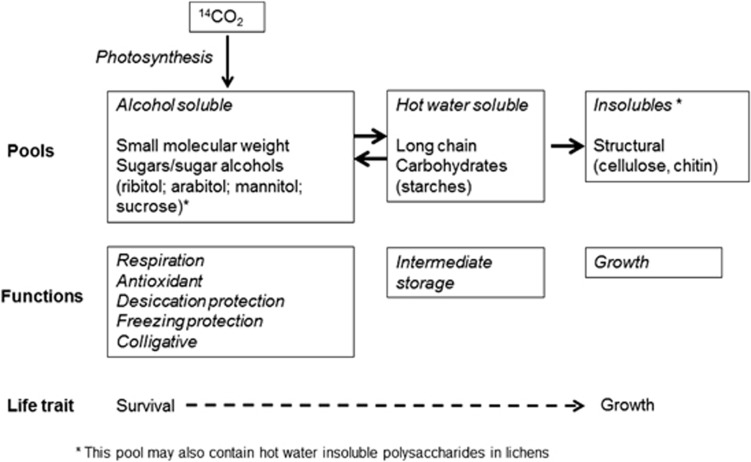
Photosynthetically fixed CO2 enters three pools, first the alcohol soluble pool mainly composed of polyols and other short-chained sugars (Richardson and Smith, 1968), secondly the hot water-soluble pool composed mainly of storage polysaccharides and thirdly the insoluble pool with molecules that are not alcohol nor hot water soluble and probably composed of polymeric carbohydrates such as in cell walls (Farrar, 1978). We suggest these pools to have different functions within the lichen-dominated BSC. The short-chained sugars and sugar alcohols with low-molecular weight in the alcohol fraction have been assigned to many different roles in terms of stress resistance and survival, desiccation protection (Kranner et al., 2008), freezing protection (Tearle, 1987; Roser et al., 1992) or being antioxidants (Kranner et al., 2005; Green et al., 2011; Figure 4). No such roles have been described for the hot water soluble long-chain carbohydrates and we suggest this pool to be an intermediate storage mainly to support growth but also to sustain the short-chained sugar pool if necessary (absolute transfer rate from one compartment to another is 2.5 × 10-4/h, Farrar, 1978). The insoluble material reflects the structural growth of the organisms and once carbon is stored in that pool, it cannot be transferred back to any other function. From the different allocations of fixed 14CO2 to, and the suggested functions of, these pools we can identify different life traits in the BSC of our study. BSC from the very extreme site Darwin store 90% of the totally fixed carbon in short-chained sugars, reflecting a high investment in stress resistance, so that these organisms emphasise survival, rather than structural growth (Figure 4). This interpretation is supported by Tearle (1987) who showed that Antarctic lichens had higher sugar+polyol pools in winter and that the polyol pool is three times higher than in temperate lichens. The less extreme the habitat, the greater the percentage of fixed carbon allocated to hot water soluble polysaccharides so this pool can therefore be treated as an indicator for growth-potential within lichens.
Figure 4.
Metabolic pools and possible contents, possible functions and overall life traits for fixed carbon allocation in lichen-dominated BSC. Arrows indicate the likely flow directions of fixed carbon between the pools.
Although there are clear advantages in the high allocation to small sugars in very extreme habitats, there are also potential risks. These first products of photosynthesis (ribitol) or the first storage products in the fungi (mainly arabitol and mannitol) are at risk to be easily leached. Such leaching can cause losses to the surrounding substrate (Tearle, 1987) that can accumulate to 65% of the total sugar pool in cryptogams (Melick and Seppelt, 1992). Dudley and Lechowicz (1987) describe losses of polyols by leaching as a general phenomenon in subarctic lichens and showed that the lichen species Umbilicaria proboscidea (L.) Schrad., which is very similar to the typical Antarctic lichen Umbilicaria aprina Nyl. had an average loss of one-tenth of its complete polyol content in the first 45 min after a rehydration event (Dudley and Lechowicz, 1987). With a loss of the short-chained sugars by leaching, the BSC investigated here would lose their respiration substrate. Such high risks appear more bearable in drier habitats as in the Darwin region, where hydration events and thus leaching are not common.
Conclusions and implications
We have identified multilevel changes in life traits of lichen-dominated BSC as responses to a range of stresses from very extreme to moderate habitats. Many of the traits, such as carbon allocation and respiration minimisation support a change from survival in the very extreme habitat, to growth in the moderate habitat.
In very stressful habitats (here represented by Site Darwin), lichens show several acclimations to improve the photosynthetic performance and have a high investment in stress resistance in terms of carbon allocation patterns. However, these changes also come with limitations and risks for the organisms. First, these lichens do not appear to have the ability of being activated by water vapour and thus lost a possible water source. Second, these lichens have a risk of leaching from the pool of short-chained sugars during rehydration events. Thirdly, these adaptations cannot be altered quickly and thus may limit chances to adapt rapidly to a changing climate.
These major changes in life trait, from survival to growth, are almost certainly not easily made by an individual species so that it is possibly not surprising that acclimation by BSC to different climates is by exchange of species rather than in physiology.
Acknowledgments
We are grateful to Antarctica New Zealand (AntNZ) for logistical support over several years as part of the Latitudinal Gradient Project coordinated by Shulamit Gordon. Logistics support was also provided by the Australian Antarctic Program, the Spanish National Antarctic Program and the US Coastguard Reserve. They are all gratefully thanked. The New Zealand Foundation for Research, Science and Technology (FRST), the University of Waikato Vice Chancellor's Fund and the Department of Biological Sciences, University of Waikato provided financial support. The research was supported by the FRST grant, ‘Understanding, valuing and protecting Antarctica's unique terrestrial ecosystems: predicting biocomplexity in Dry Valley ecosystems' and TGAG by the Spanish Education Ministry grants POL2006-08405 and CTM2009-12838-C04-01. BB and CC acknowledge the DFG Schwerpunktprogramm 1158 (BU 666/11-1). Part of the research were also funded by the ERA-Net BiodivERsA program, with the national funders German Research Foundation (DFG), Austrian Science Fund (FWF), The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), and the Spanish Ministerio de Economía y Competitividad (MINECO), part of the 2010-2011 BiodivERsA joint call. We thank Professor Neuhaus (TU Kl) for logistic support in the isotope laboratory.
The authors declare no conflict of interest.
References
- Baur A, Baur B, Fröberg L. Herbivory on calcicolous lichens: different food preferences and growth rates in two co-existing land snails. Oecologia. 1994;98:313–319. doi: 10.1007/BF00324219. [DOI] [PubMed] [Google Scholar]
- Belnap J, Büdel B, Lange OL.2003Biological soil crusts: characteristics and distributionIn: Belnap J, Lange OL, (eds)Biological soil crusts: structure, function, and management. Ecological Studies Vol. 1502nd ednSpringer: Berlin, Heidelberg; 3–30. [Google Scholar]
- Belnap J, Prasse R, Harper KT.2003Influence of biological soil crusts on soil environment and vascular plantsIn: Belnap J, Lange OL (eds)Biological soil crusts: structure, function, and management. Ecological Studies Vol. 150,2nd edn.Springer: Berlin, Heidelberg; 281–302. [Google Scholar]
- Bewley JD, Krochko JE.1982Desiccation-ToleranceIn: Lange OL, Nobel PS, Osmond CB, Ziegler H, (eds)Physiological Plant Ecology 2, Water relations and carbon assimilation, Encyclopedia of Plant Physiology Springer: Berlin; 325–378. [Google Scholar]
- Brodo IM, Sharnoff SD, Sharnoff S.2001PsoraIn: Brodo IM, Sharnoff SD, Láurie-Borque S, (eds)Lichens of North America Yale University Press: New Haven; 597–604. [Google Scholar]
- Brown DH, Snelgar WP, Green TGA. Effects of storage conditions on lichen respiration and desiccation sensitivity. Ann Bot. 1981;48:923–926. [Google Scholar]
- Büdel B.2003Biological soil crusts of European temperate and Mediterranean regionsIn: Belnap J, Lange OL, (eds)Biological soil crusts: structure, function, and management. Ecological Studies Vol. 150,2nd edn.Springer: Berlin, Heidelberg; 75–87. [Google Scholar]
- Büdel B, Darienko T, Deutschewitz K, Dojani S, Friedl T, Mohr K, et al. Southern african biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb Ecol. 2009;57:229–247. doi: 10.1007/s00248-008-9449-9. [DOI] [PubMed] [Google Scholar]
- Büdel B, Vivas M, Lange OL. Lichen species dominance and the resulting photosynthetic behavior of Sonoran Desert soil crust types (Baja California, Mexico) Ecol Proc. 2013;2:6–15. [Google Scholar]
- Campbell SE. Soil stabilization by prokaryotic desert crust: implications for precambrian land biota. Orig Life. 1979;9:335–348. doi: 10.1007/BF00926826. [DOI] [PubMed] [Google Scholar]
- Cannone N, Convey P, Guglielmin M. Diversity trends of bryophytes in continental Antarctica. Polar Biol. 2013;36:259–271. [Google Scholar]
- Carosi R, Giacomini F, Talarico F, Stump E.2007. Geology of the Byrd Glacier Discontinuity (Ross Orogen): New survey data from the Britannia Range, Antarctica. Related Publications from ANDRILL Affiliates. Paper 19, http://digitalcommons.unl.edu/andrillaffiliates/19 .
- Cary CS, McDonald IR, Barrett JE, Cowan DA. On the rocks: the microbiology of Antarctic Dry Valley soils. Nat Rev. 2010;8:129–138. doi: 10.1038/nrmicro2281. [DOI] [PubMed] [Google Scholar]
- Colesie C, Gommeaux M, Green TGA, Büdel B. Biological soil crusts in continental Antarctica: Garwood Valley, southern Victoria Land, and Diamond Hill, Darwin Mountains region. Anta Sci. 2013;26:115–123. [Google Scholar]
- Cowan DA, Green TGA, Wilson AT. Lichen metabolism 1. The use of tritium labeled water in studies of anhydrobiotic metabolism in Ramalina celastri and Peltigera polydactyla. New Phytol. 1979;82:489–503. [Google Scholar]
- Del Prado R, Sancho LG. Dew as a key factor for the distribution pattern of the lichen species Teloschistes lacunosus in the Tabernas Desert (Spain) Flora. 2007;202:417–428. [Google Scholar]
- Demetras NJ, Hogg ID, Banks JC, Adams BJ. Latitudinal distribution and mitochondrial DNA (COI) variability of Stereotydeus spp. (Acari: Prostigmata) in Victoria Land and the central Transantarctic Mountains. Anta Sci. 2010;22:749–756. [Google Scholar]
- Domaschke S, Vivas M, Sancho LG, Printzen C. Ecophysiology and genetic structure of polar versus temperate populations of the lichen Cetraria aculeata. Oecologia. 2013;173:699–709. doi: 10.1007/s00442-013-2670-3. [DOI] [PubMed] [Google Scholar]
- Dudley SA, Lechowicz MJ. Losses of polyol through leaching in subarctic lichens. Plant Physiol. 1987;83:813–815. doi: 10.1104/pp.83.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andrae B, et al. Contribution of crypotogamic covers to the global cycles of carbon and nitrogen. Nat Geosci. 2012;5:459–462. [Google Scholar]
- Farrar JF. Ecological physiology of the lichen Hypogymnia physodes, 4. Carbon allocation at low temperatures; New Phytol. 1978;81:65–69. [Google Scholar]
- Green TGA.2008Lichens in arctic, antarctic and alpine ecosystemsIn: Beck A, Lange OL, (eds)Die ökologische Rolle der Flechten. Rundgespräche der Kommission für Ökologie 36, Bayerische Akademie der Wissenschaften Verlag Dr. Friedrich Pfeil: München; 45–66. [Google Scholar]
- Green TGA, Smith DC. Lichen Physiology: 14. Differences between lichen algae in symbiosis and in isolation. New Phytol. 1974;73:753–766. [Google Scholar]
- Green TGA, Büdel B, Meyer A, Zellner H, Lange OL. Temperate rainforest lichens in New Zealand: light response of photosynthesis. New Zeal J Bot. 1997;35:493–504. [Google Scholar]
- Green TGA, Nash T-H, III, Lange OL.2008Physiological ecology of carbon dioxide exchangeIn: Nash T-H III, (ed)Lichen biology2nd edn.Cambridge University Press: Cambridge; 152–181. [Google Scholar]
- Green TGA, Sancho LG, Pintado A.2011Ecophysiology of Desiccation/Rehydration Cycles in Mosses and LichensIn Lüttge U, Beck E, Barthels D, (eds)Plant Desiccation Tolerance. Ecological Studies 215 Springer: Berlin, Heidelberg; 89–116. [Google Scholar]
- Grime JP.2002Plant strategies, vegetation processes and ecosystem propertiesSecond editionJohn Wiley & Sons Ltd.: West Sussex [Google Scholar]
- Grime JP, Pierce S. The evolutionary strategies that shape ecosystems. Wiley-Blackwell: West Sussex; 2012. [Google Scholar]
- Grube M, Cardinale M, de Castro JV, Müller H, Berg G. Species-specific structural and functional diversity of bacterial communities in lichen symbiosis. ISME J. 2009;3:1105–1115. doi: 10.1038/ismej.2009.63. [DOI] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or to defend. Q Ref Biol. 1992;67:283–335. [Google Scholar]
- Heber U, Lüttge U.2011Lichens and Bryophytes: light stress and photoinhibition in desiccation/rehydration cycles – mechanisms of photoprotectionIn: Lüttge U, Beck E, Barthels D, (eds)Plant Desiccation Tolerance. Ecological Studies 215 Springer: Berlin, Heidelberg; 212–317. [Google Scholar]
- Honegger R. The impact of different long-term storage conditions on the viability of lichen forming Ascomycetes and their green algal photobiont, Trebouxia spp. Plant Biol. 2008;5:324–330. [Google Scholar]
- Hovenden MJ, Jackson AE, Seppelt RD. Field photosynthetic activity of lichens in the Windmill Islands oasis, Wilkes Land, continental Antarctica. Phys Plant. 1994;90:567–576. [Google Scholar]
- Kappen L.1973Response to extreme environments – extreme habitats of lichensIn: Ahmadjian V, Hale ME, (eds)The Lichens Academic press: London; 311–380. [Google Scholar]
- Kappen L. Plant activity under snow and ice, with particular reference to lichens. Arctic. 1993;46:297–302. [Google Scholar]
- Körner C.2003Alpine plant life2nd edn.Springer: Berlin, Heidelberg, New York [Google Scholar]
- Kranner I, Cram WJ, Zorn M, Wornik S, Yoshimura I, Stabentheiner E, et al. Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. PNAS. 2005;102:3141–3146. doi: 10.1073/pnas.0407716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Beckett R, Hochman A, Nash T-H., III Desiccation-tolerance in lichens: a review. Bryologist. 2008;111:576–593. [Google Scholar]
- Lange OL, Kilian E. Reaktivierung der Photosynthese trockener Flechten durch Wasserdampfaufnahme aus dem Luftraum: artspezifisch unterschiedliches Verhalten. Flora. 1985;176:7–23. [Google Scholar]
- Lange OL, Kilian E, Ziegler H. Water vapor uptake and photosynthesis of lichens: Performance differences in species with green and blue-green algae as phycobionts. Oecologia. 1986;71:104–110. doi: 10.1007/BF00377327. [DOI] [PubMed] [Google Scholar]
- Lange OL, Meyer A, Zellner H, Heber U. Photosynthesis and water relations of lichen soil crusts: field measurements in the coastal fog zone of the Namib Desert. Funct ecol. 1994;8:253–264. [Google Scholar]
- Lange OL, Green TGA. Lichens show that fungi can acclimate their respiration to seasonal changes in temperature. Oecologia. 2005;142:11–19. doi: 10.1007/s00442-004-1697-x. [DOI] [PubMed] [Google Scholar]
- Lange OL, Green TGA, Melzer B, Meyer A, Zellner H. Water relations and CO2 exchange of the terrestrial lichen Teloschistes capensis in the Namib fog desert: Measurements during two seasons in the field and under controlled conditions. Flora. 2006;201:268–280. [Google Scholar]
- Melick DR, Seppelt RD. Loss of soluble carbohydrates and changes in freezing point of Antarctic bryophytes after leaching and repated freeze-thaw cycles. Anta Sci. 1992;4:399–404. [Google Scholar]
- Nash T-H, III, White SL, Marsh JE. Lichen and moss distribution and biomass in hot desert ecosystems. Bryologist. 1977;80:470–479. [Google Scholar]
- Øvstedal DO, Smith RIL. Lichens of Antarctica and South Georgia. A guide to their identification and ecology. Cambridge University Press: Cambridge; 2001. [Google Scholar]
- Palmquist K, Dahlman L, Valladares F. CO2 exchange and thallus nitrogen across 75 contrasting lichens associations from different climate zones. Oecologia. 2002;13:295–306. doi: 10.1007/s00442-002-1019-0. [DOI] [PubMed] [Google Scholar]
- Palmquist K, Dahlman L, Jonsson A, Nash T-H., III2008. In: Nash T-H III, (ed)Lichen Biology2nd edn.Cambridge University Press: New York; 183–221. [Google Scholar]
- Pannewitz S, Schlensog M, Green TGA, Sancho LG, Schroeter B. Are lichens active under snow in continental Antarctica. Oecologia. 2003;135:30–38. doi: 10.1007/s00442-002-1162-7. [DOI] [PubMed] [Google Scholar]
- Pardow A, Hartard B, Lakatos M. Morphological, photosynthetic and water relations traits underpin the contrasting success of two lichen groups at the interior and edge forest fragments. AoB Plants. 2010. 2010. p. plq004. [DOI] [PMC free article] [PubMed]
- Pointing SB, Belnap J. Microbial colonization and controls in dryland systems. Nat Rev Microbiol. 2012;10:551–562. doi: 10.1038/nrmicro2831. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. Biomass allocation to leaves, stems and roots: meta-analysis of interspecific variation and environmental control. New Phytol. 2012;193:30–50. doi: 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- Reynolds JF, Stafford-Smith DM. Global Desertification: Do Humans Cause Deserts? Dahlem Workshop Report 88. Dahlem University Press: Berlin; 2002. [Google Scholar]
- Richardson DHS, Smith DC. Lichen physiology; 9: Carbohydrate movement from the Trebouxia symbiont of Xanthoria aureola to the fungus. New Phytol. 1968;67:61–68. [Google Scholar]
- Roser DJ, Melick DR, Ling HU, Seppelt RD. Polyol and sugar content of terrestrial plants from continental Antarctica. Anta Sci. 1992;4:413–420. [Google Scholar]
- Sancho LG, Green TGA, Pintado A. Slowest to fastest: extreme range in lichen growth rates supports their use as an indicator of climate change in Antarctica. Flora. 2007;202:667–673. [Google Scholar]
- Schroeter B, Scheidegger C. Water relations in lichens at subzero temperatures: structural changes and carbon dioxide exchange in the lichen Umbilicaria aprina from continental Antarctica. New Phytol. 1995;131:273–285. [Google Scholar]
- Schroeter B, Green TGA, Pannewitz S, Schlensog M, Sancho LG. Fourteen degrees of latitude and a continent apart: comparison of lichen activity over two years at continental and maritime Antarctic sites. Anta Sci. 2010;22:681–690. [Google Scholar]
- Singh R, Ranjan S, Nayaka S, Pathre UV, Shirke PA. Functional characteristics of a fruticose type of lichen, Stereocaulon foliosum Nyl. in response to light and water stress. Acta Physiol Plant. 2013;5:1605–1615. [Google Scholar]
- Søchting U, Castello M. The polar lichens Caloplaca darbishirei and C. soropelta highlight the direction of bipolar migration. Polar Biol. 2012;35:1143–1149. [Google Scholar]
- Storey BC, Fink D, Hood D, Joy K, Shulmeister J, Riger-Kusk M, et al. Cosmogenic nuclide exposure age constraints on the glacial history of the Lake Wellman area, Darwin Mountains, Antarctica. Anta Sci. 2010;22:603–618. [Google Scholar]
- Tearle PV. Cryptogamic carbohydrate release and microbial response during spring freeze-thaw cycles in Antarctic fellfield fines. Soil Biol Biochem. 1987;19:381–390. [Google Scholar]
- Warren-Rhodes KA, Rhodes KL, Pointing SB, Ewing SA, Lacap DC, Gómez-Silva B, et al. Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microb Ecol. 2006;52:389–398. doi: 10.1007/s00248-006-9055-7. [DOI] [PubMed] [Google Scholar]
- Wynn-Williams DD, Holder JM, Edwards HGM.2000Lichens at the limits of life: past perspectives and modern technologyIn Schroeter B, Schlensog M, Green TGA, (eds)New aspects in cryptogamic research Cramer: Berlin; 275–288. [Google Scholar]