Introduction
The platinum (II) analog, dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt), the oxaliplatin parent complex, is a potent chemotherapeutic with a wide spectrum of anticancer activity, low toxicity and lack of cross resistance in many cisplatin-resistant cancers1,2. However, its clinical use is restrained by its poor solubility, unfavorable pharmacokinetics and various non-target side effects such as, cumulative peripheral distal neurotoxicity and acute dysesthesias3,4. Polymer micelles with intramicellar cross-linking for structural reinforcement and polyanionic core for platinum (II) encapsulation offer a novel macromolecular platform for carrier based delivery of such compounds. These drug-carrier conjugates acquire new physicochemical, biochemical and pharmacological characteristics, thereby offering the possibly of reducing side effects, increasing drug bioavailability at the target sites and minimizing development of drug resistance5,6. In this study block ionomer complexes (BIC) of poly(ethylene oxide)-b-poly(methacrylic acid) (PEO-b-PMA) and divalent metal cations (Ca2+) were utilized as templates for the synthesis of cross-linked (cl)-micelles7,8. DACHPt was loaded into the cross-linked core of the polymeric micelles. The physicochemical properties, loading efficacy, drug release from the polymer micelles and in vitro cytotoxicity of the formulation were investigated.
Experimental
Materials
Poly(ethylene oxide)-b-poly(methacrylic acid) (PEO-b-PMA) diblock copolymer (Mw/Mn = 1.45) was purchased from Polymer Source Inc., Canada. The block lengths were 170 and 180 repeating units for PEO and PMA, respectively. The concentration of carboxylate groups in the copolymer samples was estimated by potentiometric titration. Dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt), silver nitrate (AgNO3), 1,2-ethylenediamine (ED), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), were purchased from Sigma–Aldrich (St Louis, MO) and were used as received. All other chemicals were of reagent grade and used without further purification.
Synthesis of cross-linked polymer micelles
The general procedure for synthesis of cl-micelles has been described earlier 7,8. Briefly, BIC of PEO-b-PMA copolymer and divalent metal cations (PEO-b-PMA/Ca2+) were prepared by mixing aqueous solutions of PEO-b-PMA and CaCl2 at a molar ratio of [Ca2+]/[COO-]=1.3. EDC was added to activate the carboxylic acid groups of PMA followed by cross-linking using aqueous solution of ED (overnight, RT). The extent of degree of cross-linking was controlled by the ratio of amine functional groups to carboxylic acid groups and was targeted to be 20%. EDTA (1.5 molar excess to [Ca2+]) was added after completion of the reaction, followed by dialysis to remove Ca2+ ions and byproducts of the cross-linking reaction.
Preparation of DACHPt loaded cl-micelles
DACHPt (5 mM) was suspended in distilled water and mixed with silver nitrate ([AgNO3]/[DACHPt]=1) for 24 h to form the aqueous complex (dark, RT). AgCl precipitates were removed by centrifuging at 3000 rpm for 10 min followed by filtration through a 0.22 μm filter. Drug loading was performed by simple mixing of aqueous dispersion of cross-linked micelles (cl-micelles) with aqueous solution of DACHPt followed by incubation for 48 h (RT). Unbound DACHPt was removed by ultrafiltration using Centricon® Plus-20 centrifugal filter units (MWCO 30,000Da, Millipore). Initially for loading optimization, drug loading was performed at different pH (6.0, 7.0, 8.0 and 9.0) and a fixed R = 0.5 (where R = molar ratios of DACHPt to carboxylate groups of cl-micelles) and then at fixed pH of 7.0 and varying R (0.25, 0.5, 1, and 1.5). For all further experiments pH of loading was 7.0 and the R was 0.5.
Characterization of cross-linked micelles
Particles were characterized by dynamic light scattering (DLS) (zeta-potential (ζ), effective hydrodynamic diameters (Deff), polydispersity indices (PDI)), and atomic force microscopy (AFM) as previously described7.
Release studies
The release studies were performed in phosphate buffered saline (PBS, pH 7.4, 0.15 M NaCl) or acetate buffered saline (ABS, pH 5.5, 0.15 M NaCl) by dialysis method with membrane of 3,500 Da cutoff. The drug concentrations in loaded micelles, release studies or otherwise were measured by inductively coupled plasma mass spectrometry (ICP-MS).
In vitro cytotoxicity studies
Cells seeded in 96-well plates (10,000 cells/well) 24 h before the experiment treated with drugs (0 to 300 μg/ml DACHPt), drug loaded micelles for 24, 48 or 72 h and then cultured for additional 24 h in drug-free media at 37°C. Cytotoxicity was determined by standard colorimetric MTT assay9 and the IC50 values were calculated using GraphPad Prism Software.
Results and Discussion
Preparation of DACHPt loaded cl-micelles
Polymer micelles with cross-linked ionic cores were prepared by using BIC of PEO-b-PMA copolymer and divalent metal cations as templates7-8. The resulting cl-micelles represented hydrophilic nanospheres of core-shell morphology. The core comprised a network of the cross-linked polyanions (PMA), which was surrounded by the shell of hydrophilic PEO chains. DACHPt was immobilized in the micelles by simple mixing with cl-micelles. DACHPt incorporation into micelles increased from ∼ 12% at pH 6.0 to ∼ 23% at pH 7.0, above which there was only a marginal increase in drug loading (Fig. 1A). However, the formulations prepared at pH > 7.0 were not stable for a prolonged period and resulted in formation of black colored precipitates within 2 days. Therefore pH 7.0 was chosen as the optimal pH value for drug loading. Further, the molar ratio of DACHPt to carboxylate groups (R) of micelles was varied at this optimal pH from ca. 0.25 to 1.5. Above R = 0.5 there was no appreciable increase in drug loading (Fig. 1B) and a significant decrease in loading efficiency (percentage of drug incorporated into the micelles for a given amount added to the mixture). The R = 0.5 was hence chosen as the optimal ratio for DACHPt loading.
Figure 1.
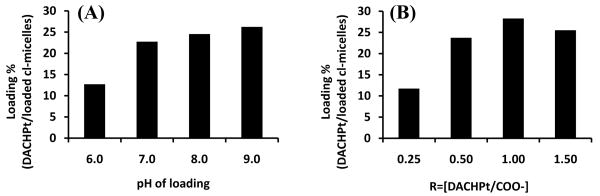
Preparation of DACHPt loaded cl-micelles. (A) Effect of pH on drug loading. (B) Effect of varying molar ratios of DACHPt to carboxylate groups (R) on drug loading.
Physicochemical characterization of cl-micelles
The size, ζ-potential, and composition of micelles were determined by a combination of physicochemical methods (Table 1). The particle had a size below 200 nm and a net negative zeta-potential. The drug loaded micelles were stable for a prolonged period of time and did not exhibit any aggregation for more than a month.
Table 1. Physicochemical properties of DACHPt-loaded cl-micellesa.
| Deff (nm) | PDb | ζ-potential | LCc (%) | LEd (%) | |
|---|---|---|---|---|---|
| Empty cl-micelles | 170 ± 20 | 0.12 | -38 ± 8 | n.a | n.a |
| DACHPt loaded micelles | 154 ± 10 | 0.10 | -30 ± 5 | 25 | 45 |
Values determined at pH 7.0
PD: Polydispersity index
LC: Loading capacity (percentage of drug by weight incorporated into drug loaded micelles)
LE: Loading efficiency (percentage of drug incorporated into the micelles for a given amount added to the mixture)
The morphology of the micelles was further studied by AFM. Both empty (Fig. 2A) and DACHPt loaded cl-micelles (Fig. 2B) appeared to be spherical particles.
Figure 2.
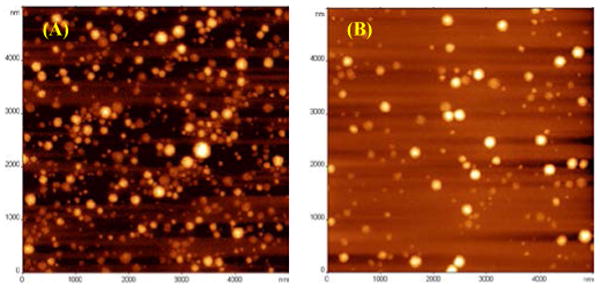
Tapping mode AFM images of cl-micelles in air, (A) empty micelles and (B) DACHPt loaded micelles.
The average height and diameter of particles is shown in table 2. Upon drug incorporation, there was an overall decrease in both height and diameter of the micelles. This could be due to the neutralization of negatively charged PMA chains and a partial collapse of the micelle structure.
Table 2. Dimensions of DACHPt-loaded cl-micelles analyzed by AFM.
| Mean height (nm) | Mean diameter (nm) | |
|---|---|---|
| Empty cl-micelles | 6.17 ± 1.14 | 96.07 ± 57.6 |
| DACHPt loaded micelles | 2.88 ± 0.84 | 91.29 ± 59.9 |
Release of DACHPt from cl-micelles
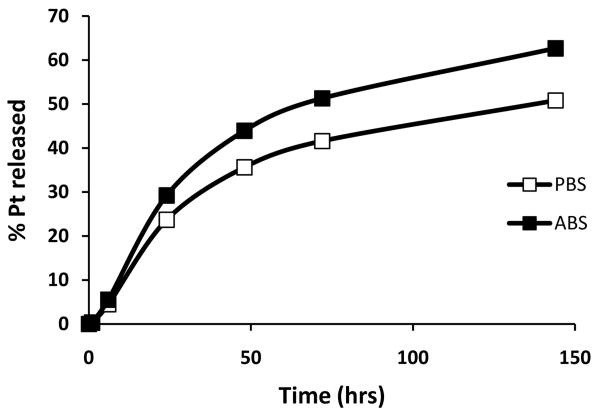
The release behavior of DACHPt from cl-micelles was studied using equilibrium dialysis method, in either phosphate buffered saline (PBS, pH 7.4) or acetate buffer saline (ABS, pH 5.5) at 37 °C (Fig. 3). The drug-carrier interaction was essentially reversible under these conditions with a slow and sustained drug release profile and lack of any burst release. The drug release was responsive to pH as seen by accelerated release under acidic conditions. This is likely due to polyanionic nature of the micelle core, protonation of which under acidic conditions could lead to faster drug release. Compared to physiological pH (7.4), intratumoral space and inflammatory tissues (pH≈6.8-7.2) as well as the endosomal and lysosomal compartments of cells (pH≈4–6) are known to be acidic. In principle, the observed release characteristics are therefore of an advantage. While systemic release of the drug from these micelles can be expected to be slower, a pH triggered accelerated release of the drug payload can be expected when they encounter a low pH in pathological tissue.
Figure 3.
Drug release profile from DACHPt loaded cl-micelles in PBS (□) or ABS (■) at 37 °C.
In vitro cytotoxicity
The cytotoxicity of DACHPt-loaded cl-micelles was evaluated against human ovarian cancer cell line A2780. Cells were exposed to various doses of either drug loaded micelles, free oxaliplatin or empty micelles (0-200 μg/ml on DACHPt basis) for 24 h, 48 h or 72 h. IC50 values for each formulation were calculated based on four parameter log fit model and are summarized in table 3. The cytotoxic activity of DACHPt loaded micelles was solely mediated by the released drug as empty polymer micelles did not affect cell survival at the tested concentration range (up to 100 μg/mL). The cytotoxicity data further provides evidence of the reversible nature of drug interaction with the carrier, with release of biologically active Pt(II) complexes under physiological conditions.
Table 3. In vitro cytotoxicity of free oxaliplatin and DACHPt-loaded micelles against A2780 ovarian cancer cell line (n=3).
| IC50 (DACHPt equivalents in μg/mL) | ||
|---|---|---|
|
| ||
| Treatment | Oxaliplatin | DACHPt loaded micelles |
| 24h | 0.982 ± 0.05 | 0.812 ± 0.11 |
| 48h | 0.726 ± 0.07 | 0.433 ± 0.09 |
| 72h | 0.517 ± 0.15 | 0.114 ± 0.07 |
Conclusions
Hydrophilic polymer micelles with cross-linked ionic cores were evaluated as potential drug carriers of oxaliplatin parent complex, DACHPt. The ionic character of the cores allowed to achieve high level of DACHPt loading (up to 25 w/w%) via polymer-metal complex formation. These DACHPt-loaded cl-micelles were shown to be very stable and exhibited sustained pH-dependent release of platinum. DACHPt-loaded micelles exhibited considerably higher in vitro cytotoxicity than oxaliplatin which increased with exposure time as a result of the release of platinum complexes from cl-micelles. Therefore, we believe that polymer micelles with cross-linked ionic cores are promising supramolecular carriers that may allow to control the biodistribution and pharmacokinetics of the drug to improve the therapeutic outcomes.
Acknowledgments
This work was supported by NIH grant CA 116590.
References
- 1.Lebwohl D, Canetta R. Eur Journal Cancer. 1998;34:1522. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 2.Kidani Y, Inagaki K, Tsukagoshi S. Gann. 1976;67:921. [PubMed] [Google Scholar]
- 3.Hartmann JT, Lipp HP. Expert Opin Pharmacother. 2003;4:889. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- 4.McKeage MJ. Drug Safety. 1996;14:180. [Google Scholar]
- 5.Kataoka K, Harada A, Nagasaki Y. Adv Drug Deliv Rev. 2001;47:113. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 6.Bronich TK. Pharm Res. 2010 doi: 10.1007/s11095-010-0270-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Bronich TK, et al. J Am Chem Soc. 2005;127:8236. doi: 10.1021/ja043042m. [DOI] [PubMed] [Google Scholar]
- 8.Bontha S, et al. J Control Release. 2006;114:163. doi: 10.1016/j.jconrel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann T. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]