Abstract
Background:
Compromised perfusion in autologous breast reconstruction results in fat necrosis and flap loss. Increased flap weight with fewer perforator vessels may exacerbate imbalances in flap perfusion. We studied deep inferior epigastric perforator (DIEP) and muscle-sparing transverse rectus abdominis myocutaneous (MS-TRAM) flaps to assess this concept.
Methods:
Data from patients who underwent reconstruction with DIEP and/or MS-TRAM flaps between January 1, 2010 and December 31, 2011 (n = 123) were retrospectively reviewed. Patient demographics, comorbidities, intraoperative parameters, and postoperative outcomes were collected, including flap fat necrosis and donor/recipient site complications. Logistic regression analysis was used to examine effects of flap weight and perforator number on breast flap fat necrosis.
Results:
One hundred twenty-three patients who underwent 179 total flap reconstructions (166 DIEP, 13 MS-TRAM) were included. Mean flap weight was 658 ± 289 g; 132 (73.7%) were single perforator flaps. Thirteen flaps (7.5%) developed fat necrosis. African American patients had increased odds of fat necrosis (odds ratio, 11.58; P < 0.001). Odds of developing fat necrosis significantly increased with flap weight (odds ratio, 1.5 per 100 g increase; P < 0.001). In single perforator flaps weighing more than 1000 g, six (42.9%) developed fat necrosis, compared to 14.3% of large multiple perforator flaps.
Conclusions:
Flaps with increasing weight have increased risk of fat necrosis. These data suggest that inclusion of more than 1 perforator may decrease odds of fat necrosis in large flaps. Perforator flap breast reconstruction can be performed safely; however, considerations concerning race, body mass index, staging with tissue expanders, perforator number, and flap weight may optimize outcomes.
Reconstruction with autologous tissue remains a sound option for many women following mastectomy.1,2 Among the strengths of autologous reconstruction is achievement of a natural consistency and pleasing aesthetic outcome. However, the main limitation can be donor site morbidity following flap harvest.3 Modifications in surgical technique and flap design using the muscle-sparing transverse rectus abdominus myocutaneous (MS-TRAM) flap and the deep inferior epigastric perforator (DIEP) flap have decreased donor site morbidity.4–8 However, advancements in preserving the integrity of the rectus muscle in the abdominal wall, that is, by focusing on single perforator dissection, may result in compromised flap vascularity and an increase in perfusion-related flap complications.5,9
Fat necrosis is a consequence of either inadequate arterial inflow or relatively poor venous outflow in a DIEP flap. Development of fat necrosis requiring reoperation can result in significant aesthetic deformity and emotional and financial costs: treatment often requires breast imaging studies, tissue biopsy, and/or excision and may cause significant anxiety in patients with a history of breast cancer.
Rates of fat necrosis documented in the literature range widely, from 2% to 62.5%.4,5,9–12 The roles of flap type, perforator number, donor site location, and flap size on the development of fat necrosis and complications continue to be debated in the literature. To our knowledge, no previous study has analyzed the interaction of perforator number and flap weight and their combined effect on fat necrosis in breast reconstruction with abdominal perforator-based free flaps. We hypothesized that increasing flap weight results in increased risk of fat necrosis. We also hypothesized that flaps with larger weights require more perforators to optimize vascular perfusion and that inclusion of more than one perforator may protect larger flaps from fat necrosis. Therefore, we retrospectively reviewed our patients to investigate the associations between flap weight and number of perforators with rates of fat necrosis and other associated complications in DIEP and MS-TRAM free flaps.
PATIENTS AND METHODS
In a study approved by the Johns Hopkins Medicine Institutional Review Board, all patients who underwent breast reconstruction with autologous abdominal perforator free flaps between January 1, 2010 and December 31, 2011 were retrospectively reviewed. Patients who underwent bilateral or unilateral reconstruction with DIEP or perforator-based MS-TRAM were included. Patients were excluded if they underwent reconstruction with true free TRAM or superficial inferior epigastric artery (SIEA) flaps, had <30 days of follow-up, or had an unrecorded flap weight.
We collected patient demographics, comorbidities, and surgical details (Table 1), intraoperative and flap parameters (Table 2), and postoperative complications (Table 3) both at the abdominal and breast sites. Intraoperative parameters included flap weight, perforator number, timing of reconstruction, flap type, and ischemia time. Postoperative outcomes included donor flap fat necrosis along with donor and recipient site complications. Fat necrosis was defined in this study as palpable firmness in the flap tissue lasting at least 3 months postoperatively. All cases of fat necrosis in this study were confirmed by ultrasound. Treatment of fat necrosis was simple observation, percutaneous suctioning with small cannulas, or direct excision. Minor complications of the breast and abdomen included abscess, seroma, hematoma, wound dehiscence or delayed healing requiring conservative management, and infection requiring antibiotics. Additional site-specific breast complications included partial flap failure requiring debridement and mastectomy skin necrosis, whereas abdominal donor site-specific complications included hernia and bulge. Major complications included emergent exploration of the flap and revision of the anastomosis, ICU admission, and venous thromboembolism. Mean follow-up time was 290 days (SD ± 172; range, 36–842).
Table 1.
Patient Demographics, Comorbidities, and Surgical Details
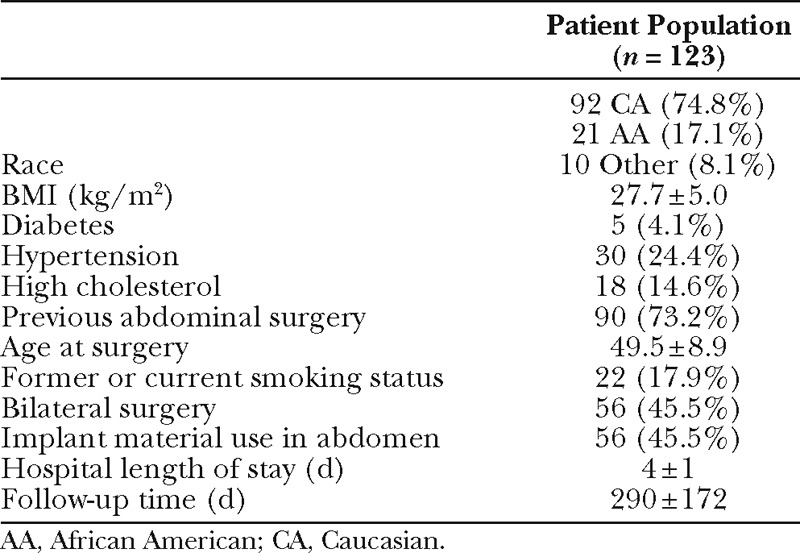
Table 2.
Intraoperative and Flap Parameters
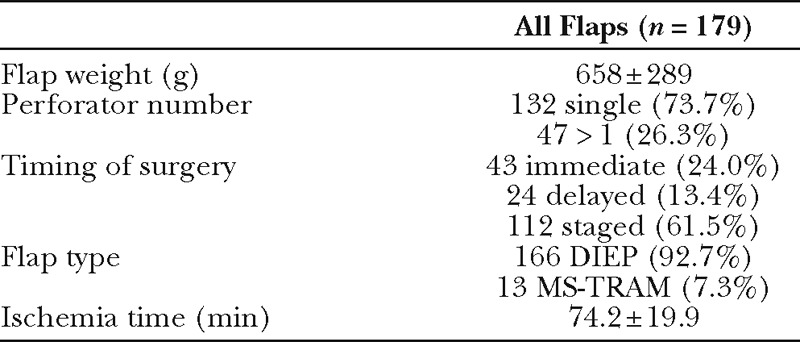
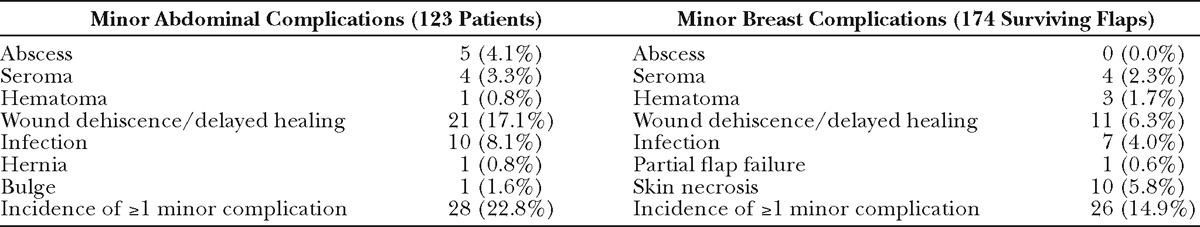
Table 3.
Postoperative Complications at the Abdominal Donor Site and Breast Recipient Site
Statistical Analysis
All data were managed using REDCap Software13 (Version 4.13.1—© 2012 Vanderbilt University). Statistical analysis was performed in Stata, Version 11.0 (StataCorp LP, College Station, TX). Analysis was performed both between patients and between reconstructed breasts. Frequencies were calculated for categorical and binary variables, and mean and standard deviation are provided for continuous variables. Comparisons among groups with or without fat necrosis and with or without incidence of minor donor or recipient site complications were calculated using Fisher’s exact test for categorical and binary variables and the Wilcoxon rank sum test for continuous variables. P-values less than 0.05 were considered statistically significant. For breast-dependent outcomes, standard errors were adjusted for within-patient correlation using clustering for bilateral operations within simple logistic regressions. Odds ratios (ORs) were calculated using simple logistic regressions and are reported with a 95% confidence interval (CI). Multivariate logistic regression was used to compare the odds of fat necrosis among flaps with weight more than 1 kg to those less than 1 kg separately by the number of perforators (1 or 2 or more). Specifically, the model included an indicator for flap weight (more than vs less than 1 kg), an indicator for number of perforators (1 vs 2 or more), and the interaction.
Surgical Technique
DIEP and MS-TRAM flaps in this series were harvested as previously described in the literature.14 Preoperative CT angiograms were routinely performed to aid surgical planning.15 Perforator number was selected by clinical judgment of the attending surgeon(s) to optimize flap harvest and perfusion. Clinical judgment along with temporary vascular clamping was used to identify the minimum number of perforators needed to perfuse the entire flap. Flaps were considered to be MS-TRAM when a small cuff of muscle was taken around a single perforator or between multiple perforators. In all flaps, the surgeon clearly determined and documented the number of perforators. Flaps were weighed before transfer to the recipient site. Flap tissue removed during inset was also weighed and final total flap weight recorded and used in the final comparison of perforator number and flap weight. All flaps were inset in a tension-free closure.
RESULTS
One hundred twenty-three patients who underwent 179 total flap reconstructions were included in the overall analysis (bilateral reconstruction n = 56, unilateral reconstruction n = 67). Median hospital length of stay was 3 days (range, 3–16 d). Characteristics of all 179 flaps are described in Table 2. Of the 47 flaps (26.3%) harvested on multiple perforators, 35 included 2 perforators, 9 included 3 perforators, 2 included 4 perforators, and 1 included 5 perforators. The majority of flaps were DIEP flaps (92.7%) undergoing planned staged reconstruction following removal of tissue expanders (62.6%).
A single flap was not transferred due to pedicle compromise from an undiagnosed DIEA transection related to outpatient gynecological surgery. Four flaps were removed due to thrombosis postoperatively. These five failed flaps were DIEP flaps staged with tissue expanders, 80% of which (n = 4) were single perforator flaps; all patients with failed flaps had undergone previous abdominal surgery. Failed flap weights ranged from 528 to 1075 g (mean, 756 ± 231 g). Failed flaps (n = 5) and patients in whom no flaps survived (n = 3) were removed from analysis of fat necrosis and minor breast complications but were included in overall population descriptions and analysis of abdominal complications. Other major outcomes included venous thromboembolism in 4.9% of patients (n = 6), one of whom was admitted to the ICU and diagnosed with heparin-induced thrombocytopenia (this patient lost both flaps secondary to thrombosis). Two other patients were admitted to the ICU postoperatively for respiratory distress and Clostridium difficile sepsis, respectively.
Individual breast and abdominal complication rates are reported in Table 3. Patients or flaps with at least 1 minor complication were analyzed in aggregate due to small samples of individual outcomes. Patients with and without incidence of minor breast complications were comparable in terms of race, BMI, comorbidities, and age (data not shown). Comparison of flaps with and without minor recipient site complications revealed timing of reconstruction as a protective factor: of the flaps with minor complications, immediate reconstruction comprised a greater proportion and staged reconstruction comprised a smaller proportion, as compared to the total population (OR, 0.52; P = 0.006; Table 4). A comparison of patients with and without incidence of minor abdominal complication (Table 5) revealed BMI as a significant risk factor, with a 1.11 increased odds of abdominal complication per unit increase in BMI (P= 0.014). Hypertension was marginally statistically significant (P = 0.047).
Table 4.
Comparison of All Surviving Flaps (n = 174) with and without Minor Recipient Site Complications
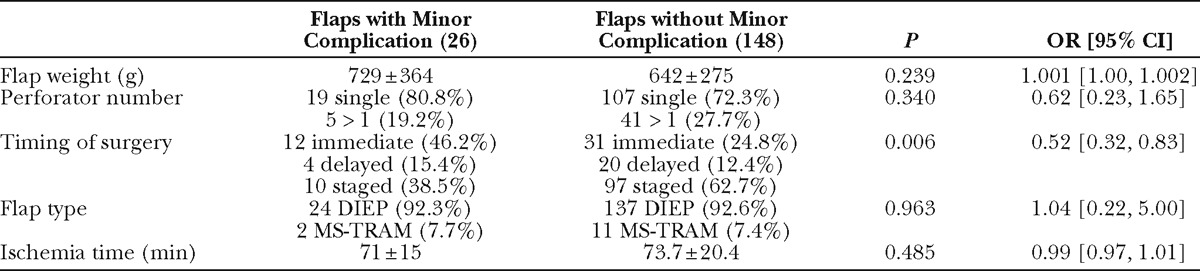
Table 5.
Comparison of All Patients (n=123) with and without Minor Donor Site Complications
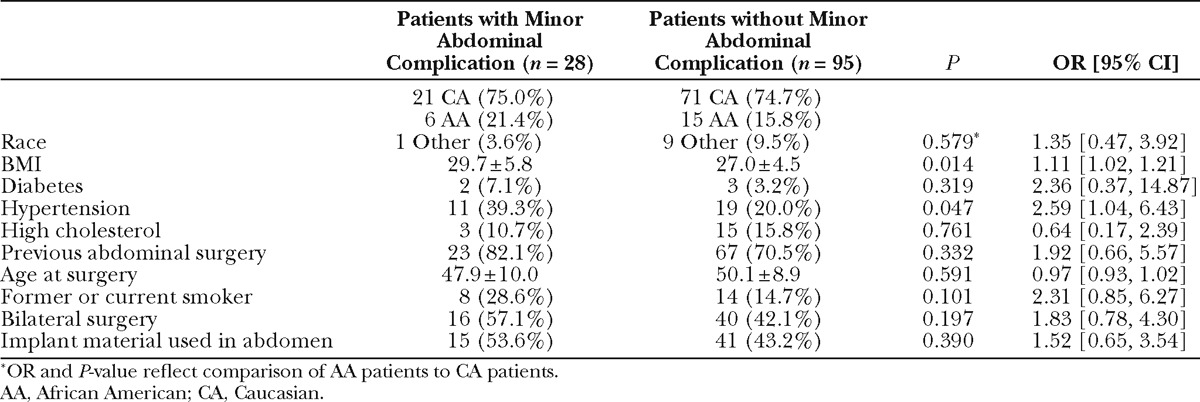
Fat necrosis occurred in 13 flaps in 11 patients. Comparison of patient characteristics in patients with and without incidence of fat necrosis demonstrated an association with race and fat necrosis: the odds of fat necrosis in African American subjects was nearly 12 times the odds for Caucasian subjects (OR, 11.58; P < 0.001; Table 6). The odds of fat necrosis increased with BMI, although this association did not reach statistical significance (P = 0.088). In a multivariate logistic regression including both race and BMI, race was independently associated with fat necrosis (OR, 9.37; P = 0.003). Patients with and without fat necrosis were comparable in relation to comorbidities, age, previous surgery, and proportion of bilateral procedures. Comparison of flap characteristics between flaps with and without fat necrosis revealed flap weight as a highly significant predictor of fat necrosis, with an OR of 1.004 per gram increase in flap weight (P < 0.001, Table 7). This correlates to an OR of 1.5 per 100 g increase in flap weight. Figure 1 depicts weights of flaps with and without incidence of fat necrosis; the trend line indicates flap probability of fat necrosis depending on flap weight. Fat necrosis was also highly related to incidence of another minor breast complication (P =0.009). Forty-six percent of flaps with fat necrosis also had wound dehiscence or delayed wound healing. Interestingly, in the overall patient population, no difference in fat necrosis risk was seen in flaps with one vs multiple perforators. Additionally, although our data did not include perforator location for all flaps, Fisher’s exact analysis of 160 flaps showed no difference in rates of fat necrosis among flaps based on medial vs lateral perforators (P = 0.5).
Table 6.
Comparison of All Patients with Surviving Flaps (n = 120) with and without Fat Necrosis
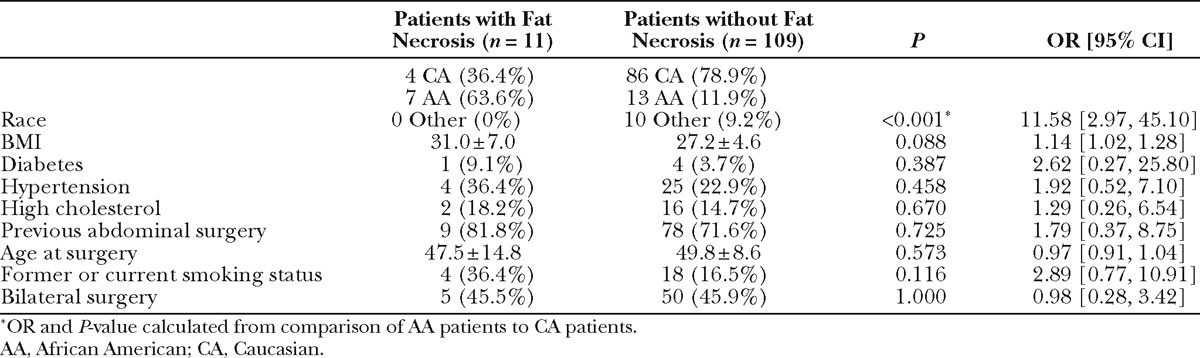
Table 7.
Comparison of All 174 Surviving Flaps with and without Fat Necrosis
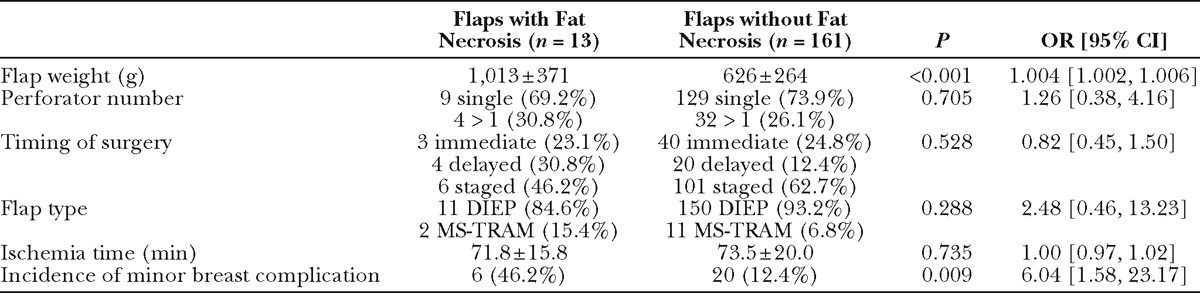
Fig. 1.
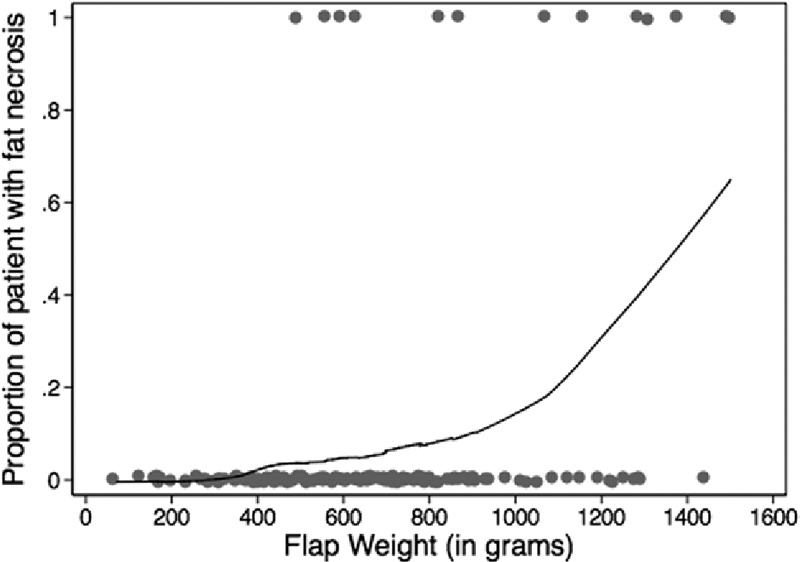
Probability of fat necrosis by flap weight.
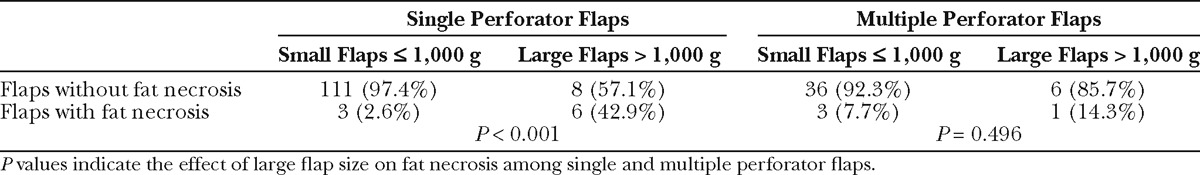
Further analysis was performed by comparing the effect of large flap size (flaps > 1000 g) on fat necrosis among single perforator flaps and flaps with more than 1 perforator. Among single perforator flaps, 42.9% of flaps weighing more than 1000 g had fat necrosis, compared to only 2.6% of flaps weighing less than or equal to 1000 g. The effect of large flap size was significant (P < 0.001, Table 8). Among flaps with multiple perforators, 14.3% of flaps weighing more than 1000 g had fat necrosis, and the effect of large flap size was not significant (P = 0.496). Logistic regression of fat necrosis on perforator number, more than 1000 g flap size, and the interaction of perforator number and flap size was performed. Among single perforator flaps, odds of fat necrosis was 27.8 in large flaps compared to flaps less than or equal to 1000 g (P < 0.001), whereas the OR of multiple perforator flaps more than 1000 g compared to flaps less than or equal to 1000 g was 1.9, showing no significance. A comparison among large flaps (>1000 g) suggested that inclusion of 2 or more perforators may be protective compared to single perforator flaps, but due to the low incidence of fat necrosis and subsequently small sample size, this was not a statistically significant finding (OR = 0.22, P = 0.173). However, the interaction of flap weight and perforator number trended toward significance (P= 0.059).
Table 8.
Comparison of Flap Size and Fat Necrosis in Single and Multiple Perforator Flaps
DISCUSSION
Reconstruction with autologous tissue is an integral part of treatment for many women with breast cancer.1 As such, flaps must be designed to optimize reconstructive outcomes. Knowledge of flap characteristics, including flap size and perforator number, will allow surgeons to minimize fat necrosis, which can have important and lasting negative effects in breast reconstruction.
The effect of perforator number on fat necrosis has been widely debated in the literature. Recent studies by Baumann et al11 and Bozikov et al16 concluded that the risk of fat necrosis increases with decreasing perforator number. Similarly, studies have shown increased fat necrosis in DIEP flaps, compared to free TRAM flaps, potentially due to fewer perforators and a less robust blood supply.12,17 Still other studies argue that perforator number is not a significant predictor of fat necrosis.18 Each of these studies lacked analysis of flap size and weight. Nahabedian et al4 stated that they use TRAM flaps in women with estimated breast volume more than 1000 ml because they believe that flaps of that size may be at increased risk of inadequate perfusion from a DIEP flap that includes only 1 to 3 perforators. This practice is based on unpublished experience, and the authors state that data regarding large-volume 1- or 2-perforator DIEP flaps have not been established. In this study, we sought to elicit the relationship between flap weight, perforator number, and fat necrosis and to determine whether the effect of one depends on the other.
Our study population included 13 flaps that were categorized as MS-TRAM flaps. In each of these MS-TRAM flaps, the surgeon clearly determined and documented the number of perforators. Twelve of these flaps contained 2 perforators and 1 flap was based on a single perforator. These flaps were included based on our conclusion that the inclusion of a small cuff of muscle in these flaps does not add any meaningful variation to the main variables, perforator number, and flap weight. Previous studies have indicated that the degree of muscle-sparing does not significantly impact the outcomes of fat necrosis.4,9,18 Studies have further specified that comparison of MS-2 to MS-3 (completely muscle-sparing DIEP flaps) shows no difference in flap-related outcomes.4,19,20
Our results revealed a marked increase in risk of fat necrosis with increasing flap weight, particularly in flaps weighing more than 1 kg (Fig. 1). Flap weight and BMI are highly correlated (P < 0.001), providing explanation for the trend of increased BMI in patients with fat necrosis. We hypothesize that the relationship between flap size and fat necrosis is related to both vascular perfusion and drainage. Although all efforts were made to close flaps in a tension-free major and to preserve the integrity of the vascular pedicle, it could be that increased flap weight compromises venous drainage due to the location of the pedicle at the bottom of the flap. However, we did not further examine the role of venous drainage in our study. To further elicit the role of vascular perfusion, we stratified our analysis of perforator number among flaps of different sizes. To our knowledge, flap weight and correlation with fat necrosis data were not analyzed in previous studies that examined the effects of perforator number on fat necrosis. After choosing to categorize flaps as either large (>1000 g) or small (≤1000 g) based on the marked increase in the proportion of flaps with fat necrosis portrayed in Figure 1, we compared these flap groups.
Current practice in many institutions where the DIEP flap procedure is available is to include as few perforators that adequately perfuse the flap as possible. Poiseuille’s law dictates that a single large perforator will have better flow than multiple small perforators by a factor of (Δr)4 where Δr represents the differential between the radii of the vessels. Determination of adequate perfusion is based on clinical judgment of capillary refill, dermal bleeding, and flap color. Intraoperative judgment is insufficient in some cases, as evidenced by the development of fat necrosis. We suggest that a single perforator may not be adequate in large flaps weighing more than 1 kg, based on the high incidence of fat necrosis among single perforator flaps more than 1000 g seen in this study (42.9%). Our data, in which only 1 of 7 large multiple perforator flaps had fat necrosis, suggest that inclusion of more than 1 perforator may be protective in flaps of that size. We suggest that use of a multiple perforator flap may be appropriate when the reconstruction volume is more.
Our study population revealed an increased risk of fat necrosis in African American patients nearly 12 times that of Caucasian patients. This increased risk was not due to confounding by BMI. Our clinical experience suggests that darker skin tone may increase the difficulty of clinically evaluating perfusion and venous drainage.21 We hypothesize that this may contribute to the increased odds of fat necrosis among African American patients.
The strengths of this study include accurate measurement of flap weight in all flaps before anastomosis and insetting at the recipient site. Limitations include the retrospective design, clinical bias of perforator selection, and uncertainty in identifying fat necrosis. We attempted to control for the latter by confirming all cases of fat necrosis with imaging, biopsy, or excision. The greatest limitation is the small sample size of flaps with fat necrosis due to the low incidence rate (7.5%), limiting our ability to perform robust analysis of the interaction of flap weight and perforator number.
CONCLUSIONS
Autologous breast reconstruction with perforator flaps comes with the risk of fat necrosis. African American patients had an increased risk of fat necrosis. Free DIEP flaps for breast reconstruction with increasing weight are at a significantly increased risk of fat necrosis, with odds increasing by 1.5 for every 100 g increase in flap weight. Incidence of fat necrosis was also highly correlated with wound healing complications at the recipient site. Among large flaps in this study, flaps with more than 1 perforator had a lower incidence of fat necrosis compared to single perforator flaps. As such, DIEP flaps may be optimized when balancing perforator number and increasing flap weight. A greater sample of flaps with fat necrosis may prove that inclusion of more than 1 perforator significantly decreases odds of fat necrosis among large flaps, as suggested by the data in this study. Perforator flap breast reconstruction can be performed safely; however, considerations concerning race, BMI, deliberate staging with tissue expanders, perforator number, and flap weight may potentially optimize outcomes.
ACKNOWLEDGMENTS
Publication of this article was funded in part by the Open Access Promotion Fund of the Johns Hopkins University Libraries.
Justin M. Sacks, MD, FACS
Department of Plastic and Reconstructive Surgery
Johns Hopkins University School of Medicine
601 N. Caroline Street, JHOC 8140D
Baltimore, MD 21287
E-mail: jmsacks@jhmi.edu
Footnotes
Disclosure: Justin M. Sacks is a speaker and consultant for LifeCell Corporation. The authors have no financial interest to declare in relation to the content of this article. No industry or grant monies were used to fund this study. No devices or products were used to conduct this study. Publication of this article was funded fully by the Open Access Promotion Fund of the Johns Hopkins University Libraries. This study was supported by the Johns Hopkins Department of Plastic and Reconstructive Surgery. The Article Processing Charge was paid for by the Open Access Promotion Fund of the Johns Hopkins University Libraries.
This study was approved by The Johns Hopkins Medicine Institutional Review Board.
REFERENCES
- 1.Gopie JP, Hilhorst MT, Kleijne A, et al. Women’s motives to opt for either implant or DIEP-flap breast reconstruction. JPlast Reconstr Aesthet Surg. 2011;64:1062–1067. doi: 10.1016/j.bjps.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 2.Alderman AK, Wilkins EG, Lowery JC, et al. Determinants of patient satisfaction in postmastectomy breast reconstruction. Plast Reconstr Surg. 2000;106:769–776. doi: 10.1097/00006534-200009040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Rosson GD, Magarakis M, Shridharani SM, et al. A review of the surgical management of breast cancer: plastic reconstructive techniques and timing implications. Ann Surg Oncol. 2010;17:1890–1900. doi: 10.1245/s10434-010-0913-7. [DOI] [PubMed] [Google Scholar]
- 4.Nahabedian MY, Momen B, Galdino G, et al. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475. doi: 10.1097/00006534-200208000-00015. discussion 476–477. [DOI] [PubMed] [Google Scholar]
- 5.Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg. 2009;124:752–764. doi: 10.1097/PRS.0b013e31818b7533. [DOI] [PubMed] [Google Scholar]
- 6.Blondeel N, Vanderstraeten GG, Monstrey SJ, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg. 1997;50:322–330. doi: 10.1016/s0007-1226(97)90540-3. [DOI] [PubMed] [Google Scholar]
- 7.Futter CM, Webster MH, Hagen S, et al. A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Br J Plast Surg. 2000;53:578–583. doi: 10.1054/bjps.2000.3427. [DOI] [PubMed] [Google Scholar]
- 8.Rozen WM, Rajkomar AK, Anavekar NS, et al. Post-mastectomy breast reconstruction: a history in evolution. Clin Breast Cancer. 2009;9:145–154. doi: 10.3816/CBC.2009.n.024. [DOI] [PubMed] [Google Scholar]
- 9.Andrades P, Fix RJ, Danilla S, et al. Ischemic complications in pedicle, free, and muscle sparing transverse rectus abdominis myocutaneous flaps for breast reconstruction. Ann Plast Surg. 2008;60:562–567. doi: 10.1097/SAP.0b013e31816fc372. [DOI] [PubMed] [Google Scholar]
- 10.Peeters WJ, Nanhekhan L, Van Ongeval C, et al. Fat necrosis in deep inferior epigastric perforator flaps: an ultrasound-based review of 202 cases. Plast Reconstr Surg. 2009;124:1754–1758. doi: 10.1097/PRS.0b013e3181bf7e03. [DOI] [PubMed] [Google Scholar]
- 11.Baumann DP, Lin HY, Chevray PM. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2010;125:1335–1341. doi: 10.1097/PRS.0b013e3181d4fb4a. [DOI] [PubMed] [Google Scholar]
- 12.Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:576–583. doi: 10.1097/00006534-200009030-00008. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkat R, Lee JC, Rad AN, et al. Bilateral autologous breast reconstruction with deep inferior epigastric artery perforator flaps: review of a single surgeon’s early experience. Microsurgery. 2012;32:275–280. doi: 10.1002/micr.21948. [DOI] [PubMed] [Google Scholar]
- 15.Rosson GD, Williams CG, Fishman EK, et al. 3D CT angiography of abdominal wall vascular perforators to plan DIEAP flaps. Microsurgery. 2007;27:641–646. doi: 10.1002/micr.20423. [DOI] [PubMed] [Google Scholar]
- 16.Bozikov K, Arnez T, Hertl K, et al. Fat necrosis in free DIEAP flaps: incidence, risk, and predictor factors. Ann Plast Surg. 2009;63:138–142. doi: 10.1097/SAP.0b013e31818937d4. [DOI] [PubMed] [Google Scholar]
- 17.Sailon AM, Schachar JS, Levine JP. Free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps for breast reconstruction: a systematic review of flap complication rates and donor-site morbidity. Ann Plast Surg. 2009;62:560–563. doi: 10.1097/SAP.0b013e31819faf0d. [DOI] [PubMed] [Google Scholar]
- 18.Garvey PB, Salavati S, Feng L, et al. Perfusion-related complications are similar for DIEP and muscle-sparing free TRAM flaps harvested on medial or lateral deep inferior epigastric Artery branch perforators for breast reconstruction. Plast Reconstr Surg. 2011;128:581e–589e. doi: 10.1097/PRS.0b013e318230c122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeishi M, Fujimoto M, Ishida K, et al. Muscle sparing-2 transverse rectus abdominis musculocutaneous flap for breast reconstruction: a comparison with deep sinferior epigastric perforator flap. Microsurgery. 2008;28:650–655. doi: 10.1002/micr.20563. [DOI] [PubMed] [Google Scholar]
- 20.Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: is there a difference? Plast Reconstr Surg. 2005;115:436–444. doi: 10.1097/01.prs.0000149404.57087.8e. discussion 445–446. [DOI] [PubMed] [Google Scholar]
- 21.Rosson GD, Singh NK. Innovative microsurgery: interesting scenarios in perforator flap breast reconstruction: the bullet proof flap, contralateral IMA, adding the SIEV, and 3D CT scans. Reconstr Microsurg Newslett. 2006;17:13–15. [Google Scholar]


