Abstract
Background:
Measurement of transcutaneous oxygen pressure (TcPO2) and transcutaneous carbon dioxide pressure (TcPCO2) has been used for free flap monitoring. Because these values are obtained with sensor probes heated to 44°C, there is potential for low-temperature burns on skin flaps. We measured TcPO2 and TcPCO2 at 37°C in both animals and humans to determine the feasibility and safety of the procedure as a postoperative flap monitoring method.
Methods:
Twelve epigastric island flaps were elevated in rabbits, and TcPO2 and TcPCO2 were measured at 37°C before and after ligation of the pedicles. In addition, TcPO2 and TcPCO2 at 37°C were measured in healthy men. Subsequently, the method was applied to postoperative monitoring of free flaps in 49 clinical cases.
Results:
TcPO2 and TcPCO2 values were significantly affected by the experimental flap elevation. A rapid increase in TcPCO2 was observed with both arterial and venous occlusion. In the healthy men, TcPO2 and TcPCO2 were measurable at all skin surface sites. In the clinical cases of free flap transfer, TcPO2 values remained very low for at least 72 hours. TcPCO2 values ranged from 40 to 70 mm Hg for 72 hours in more than 80% of cases. In 2 cases, TcPCO2 values increased to more than 90 mm Hg, and exploration surgery was performed. These compromised flaps were saved by reanastomosis of the veins.
Conclusions:
Continuous monitoring of TcPCO2 at 37°C can provide objective information and alert doctors and nurses to the need for checking the free flap.
The success rate for free tissue transfer is now more than 95%1,2 because of improved procedures and increased knowledge of vascular supply patterns of the flaps. Initial success rates are approaching 100%. Nevertheless, a transferred flap can be lost, and microsurgeons expend considerable effort to salvage the failing flap or at least manage the flap conservatively.3 The importance of postoperative flap monitoring for early detection of circulatory disturbance is therefore understood. Although many monitoring methods have been reported, the ideal monitoring method for detecting flap compromise has not been determined.4
Flap monitoring methods include transcutaneous oxygen pressure (TcPO2) measurement5,6 and transcutaneous carbon dioxide pressure (TcPCO2) measurement.7 The instrument for measuring TcPO2 and TcPCO2 was originally developed to measure partial pressure of arterial oxygen (PaO2) and partial pressure of arterial carbon dioxide (PaCO2) with a skin probe heated to 44°C. However, a low-temperature skin burn can result when TcPO2 and TcPCO2 are monitored continuously at 44°C.5,7
The aim of this 3-part study was to investigate the feasibility of monitoring TcPO2 and TcPCO2 in skin flaps with a probe heated to 37°C. TcPO2 and TcPCO2 were first measured at 37°C in rabbit epigastric island flaps that were subjected to arterial or venous occlusion. TcPO2 and TcPCO2 were then measured at 37°C at 8 different surface skin sites in healthy human adults. Subsequently, a clinical study was conducted in which TcPO2 and TcPCO2 were measured at 37°C in 49 free flaps, two of which proved to be compromised.
MATERIALS AND METHODS
Measurements in the experimental and clinical studies were performed with a TCM4 monitor (Radiometer Medical ApS, Copenhagen, Denmark). TcPO2 and TcPCO2 are measured simultaneously with the device’s sensor; the probe, which allows measurement of TcPO2 and TcPCO2 at the same site and the same time, contains a Clark-type electrode for measurement of TcPO2 and a pH-sensitive glass electrode for measurement of TcPCO2. The probe, which was fixed to the skin surface with adhesive tape, was thermostatically adjusted to 37°C. Room temperature was controlled between 18°C and 24°C during measurements in both the animal and clinical studies. Experimental procedures were approved by the institutional animal care and use committee of The University of Tokushima and were carried out in accordance with committee guidelines. The human subjects were fully informed of the reason for and the importance of TcPO2 and TcPCO2 measurement.
Part 1: Animal Experiment For Testing The Effects Of Flap Elevation And Pedicle Occlusion By Low-Temperature Measurement Of Tcpo2 And Tcpco2
Twelve male Japanese white rabbits weighing 3.0–3.5 kg were used for the study. All were maintained under standard housing conditions and allowed water and standard dry rabbit feed ad libitum. The rabbits were anesthetized with intravenous pentobarbital (Somnopentyl) (64.8 mg/mL, Kyoritsu Seiyaku, Tokyo, Japan), and an epigastric island flap (17 cm × 5 cm) was elevated in each rabbit on the basis of the superficial inferior epigastric artery and vein, according to a well-described technique.8–10 After the flap was sewn back into its original place, the probe was placed on the skin 4 cm away from the distal edge of the flap. The animals were divided into 2 groups for arterial or venous occlusion produced by ligation of the superficial inferior epigastric artery or vein. TcPO2 and TcPCO2 were measured immediately before and 20 minutes after flap elevation (before ligation of the vascular pedicle) and at 5, 10, 20, 30, 60, 90, and 120 minutes after ligation of the vascular pedicle.
Part 2: Study Of Low-Temperature Tcpo2 And Tcpco2 Measurement At Different Body Sites In Healthy Humans
We measured TcPO2 and TcPCO2 at 8 superficial sites in 10 healthy male volunteers (age range, 24–36 y; mean age, 30.4 y): the forehead, cheek, back, abdomen, volar aspect of the forearm, lateral aspect of the thigh, posterior aspect of the leg, and anterior aspect of the tibia. We did this to investigate whether measurement of these values with a probe adjusted thermostatically to 37°C was possible at various donor sites.
Part 3: Low-Temperature Monitoring Of Tcpo2 And Tcpco2 In Clinical Cases Of Free Flap Transfer
This part of the study comprised 49 patients [32 men and 17 women ranging in age from 19 to 84 y (mean, 54.8 y)] undergoing free flap transfer at Tokushima University Hospital between January 2002 and July 2011. All 49 patients were of normal nutritional status before surgery. The free flaps comprised 23 anterolateral thigh flaps, 10 latissimus dorsi musculocutaneous flaps, 5 fibular osteocutaneous flaps, 4 medial plantar flaps, 4 thoracodorsal artery perforator flaps, 1 radial forearm flap, 1 deep inferior epigastric perforator flap, and 1 transverse rectus abdominis musculocutaneous flap. TcPO2 and TcPCO2 were measured continuously for at least the first 72 hours after the transfer. That is, values were recorded immediately upon the patient’s postsurgical transfer to the intensive care unit and then every hour on postoperative day 1, every 2 hours on postoperative day 2, every 4 hours on postoperative day 3, and thereafter.
STATISTICAL ANALYSIS
Measured values are shown as median (range). In the animal flap experiment, differences between values before and after flap elevation and differences between values before and after ligation of vascular pedicles were analyzed by Wilcoxon signed-rank test for nonparametric data. In the clinical free flap study, values recorded at 24, 48, and 72 hours after the transfer were compared to values recorded immediately after the transfer, and differences were analyzed by Wilcoxon signed-rank test for nonparametric data.
RESULTS
Part 1: Animal Experiment for Testing the Effects of Flap Elevation and Pedicle Occlusion by Low-temperature Measurement of TcPO2 and TcPCO2
In the rabbit epigastric flaps subjected to arterial occlusion (n = 6), TcPO2 before flap elevation [44.5 (30–68) mm Hg] decreased significantly to 3.5 (2–8) mm Hg after elevation (P = 0.031). After arterial ligation, TcPO2 decreased significantly to 2 (1–3) mm Hg at 5 minutes (P = 0.031), and it remained below 2.0 mm Hg to the end of the study period. In this group, TcPCO2 before flap elevation [57 (53–76) mm Hg] increased significantly to 85.5 (67–105) mm Hg after elevation (P = 0.031). After arterial ligation, TcPCO2 increased significantly to 98 (71–120) mm Hg at 5 minutes (P = 0.031), and it increased gradually to as much as 145.5 (128–168) mm Hg by 90 minutes (Fig. 1A).
Fig. 1.
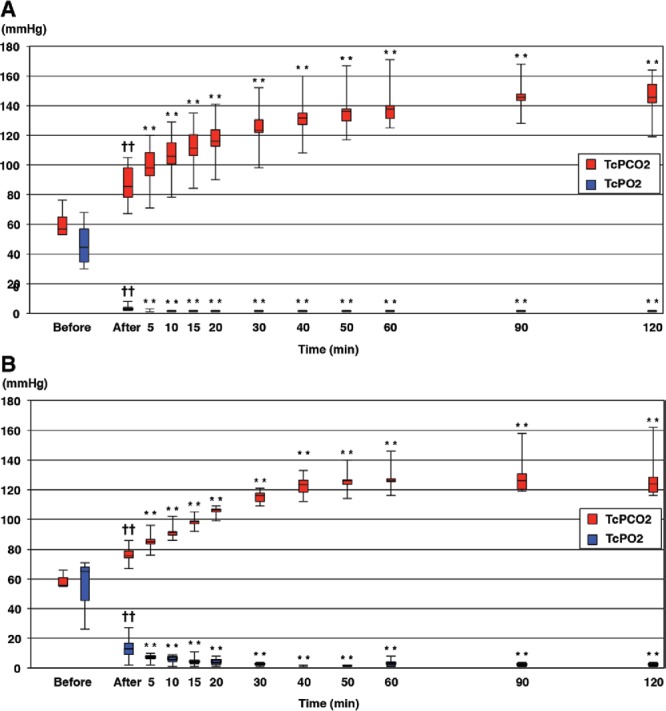
TcPO2 and TcPCO2 values in rabbit epigastric flaps. Values are expressed as median and range. “Before” refers to before flap elevation, “after” refers to 20 min after flap elevation (before ligation of vascular pedicle), and “time” refers to elapsed time after ligation of vascular pedicle. A, Graph showing TcPO2 and TcPCO2 values before and after rabbit epigastric flap elevation and arterial occlusion. B, Graph showing TcPO2 and TcPCO2 values before and after rabbit epigastric flap elevation and venous occlusion. ††P < 0.01 vs values before flap elevation, by Wilcoxon signed-rank test. **P < 0.01 vs values before ligation of the artery or vein, by Wilcoxon signed-rank test.
In the flaps subjected to venous occlusion (n = 6), TcPO2 before flap elevation [65 (26–71) mm Hg] decreased significantly to 13 (2–27) mm Hg after elevation (P = 0.031). After venous ligation, TcPO2 decreased significantly to 7 (2–10) mm Hg at 5 minutes (P = 0.031), and it remained below 7.0 mm Hg to the end of the study period. In this group, the TcPCO2 value before flap elevation [56 (55–66) mm Hg] increased significantly to 75.5 (67–86) mm Hg after elevation (P = 0.031). After venous ligation, TcPCO2 increased significantly to 85 (76–96) mm Hg at 5 minutes (P = 0.031), and it continued to increase to as much as 126 (119–158) mm Hg by 90 minutes (Fig. 1B).
Part 2: Study of Low-temperature TcPO2 and TcPCO2 Measurement at Different Body Sites in Healthy Adults
Among the 10 healthy subjects in whom different monitoring sites were investigated, TcPO2 was lower on the face than at other sites. TcPCO2 was higher on the face (up to 70 mm Hg) than elsewhere (values elsewhere never exceeded 60 mm Hg) (Fig. 2).
Fig. 2.
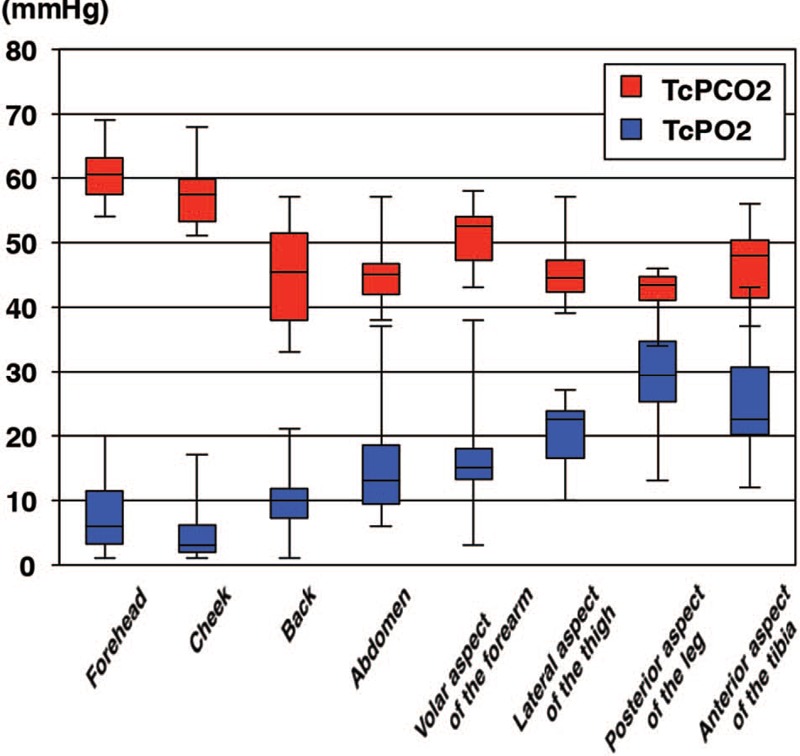
Boxplot showing TcPO2 and TcPCO2 values (median, upper and lower quartiles, and range) at 8 different sites in healthy adults. Values were obtained with probes thermally controlled at 37°C.
Part 3: Low-temperature Monitoring of TcPO2 and TcPCO2 in Clinical Cases of Free Flap Transfer
None of the 49 patients who underwent free flap transfer died or suffered a critical setback owing to the original defect during the intraoperative period. There were no complications associated with use of the probe, and presence of the probe elicited no complaints from the patients.
Median TcPO2 was 4 (1–47) mm Hg immediately after flap transfer (Fig. 3A). TcPO2 values immediately after transfer and at 24, 48, and 72 hours after transfer were below 10 mm Hg in 71.4%, 87.8%, 86.7%, and 93.9% of patients, respectively. Median TcPCO2 was 56 (40–85) mm Hg immediately after flap transfer (Fig. 3B). TcPCO2 values immediately after transfer and at 24, 48, and 72 hours after transfer ranged between 40 and 70 mm Hg in 81.7%, 85.4%, 93.3%, and 84.8% of patients, respectively. TcPO2 and TcPCO2 values at 24, 48, and 72 hours after transfer decreased compared with the values immediately after transfer.
Fig. 3.
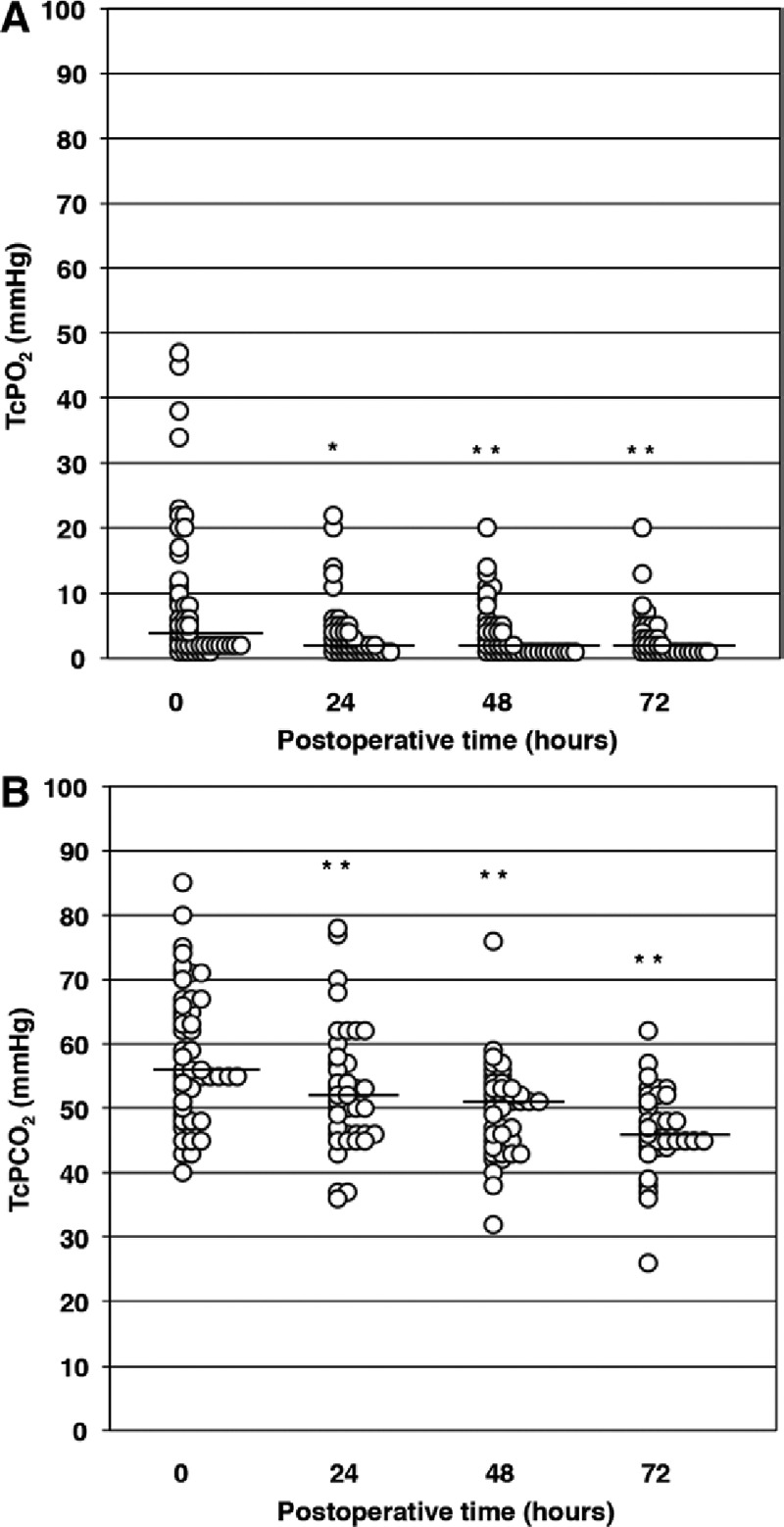
Clinical postoperative TcPO2 and TcPCO2 values obtained with probes thermally controlled at 37°C. Circles represent individual patients. A, Graph of TcPO2 values obtained immediately after surgery (0 h) and at 24-h intervals thereafter. Bars indicate the median values at each time point. B, Graph of TcPCO2 values obtained immediately after surgery (0 h) and at 24-h intervals thereafter. Bars indicate the median values at each time point. *P < 0.05 vs values at 0 h (ie, immediately after surgery), by Wilcoxon signed-rank test. **P < 0.01 vs values at 0 h (ie, immediately after surgery), by Wilcoxon signed-rank test.
The lowest and highest TcPO2 and TcPCO2 values obtained during the first 72 hours after transfer in each case are shown in Figures 4A and B, respectively. The lowest TcPO2 values ranged from 0 to 20 mm Hg and were less than 10 mm Hg in 96% (47/49) of flaps. The lowest TcPCO2 values ranged from 23 to 64 mm Hg. The highest TcPO2 values ranged from 2 to 47 mm Hg, and the highest TcPCO2 values ranged from 45 to 112 mm Hg. Two cases in which the TcPCO2 values increased to 92 and 112 mm Hg required exploration.
Fig. 4.
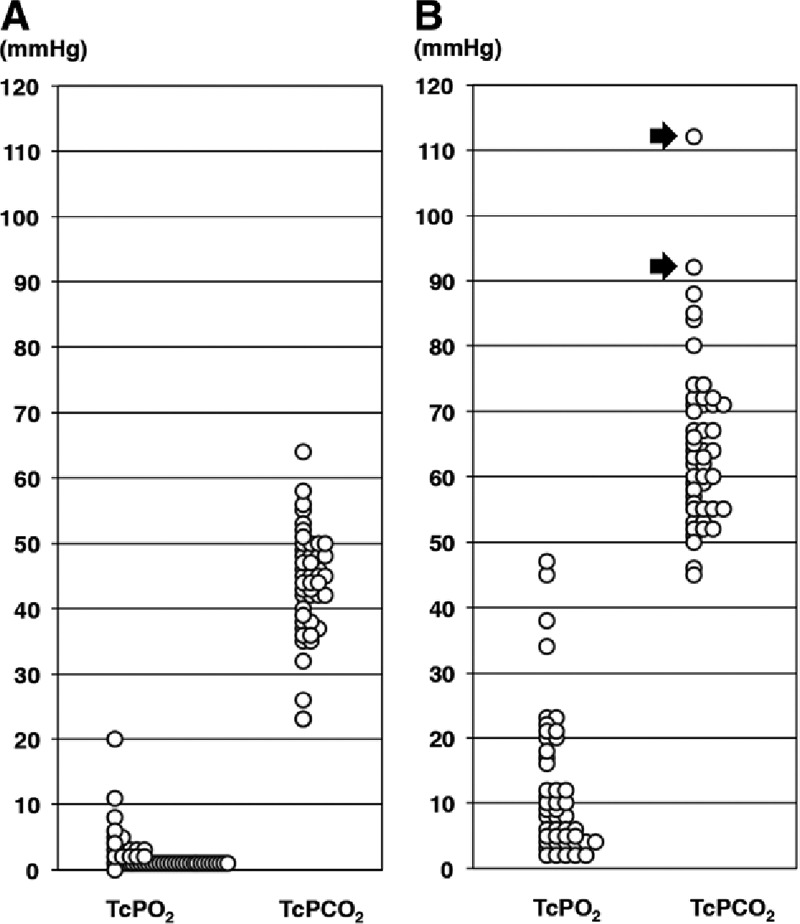
Highest and lowest clinical TcPO2 and TcPCO2 values measured at 37°C. Circles represent individual patients. A, Graph showing the lowest TcPO2 and TcPCO2 values in the 49 free flaps. B, Graph showing the highest TcPO2 and TcPCO2 values in the 49 free flaps. Two patients with a TcPCO2 value more than 90 mm Hg (arrows) underwent exploration.
A sudden increase in TcPCO2 was noted postoperatively in 2 patients, one at 6 hours and the other at 60 hours after flap transfer (Table 1 and Figs. 5–7). Immediate exploration was performed, and venous thrombi were removed in both cases. TcPCO2 decreased immediately after venous reanastomosis. The transferred flaps were rescued and fully survived. All flaps that survived the initial operation showed TcPCO2 values below 88 mm Hg. We experienced only 2 cases of flap compromise; however, both positive predictive value and negative predictive value were 100% when TcPCO2 exceeding 90 mm Hg was used to determine that revision surgery was necessary (Fig. 4B). We could not confirm significant changes in TcPO2 values after the reanastomoses because the values remained below 10 mm Hg. Accordingly, the decrease in TcPO2 values could not be used to detect the circulatory disturbance.
Table 1.
Details of the 2 Cases Requiring Exploration

Fig. 5.
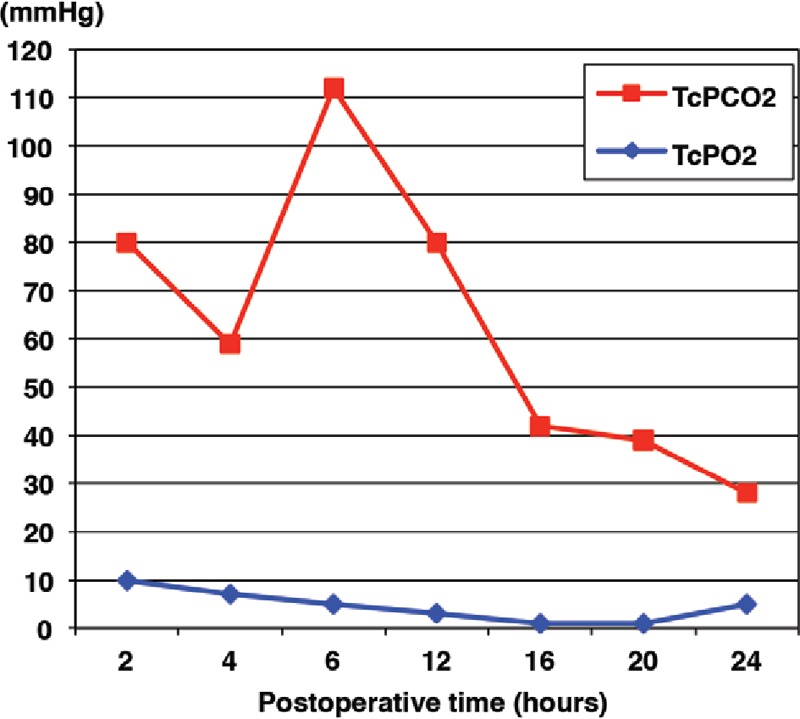
Graph showing TcPO2 and TcPCO2 values after transfer in a case requiring exploration. Serious circulatory disturbance was suspected because of the sudden increase in TcPCO2 to more than 90 mm Hg 6 h after flap transfer. Exploration was performed immediately, and a venous thrombus was removed. TcPCO2 decreased promptly, and the flap survived completely. TcPO2 did not change significantly before or after exploration.
Fig. 7.
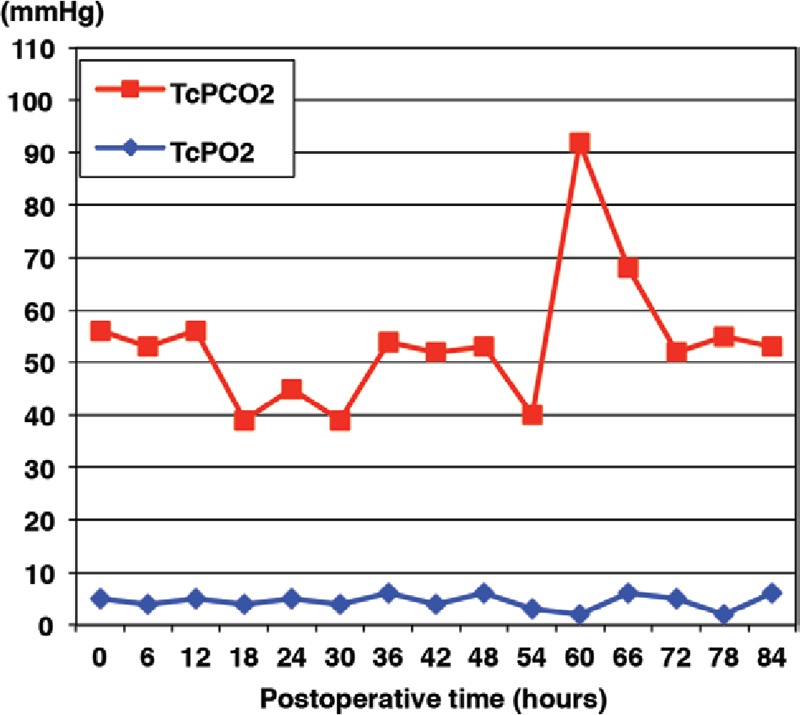
Graph showing TcPO2 and TcPCO2 values after flap transfer in the second case requiring exploration. TcPCO2 increased suddenly to 92 mm Hg at 60 h after transfer. Exploration was performed, and a venous thrombus was removed. The TcPCO2 decreased promptly, and the flap survived completely. TcPO2 did not change significantly before or after exploration.
DISCUSSION
Free tissue transfer depends on the patency of pedicle vessels for survival. The sooner the circulatory disturbance in the flap is detected, the sooner the exploration and rescue can be performed. Thus, numerous techniques have been investigated and used in search of the ideal free flap monitoring method. Two basic types of measurement methods exist. First, blood flow through the vascular pedicle can be monitored by means of an implantable Doppler probe or by color duplex sonography.11 Second, blood flow in the peripheral circulation can be assessed by observation of flap skin color,12 by capillary refilling, by monitoring with a handheld Doppler probe, by near-infrared spectroscopy,13 by use of a laser Doppler flowmeter,14,15 or by measuring the temperature of flaps,16 tissue oxygen tension,17,18 and TcPO2 and TcPCO2 with a probe heated to 44°C.5–7
The TcPO2 and TcPCO2 probe detects oxygen and carbon dioxide coming from capillaries in the dermis through the epidermis and corneum. The oxygen is consumed by the epidermal cells and by the TcPO2 probe on the corneum.19 The carbon dioxide is produced by the epidermal cells and is not consumed by the probe. This explains why the TcPO2 is lower than the PaO2 and the TcPCO2 is higher than the PaCO2 when TcPO2 and TcPCO2 are measured by unheated probes. When a heated probe is used, the skin temperature reaches approximately 44°C, making the TcPO2 value closer to the PaO2 value. The TcPCO2 value measured at 44°C becomes higher than that measured at 37°C. Although a heated electrode is not required to measure TcPCO2, the measurement is usually carried out under heated conditions for faster reactivity of TcPCO2 upon the change in PaCO2.20 A low-temperature burn may result from heating the skin to 44°C for more than 3 hours during continuous monitoring.5,7 Therefore, the monitoring site must be altered every 3–4 hours to avoid thermal injury, and the electrode must be recalibrated before placement. Frequent reattachment often causes inaccurate monitoring due to air bubbles trapped in the electrode, improper placement, or a damaged sensor membrane.
Our experimental study revealed that flap elevation and ligation of the vascular pedicle affected the TcPO2 and TcPCO2 values. This suggests that the measurement of both values can perhaps detect any circulatory disturbance caused by arterial or venous ischemia. However, the TcPO2 values remain very low after flap elevation. Trends in the changing TcPO2 and TcPCO2 values were quite similar between arterial occlusion and venous occlusion. Thus, it is difficult to differentiate between the 2 types of occlusion through TcPO2 and TcPCO2 measurements.
In our study of different monitoring sites, TcPO2 and TcPCO2 values obtained at all measurement sites at 37°C were lower than previously reported TcPO2 and TcPCO2 values obtained at 44°C or 45°C.19,21 With measurement at 37°C, TcPO2 and TcPCO2 values on the face differed from those on other body surfaces. These differences are also found with measurement at 44°C.19 Thus, the TcPO2 and TcPCO2 values of skin flaps obtained from various donor sites can be measured at 37°C.
TcPO2 values on clinical free flaps are characteristically low in comparison to TcPCO2 values. TcPO2 values on the skin are reported to vary not only in relation to the actual perfusion situation, density of the capillary network, and temperature but also in relation to regional factors, that is, thickness of the stratum corneum and density of the subdermal sebaceous glands.19,22–24 Flap elevation decreased TcPO2 values in our animal study. The low TcPO2 values may reflect postoperative edema of the flap skin or the vasospasm that occurs during elevation of the flap.7,8,25
TcPCO2 values are measurable at 37°C even after clinical free flap elevation. TcPCO2 is not readily influenced by the cutaneous factors described above.20,23 We suppose that TcPCO2 is influenced by the severe circulatory disturbance such as venous or arterial ischemia rather than the cutaneous factors.7 Reasons for the decrease in TcPO2 and TcPCO2 we observed every 24 hours after the transfer are not clear. The decrease in TcPCO2 may reflect a settling down of the circulatory condition, whereas the decrease in TcPO2 may mean continuing edema of the flap skin.
When critical circulatory failure occurs in the free flap, we can expect to detect the failure by the decrease in TcPO2 and increase in TcPCO2. The lowest TcPO2 values measured in the 2 patients undergoing removal of a thrombus in the vascular pedicle and those measured in patients who did not require exploration were very low and not distinguishable. The highest TcPCO2 values measured in the 47 patients who did not require exploration were less than 90 mm Hg. The highest values measured in the 2 patients who underwent reoperation were more than 90 mm Hg, and these values decreased to less than 80 mm Hg after removal of the thrombi. This means a line can be drawn at 90 mm Hg of TcPCO2 to indicate exploratory surgery. This value (90 mm Hg) is the same as that reported in a previous study performed at 44°C.7 TcPCO2 of more than 90 mm Hg is the critical limit for circulatory failure of a free flap.
The TcPCO2 monitoring method does not involve direct observation of blood flow to determine the viability of skin flaps. A TcPCO2 of 90 mmHg might not be definitive for a diagnosis of flap failure; TcPCO2 values in some of our flaps that survived were very close to this cutoff value in the present study. Clinical assessment that includes the pinprick test and observation of flap color remains the gold standard for evaluation of circulatory failure. We believe that final judgment for exploration must include clinical assessment. However, changes in the skin color of the flap can seem vague and assessment is subjective, especially for an untrained doctor or nurse, and such assessment does not equal continuous nor quantitative monitoring. Monitoring of TcPCO2 at 37°C can be performed continuously without burning the skin. When an alarm is set for a TcPCO2 value of 90 mm Hg, even an untrained doctor or nurse can be alerted to check for circulatory failure because the critical TcPCO2 value is clear, objective, and easily understood.
CONCLUSIONS
In our study, TcPO2 and TcPCO2 values measured at 37°C were affected by flap elevation and arterial and venous ischemia. However, the TcPO2 value was not particularly useful for detecting circulatory failure of free flaps because this value remained very low after free flap transfer. On the basis of our study findings, we suggest a TcPCO2 value of 90 mm Hg to be the point at which free flap failure is indicated. The measurement procedure is noninvasive, safe, continuous, quantitative, easy to understand, and commercially available. We believe that this method is useful as an initial alert to the possibility of flap compromise and the need for further checking and that it can reduce the risk of flap failure by early detection of circulatory disturbance in the transferred flap.
Fig. 6.
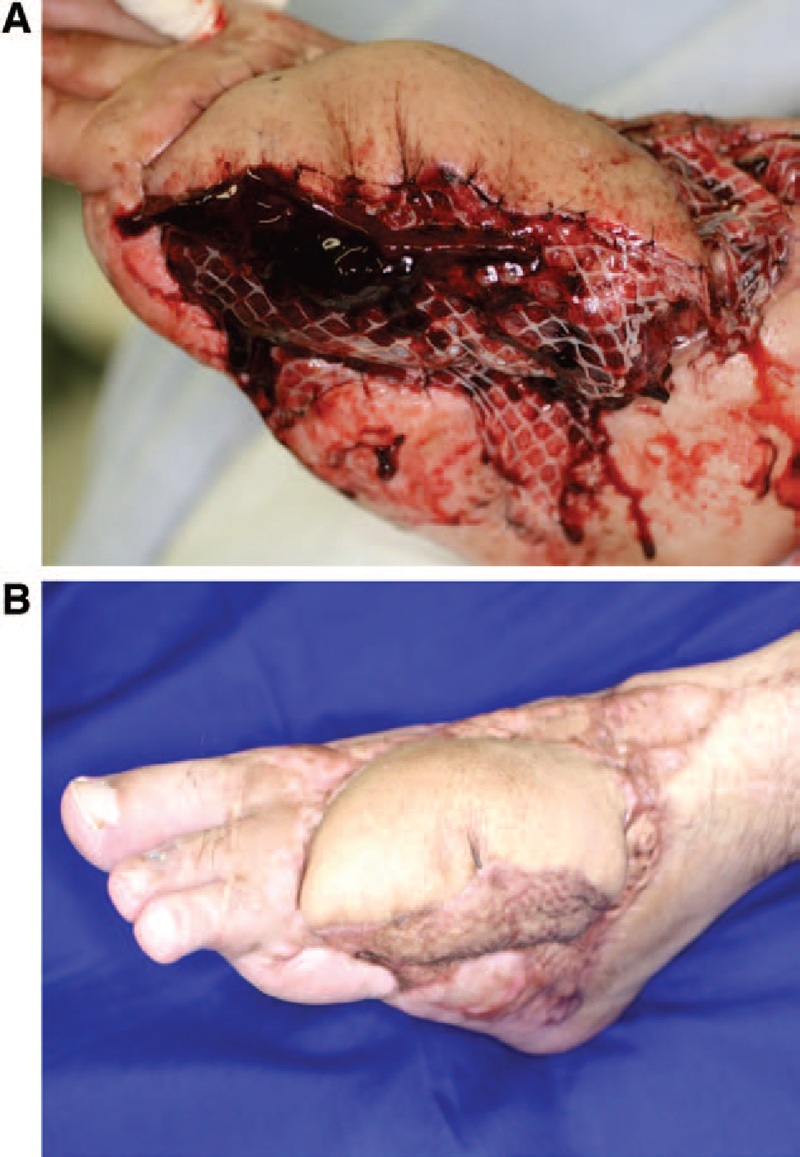
Photographs obtained in the case charted in Figure 5. A, Photograph taken before exploration. The color of the skin flap before exploration is normal and not indicative of the underlying congestion. B, Photograph taken 6 mo later.
Ichiro Hashimoto, MD, PhD
Department of Plastic and Reconstructive Surgery
The University of Tokushima Graduate School
Kuramoto-cho
Tokushima 770–8503, Japan.
E-mail: ihplast@clin.med.tokushima-u.ac.jp.
Footnotes
Drs. Abe and Hashimoto contributed equally to this work and should be considered co-first authors.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported by departmental resources. The Article Processing Charge was paid for by department resources.
REFERENCES
- 1.Hidalgo DA, Disa JJ, Cordeiro PG, et al. A review of 716 consecutive free flaps for oncologic surgical defects: refinement in donor-site selection and technique. Plast Reconstr Surg. 1998;102:722–732. discussion 733. [PubMed] [Google Scholar]
- 2.Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–721. doi: 10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 3.Weinzweig N, Gonzalez M. Free tissue failure is not an all-or-none phenomenon. Plast Reconstr Surg. 1995;96:648–660. doi: 10.1097/00006534-199509000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Furnas H, Rosen JM. Monitoring in microvascular surgery. Ann Plast Surg. 1991;26:265–272. doi: 10.1097/00000637-199103000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Achauer BM, Black KS, Litke DK. Transcutaneous PO2 in flaps: a new method of survival prediction. Plast Reconstr Surg. 1980;65:738–745. doi: 10.1097/00006534-198006000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Svedman P, Jacobsson S, Ponnert L, et al. Transcutaneous oxygen tension in flaps. Chir Plast. 1982;6:201–207. doi: 10.3109/02844318209006581. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto I, Nakanishi H, Takiwaki H, et al. Flap monitoring by transcutaneous PO2 and PCO2: importance of transcutaneous PCO2 in determining follow-up treatment for compromised free flaps. J Reconstr Microsurg. 2007;23:269–274. doi: 10.1055/s-2007-985208. [DOI] [PubMed] [Google Scholar]
- 8.Raskin DJ, Nathan R, Erk Y, et al. Critical comparison of transcutaneous PO2 and tissue pH as indices of perfusion. Microsurgery. 1983;4:29–33. doi: 10.1002/micr.1920040110. [DOI] [PubMed] [Google Scholar]
- 9.Gould JS, Sully L, O’Brien BM, et al. The effects of combined cooling and perfusion on experimental free-flap survival in rabbits. Plast Reconstr Surg. 1985;76:104–109. doi: 10.1097/00006534-198507000-00017. [DOI] [PubMed] [Google Scholar]
- 10.May JW, Jr, Chait LA, O’Brien BM, et al. The no-reflow phenomenon in experimental free flaps. Plast Reconstr Surg. 1978;61:256–267. doi: 10.1097/00006534-197802000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Swartz WM, Jones NF, Cherup L, et al. Direct monitoring of microvascular anastomoses with the 20-MHz ultrasonic Doppler probe: an experimental and clinical study. Plast Reconstr Surg. 1988;81:149–161. doi: 10.1097/00006534-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DH, Girling M, Mott G. Experience in monitoring the circulation in free-flap transfers. Plast Reconstr Surg. 1981;68:543–555. doi: 10.1097/00006534-198110000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Scheufler O, Andresen R. Tissue oxygenation and perfusion in inferior pedicle reduction mammaplasty by near-infrared reflection spectroscopy and color-coded duplex sonography. Plast Reconstr Surg. 2003;111:1131–1146. doi: 10.1097/01.PRS.0000046615.36917.3E. [DOI] [PubMed] [Google Scholar]
- 14.Liss AG, Liss P. Use of a modified oxygen microelectrode and laser-Doppler flowmetry to monitor changes in oxygen tension and microcirculation in a flap. Plast Reconstr Surg. 2000;105:2072–2078. doi: 10.1097/00006534-200005000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Heller L, Levin LS, Klitzman B. Laser Doppler flowmeter monitoring of free-tissue transfers: blood flow in normal and complicated cases. Plast Reconstr Surg. 2001;107:1739–1745. doi: 10.1097/00006534-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Cohn KH, May JW., Jr Thermal-energy dissipation: a laboratory study to assess patency in blood vessels. Plast Reconstr Surg. 1982;70:475–480. [PubMed] [Google Scholar]
- 17.Mahoney JL, Lista FR. Variations in flap blood flow and tissue PO2: a new technique for monitoring flap viability. Ann Plast Surg. 1988;20:43–47. doi: 10.1097/00000637-198801000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Hirigoyen MB, Blackwell KE, Zhang WX, et al. Continuous tissue oxygen tension measurement as a monitor of free-flap viability. Plast Reconstr Surg. 1997;99:763–773. doi: 10.1097/00006534-199703000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Takiwaki H, Arase S, Nakanishi H, et al. Transcutaneous PO2 and PCO2 measurements in various skin lesions. J Dermatol. 1991;18:311–313. doi: 10.1111/j.1346-8138.1991.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 20.Rochat MC, Payne JT, Pope ER, et al. Evaluation of skin viability in dogs, using transcutaneous carbon dioxide and sensor current monitoring. Am J Vet Res. 1993;54:476–480. [PubMed] [Google Scholar]
- 21.Orenstein A, Mazkereth R, Tsur H. Mapping of the human body skin with the transcutaneous oxygen pressure method. Ann Plast Surg. 1988;20:419–425. doi: 10.1097/00000637-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Takiwaki H, Nakanishi H, Shono Y, et al. The influence of cutaneous factors on the transcutaneous pO2 and pCO2 at various body sites. Br J Dermatol. 1991;125:243–247. doi: 10.1111/j.1365-2133.1991.tb14748.x. [DOI] [PubMed] [Google Scholar]
- 23.Takiwaki H. Transcutaneous PO2 and PCO2 measurement in dermatology. Acta Derm Venereol Suppl (Stockh) 1994;185:21–25. [PubMed] [Google Scholar]
- 24.Wolff KD, Kolberg A, Mansmann U. Cutaneous hemoglobin oxygenation of different free flap donor sites. Plast Reconstr Surg. 1998;102:1537–1543. doi: 10.1097/00006534-199810000-00029. [DOI] [PubMed] [Google Scholar]
- 25.Caselli A, Latini V, Lapenna A, et al. Transcutaneous oxygen tension monitoring after successful revascularization in diabetic patients with ischaemic foot ulcers. Diabet Med. 2005;22:460–465. doi: 10.1111/j.1464-5491.2005.01446.x. [DOI] [PubMed] [Google Scholar]


