Abstract
Background:
The latissimus dorsi (LD) muscle flap has been widely used in facial reanimation surgery. However, there are no standards to what degree the muscle flap may be safely thinned because the three-dimensional positional relationship of thoracodorsal artery, vein, and nerve inside the LD muscle is poorly understood.
Methods:
From 18 formalin-fixed cadavers, we made 36 transparent specimens of LD muscles using a newly developed decoloration technique. In 26 specimens, nerve staining (Sihler’s staining method) and silicone rubber (Microfil) injection to the thoracodorsal artery were performed, and the relationship of the artery and the vein was examined in 10 specimens.
Results:
The thoracodorsal artery and vein always ran parallel in a deeper layer compared to the nerve. The thoracodorsal nerve constantly existed in a deeper layer than half (50%) of the muscle in the range of use of the muscle flap in facial reanimation surgery.
Conclusions:
The thoracodorsal nerves ran in a shallower layer, and the depth to the nerve in the muscle flap in actual facial reanimation surgery is safe enough to avoid damage to the nerves. The LD muscle may be thinned to half its original thickness safely.
Facial reanimation surgery using a free muscle flap for facial paralysis, in which gracilis muscle flap was used, was first reported by Harii1 in 1976. Subsequently, many muscle flaps for the surgery have been reported, such as latissimus dorsi (LD) muscle,2–4 rectus abdominis muscle,5 rectus femoris muscle,6 abductor hallucis muscle,7 internal oblique muscle,8 biceps femoris muscle,9 and pectoral minor muscle.10 Currently, in Asian countries, the LD muscle flap is the most commonly used among these muscle flaps because it has a long vascular pedicle and nerve. However, the LD muscle flap has 1 problem, that is, its thickness. In surgery, if the LD muscle flap is to be transplanted without thinning in the subcutaneous pocket of the cheek, (1) insertion of the muscle flap inside the pocket may be difficult due to its thickness, (2) convex deformation occurs due to its thickness, and (3) contraction of the muscle flap is too strong, and problems such as facial expression becoming asymmetrical in shape when the patient smiles occur. Therefore, in many cases, a method to shave the LD muscle flap before transplanting is followed, leading to substantially satisfactory results being stably obtained.2,3 However, there are no reports clearly indicating to what degree the muscle flap may be safely shaven. The purpose of this study is to anatomically investigate the three-dimensional route of the thoracodorsal artery, vein, and nerve inside the LD muscle used for facial reanimation and to clarify the degree to which the muscle flap may be safely shaven.
METHODS
Materials
The research materials were the LD muscles sampled from 36 sides of 18 preserved cadavers (the duration of formalin fixation is approximately 2–20y). While preserving the thoracodorsal artery, vein, and nerve, the LD muscle, including the thoracodorsal aponeurosis in the medial side and the iliac crest in the caudal side of the muscle, wassampled.
Making a Transparent Specimen of the LD Muscle and Coloring the Thoracodorsal Artery, Veins, and Nerve
Sampling and Fixing the LD Muscle
The sampled LD muscle was fixed in 70% alcohol for 3 weeks.
Maceration and Decoloration
1.After removing the fat and fascia from the surface layer of the LD muscle, it was rinsed with tap water for 30 minutes to 1 hour.
2.The LD muscle was immersed in 3% KOH solution (100 ml distilled water with 0.2–1 ml of 3% hydrogen peroxide solution added) for 5–7 weeks while stirring the solution several times a day. The solution was appropriately exchanged if it became opaque and polluted during this period. Actually, the solution was exchanged every day for the first 4 days following commencement and exchanged once every 3–7 days, for a total of 7–10 times, thereinafter. Silicone rubber injection to the thoracodorsal blood vessel was carried out at the stage when the solution started becoming clear to some degree and the muscle became decolorized and white.
Filling the Thoracodorsal Artery by Silicone Rubber(Microfil)
A central venous catheter (Agyle Inc., 20G, outer diameter of 0.8 mm) was inserted from the stump of the thoracodorsal artery to the periphery as much as possible and stopped when resistance was felt. Microfil (Flow Tech, Carver, MA) was injected from this site while pulling out the catheter little by little to prevent Microfil from hardening. This filling of the artery was carried out on all 36 sides, whereas the procedure of injecting Microfil into the vein was not carried out on 26 sides among these, and the procedure of decalcification was carried out thereafter to stain the nerves.
Filling the Thoracodorsal Vein by Silicone Rubber
After injecting Microfil into the artery, infusion to the veins was carried out on 10 sides. The central venous catheter was inserted from the stump of the thoracodorsal vein to the periphery as much as possible and stopped when resistance was felt. There was reflux due to the venous valves, so Microfil could not be injected into the periphery as is; therefore, it was slowly injected, taking several minutes while applying pressure to the LD muscle in the upright position by hanging it. The procedures of decalcification, staining the nerves, and decolorization and neutralization were not carried out, but the procedure of rarefaction was carried out as is on these 10 sides with marked veins.
Decalcification (Removing Calcium from the Specimen)
After the LD muscle was rinsed with tap water for 30 minutes to 1 hour, it was immersed in Sihler’s I solution (glacial acetic acid:glycerin:1% chloral hydrate = 1:2:12) for 2 weeks while stirring the solution several times a day. The solution was exchanged once a week.
Staining the Nerves
After the LD muscle was rinsed with tap water for 30 minutes to 1 hour, it was immersed in Sihler’s II solution (Ehrlich’s hematoxylin:glycerin:1% chloral hydrate = 1:2:12) for 2 weeks while stirring the solution several times a day. The solution was exchanged once a week.
Decolorization and Neutralization
The muscle was immersed in Sihler’s I solution again. The procedure was stopped when the small nerves started to decolorize after 2–3 hours. Subsequently, it was rinsed with tap water for 30 minutes to 1 hour and then dipped in 0.05% lithium carbonate liquid. Because of this procedure, the nerve changed from purple to blue within 2–3 hours, thereby completing marking.
Rarefaction
The sample made was immersed in 40% glycerin for 3 days, then immersed in 60% glycerin for 2 days, immersed in 80% glycerin for a day, and finally put in 100% glycerin. A small amount of thymol crystal was added in 100% glycerin for embalming.
Using the methods mentioned above, a transparent specimen of the LD muscle with marked thoracodorsal artery and veins (10 sides) and thoracodorsal artery and nerves (26 sides) was prepared. Decalcification, staining the nerves, and decolorization and neutralization procedures were not carried out for 10 specimen sides with marked veins because the weak vein walls would rupture during the procedure of staining the nerves. Therefore, examination of the arteries, veins, and nerves in a single specimen was not possible.
Macroscopic (Diagonally Upward View) Observation of the Three-dimensional Route of the Thoracodorsal Artery, Veins, and Nerves and the Positional Relationship Thereof
Following were macroscopically examined by viewing the completed transparent specimen diagonally from above from the back surface side of the LD muscle:
1.The route of the thoracodorsal artery and vein and the positional relationship thereof (10 sides) and
2.The route of the thoracodorsal artery and nerves and the positional relationship thereof (26 sides).
Making a Specimen with a Vertical Section on the Muscle Flap for Use in Facial Paralysis Surgery
A specimen with a vertical section on the muscle flap used for facial paralysis surgery was made from the transparent specimen, and the positional relationship of the thoracodorsal vessels and nerve was observed from the vertical section.
Making a Specimen with a Vertical Section on the LDMuscle
The prepared transparent specimens were hardened using gelatin or agar. During facial paralysis surgery, the area closest to the anterior axillary line of the LD muscle is frequently used in many cases due to the posture (dorsal position). In actual clinical practice, the area approximately 2 cm from the site in which the thoracodorsal vessels enter the muscle and the area nearly 8 cm to the peripheral side along the descending branch are mainly used. These areas were cut away from the transparent specimen and cut in a 5 mm width, parallel to the route of the thoracodorsal vessels and nerve to make a specimen with a vertical section.
Subsequently, the route of the thoracodorsal vessels and nerves was observed using a stereoscopic microscope (model: Stemi1000, Zeiss). Furthermore, observation of the vertical section was carried out on 24 sides of the prepared transparent specimen (7 sides with the artery and veins marked and 17 sides with the artery and nerves marked).
Investigating the Depth at Which the Thoracodorsal Artery and Vein Run
Which among the thoracodorsal artery and vein run along the shallow surface of the vertical section was investigated.
Investigating the Depth at Which the Thoracodorsal Artery and Nerve Run and Measuring the Nerve Depth
On the vertical section, the distance from the muscle surface to the nerves (A) and the thickness of the entire muscle at the same site (B) were measured in 2-cm intervals from the site where the thoracodorsal nerve enters the muscle to the periphery.
Actual measurement was carried out using vernier calipers. Nerve depth was calculated as A/B × 100 (%) (Fig. 1). That is, the nerve depth indicates in what % depth the nerves run from the muscle surface in the specimen.
Fig. 1.
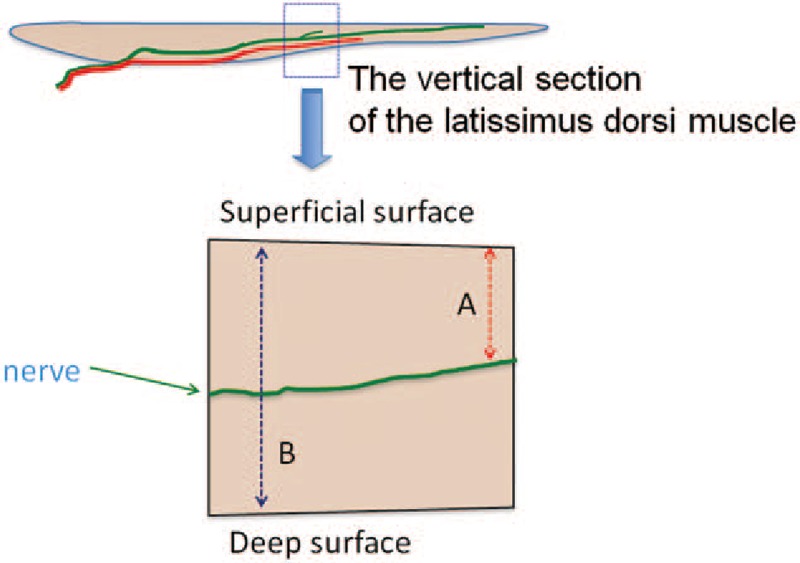
Setting the nerve depth. Nerve depth = A: the distance from the muscle surface to the nerves; B: thickness of the entire muscle × 100 (%).
RESULTS
Macroscopic (Diagonally Upward View) Observation of the Three-dimensional Route of the Thoracodorsal Artery, Veins, and Nerves and the Positional Relationship Thereof
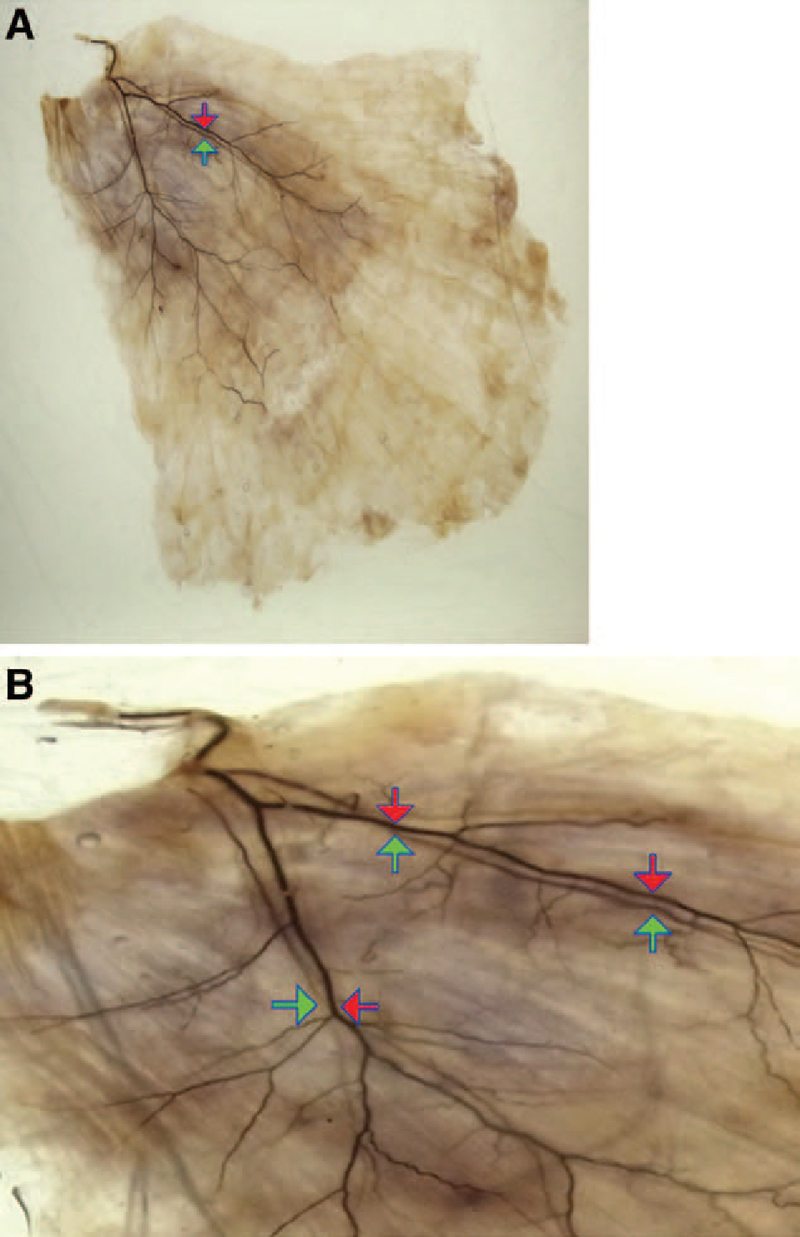
The thoracodorsal artery and vein always ran parallel to a range that may be confirmed in the 10 specimens (Figs. 2A, B). The route was in the same layer regarding depth, and which of the two ran in the shallower layer could not be accurately determined from the diagonally upward view by the naked eye.
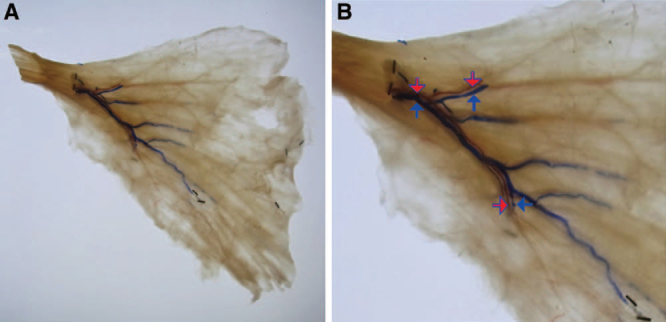
The thoracodorsal artery and nerve ran parallel to a range in which the periphery of them could possibly be confirmed in the 26 specimens. Moreover, in the muscle, the nerve constantly ran in a slightly shallower surface compared to the artery (Figs. 3A, B).
Fig. 2.
The route of the thoracodorsal artery and vein from the diagonally upward view. The artery and vein run substantially in parallel to the region in which the peripheral may be confirmed. A, The diagonally upward view from the back surface. B, The enlarged image. (The red arrow indicates the thoracodorsal artery, and the blue arrow indicates the thoracodorsal vein.)
Fig. 3.
The route of the thoracodorsal artery and nerve from a diagonally upward view: The artery and nerve run substantially in parallel to the region in which the periphery may be confirmed. The nerve passes a more superficial layer from the artery after invasion into the muscle. (The red arrow indicates the thoracodorsal artery, and the green arrow indicates the thoracodorsal nerve.) A, The diagonally upward view from the back surface. B, The enlarged image.
The Positional Relationship of the Thoracodorsal Artery, Veins, and Nerves on the Vertical Section
The artery and vein ran in the same depth in parallel until entering the muscle. Once entering the muscle, the artery ran in the same layer or a slightly shallower layer compared to the veins, and the veins did not run in a shallower layer compared to the artery in all specimens (Figs. 4A, B).
The artery and the nerve ran in the same depth in parallel before entering the muscle. Once entering the muscle, the nerve constantly ran in a shallower layer compared to the artery in all specimens (Fig. 5).
Measuring the depth of the thoracodorsal nerve running inside the muscle.
Fig. 4.
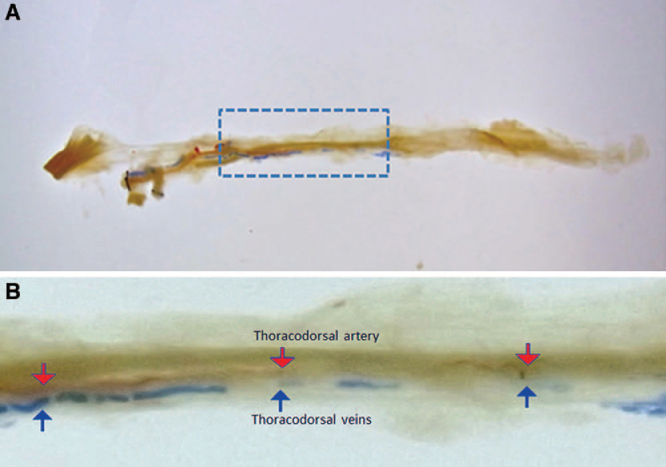
The positional relationship of the thoracodorsal artery and vein on the vertical section. The thoracodorsal artery runs in the same layer or a slightly shallower layer compared to the thoracodorsal vein in the muscle. A, The entire view of the vertical section of lattisimus dorsi muscle. B, The enlarged view of the vertical section. (The red arrow indicates the thoracodorsal artery, and the blue arrow indicates the thoracodorsal vein.)
Fig. 5.
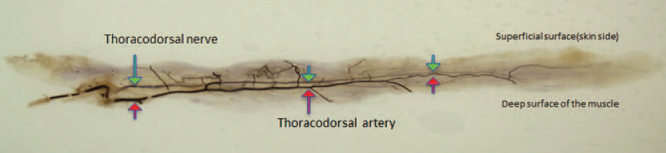
The positional relationship of the thoracodorsal artery and nerve on the vertical section. The nerve constantly runs in a shallower layer compared to the artery in the muscle, considering all specimens. (The red arrow indicates the thoracodorsal artery, and the green arrow indicates the thoracodorsal nerve.)
The nerve depth was 100% (average, 100%) at 0 cm from the site where the nerve enters the muscle to the center, toward the periphery; 100% to 68% (average, 79.6%) at 2 cm, 80% to 60% (average, 67.9%) at 4 cm, 75% to 60% (average, 66.3%) at 6 cm, 75% to 50% (average, 63.6%) at 8 cm, and 70% to 33% (average, 53.8%) at 10 cm (Fig. 6).
Fig. 6.
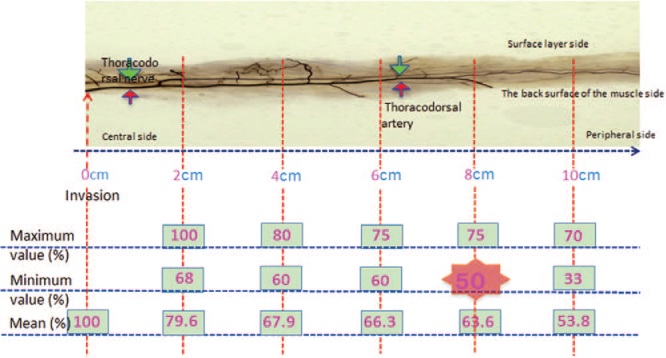
Measuring the depth of the thoracodorsal nerve running inside the muscle. The nerve depth within 8 cm ranged from 100% to 50% (average, 75.48%). That is, the thoracodorsal nerve constantly ran in a deeper layer than half (50%) the muscle within 8 cm.
In facial paralysis surgery, muscle with a maximum length of 8 cm or less is normally used from the site in which the thoracodorsal blood vessel and nerves enter the muscle toward the periphery. The nerve depth within 8 cm ranged from 100% to 50% (average, 75.48%). Thus, the thoracodorsal nerve constantly ran in a deeper layer than half (50%) of the muscle in the range of use of the muscle in facial reanimation surgery.
DISCUSSION
Various surgical procedures for the facial paralysis have been carried out until now, such as muscle flap transplantation in 2 stages following nerve graft,11–13 nonvascularized free muscle transplantation,14 and nerve grafting.15 However, recently, the method using vascularized muscle transplantation with nerve in a single stage has become the mainstream. In these reconstruction methods, LD muscle,2–4 rectus abdominis muscle,5 rectus femoris muscle,6 abductor hallucis muscle,7 internal oblique muscle,8 biceps femoris muscle,9 and smaller pectoral muscle10 have been used; however, the LD muscle flap, which comprises long nerves and vascular pedicles, is the most commonly used.
In actual surgery, the LD muscle flap is usually sampled with a rectangular shape of approximately 8–10 cm in length and 3–4.5 cm in width, toward periphery from the point where the thoracodorsal vessels and nerve enter the muscle and in an area close to the anterior axillary line.2,3 Subsequently, once the muscle flap is thinned to an appropriate thickness, it is transplanted inside the subcutaneous pocket of the cheek prepared in advance. However, this technique was based on experience, and there are no reports anatomically clarifying to what degree thinning may be carried out safely. Then we carried out this study with the purpose of clarifying this point.
At first, to investigate the distribution pattern of vessels and nerve, we decided to prepare transparent specimens of the LD muscle with the thoracodorsal artery, veins, and nerve colored at the same time. Sihler’s staining method was carried out for the nerves.16 This is a staining method invented in the late 19th century to clarify the nerve end inside muscles, with various reports still being used today including the LD muscle.17–22 We carried out the modified Sihler’s staining method reported by Liu et al.23 In addition, for coloring the vessels, Microfil was injected from the stump of thoracodorsal artery and vein when the process of maceration and decoloration of the nerve staining procedure was completed, and we tried to make specimens’ nerve and vessels colored at the same time. However, the weak vein walls sometimes ruptured during the processes of decalcification, staining, and decolorization and neutralization, causing Microfil to leak outside the veins and making it impossible to mark arteries, veins, and nerves in a single specimen at the same time. Accordingly, the procedures from decalcification to decolorization and neutralization were omitted and rarefaction was carried out, thereby allowing the transparent specimen with a rendered artery and vein to be prepared. Meanwhile, the artery did not rupture during the processes of decalcification, staining, and decolorization and neutralization, so a transparent specimen with both the artery and veins rendered could be prepared by carrying out all these procedures. By comparing and investigating these 2 types of transparent specimens, that is, 10 specimens with rendered artery and vein and 26 specimens with rendered artery and nerve, we succeeded in three-dimensionally clarifying the positional relationship of these three.
Using the transparent specimens prepared in this manner, first, the positional relationship of the thoracodorsal artery, vein, and the nerve from the diagonally upward view was macroscopically examined. We found that the thoracodorsal artery and vein ran in parallel to a range where the periphery thereof was able to be confirmed, and they substantially ran in the same depth layer. However, which of the two ran in the shallower layer could not be accurately determined by the naked eye. Meanwhile, the thoracodorsal artery and nerves ran in parallel to a range where the periphery of the artery was able to be confirmed, and inside the muscle, the nerves constantly ran in a shallower layer compared to the artery. From this result, regarding the positional relationship of the artery, veins, and nerves running inside the muscle, it was surmised that the nerves ran in the shallowest layer. An investigation into the depth was further carried out on the vertical section with the purpose of verifying this.
On the vertical section, the artery and veins ran in the same layer in parallel immediately until entering the muscle, and the artery ran in the same or a deeper layer compared to the vein after entering the muscle. Moreover, regarding the artery and nerves, in the same manner as the findings from the diagonally upward view, it was confirmed that the nerves constantly ran in a shallower layer compared to the artery after entering the muscle. Accordingly, the nerves ran in the shallowest layer among the three, and we have reached a conclusion that the depth to the nerve in the muscle flap in facial reanimation surgery is safe enough to avoid damage to the thoracodorsal nerves.
Subsequently, to determine to what degree of flap thickness thinning is possible, we measured the depth at which the thoracodorsal nerve runs inside the muscle from the vertical section of the specimen. Because there are individual differences in the thickness of the LD muscle and possible muscle thickness changes (contraction) when making transparent specimens, we provided relative ratio of the distance from the muscle surface to the nerves to the thickness of the entire muscle rather than actual numbers of them. We found that the minimum nerve depth was 50% within 8 cm from the site at which the descending branch used for facial reanimation enters into the muscle toward periphery. That is, it means that in facial reanimation surgery, the LD muscle flap may be safely thinned to half its thickness.
A couple of limitations should be considered in this study. First, the number of samples used in this study is small; therefore, a replication study using larger sample sets is needed. Second, a comparison of our findings with live LD muscle is needed, although it is technically very difficult. Third, no specimen has artery, vein, and nerve, all in one. Fourth limitation is the limited sample size from which to make statements about anatomic variability. Fifth is the assessment of only the inferior TD nerve branch along the anterior latissimus border.
CONCLUSION
In conclusion, we have clarified the three-dimensional positional relationship of the thoracodorsal artery, vein, and nerve inside the LD muscle. The present result suggests that the depth to the nerve in the muscle flap in actual facial reanimation surgery is safe enough to avoid damage to the thoracodorsal nerves, and the LD muscle could be thinned to half its original thickness from the surface. We believe that this procedure would be very useful to perform dynamic reconstruction surgery for the treatment of facial paralysis.
Kensuke Kiyokawa, MD, PhD
Department of Plastic and Reconstructive
Surgery and Maxillofacial Surgery
Kurume University School of Medicine
67 Asahi-machi, Kurume City
Fukuoka-ken 830 0011, Japan.
E-mail: prsmf@med.kurume-u.ac.jp.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Harii K. Microneurovascular free muscle transplantation for reanimation of facial paralysis. Clin Plast Surg. 1979;6:361–375. [PubMed] [Google Scholar]
- 2.Harii K, Asato H, Yoshimura K, et al. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102:941–951. doi: 10.1097/00006534-199809040-00001. [DOI] [PubMed] [Google Scholar]
- 3.Wei W, Zuoliang Q, Xiaoxi L, et al. Free split and segmental latissimus dorsi muscle transfer in one stage for facial reanimation. Plast Reconstr Surg. 1999;103:473–480. doi: 10.1097/00006534-199902000-00016. discussion 481–482. [DOI] [PubMed] [Google Scholar]
- 4.Takushima A, Harii K, Asato H, et al. One-stage reconstruction of facial paralysis associated with skin/soft tissue defects using latissimus dorsi compound flap. J Plast Reconstr Aesthet Surg. 2006;59:465–473. doi: 10.1016/j.bjps.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Koshima I, Tsuda K, Hamanaka T, et al. One-stage reconstruction of established facial paralysis using a rectus abdominis muscle transfer. Plast Reconstr Surg. 1997;99:234–238. doi: 10.1097/00006534-199701000-00036. [DOI] [PubMed] [Google Scholar]
- 6.Koshima I, Moriguchi T, Soeda S, et al. Free rectus femoris muscle transfer for one-stage reconstruction of established facial paralysis. Plast Reconstr Surg. 1994;94:421–430. doi: 10.1097/00006534-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Guo ET, Ji ZL, et al. One-stage microneurovascular free abductor hallucis muscle transplantation for reanimation of facial paralysis. Plast Reconstr Surg. 1995;96:78–85. [PubMed] [Google Scholar]
- 8.Wang W, Qi Z, Lin X, et al. Neurovascular musculus obliquus internus abdominis flap free transfer for facial reanimation in a single stage. Plast Reconstr Surg. 2002;110:1430–1440. doi: 10.1097/01.PRS.0000029809.71845.04. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi A, Maruyama Y. Neurovascularized free short head of the biceps femoris muscle transfer for one-stage reanimation of facial paralysis. Plast Reconstr Surg. 2005;115:394–405. doi: 10.1097/01.prs.0000149405.89201.9e. [DOI] [PubMed] [Google Scholar]
- 10.Harrioson DH. The pectoralis minor vascularized muscle graft for the treatment of unilateral facial palsy. Plast Reconstr Surg. 1985;75:206–216. doi: 10.1097/00006534-198502000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Manktelow RT. Free muscle transplantation for facial paralysis. Clin Plast Surg. 1984;11:215–220. [PubMed] [Google Scholar]
- 12.O’Brien BM, Franklin JD, Morrison WA. Cross-facial nerve grafts and microneurovascular free muscle transfer for long established facial palsy. Br J Plast Surg. 1980;33:202–215. doi: 10.1016/0007-1226(80)90013-2. [DOI] [PubMed] [Google Scholar]
- 13.Terzis JK. Pectoralis minor: a unique muscle for correction of facial palsy. Plast Reconstr Surg. 1989;83:767–776. [PubMed] [Google Scholar]
- 14.Thompson N. Autogenous free grafts of skeletal muscle. A preliminary experimental and clinical study. Plast Reconstr Surg. 1971;48:11–27. doi: 10.1097/00006534-197107000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Anderl H. Cross-face nerve transplant. Clin Plast Surg. 1979;6:433–449. [PubMed] [Google Scholar]
- 16.Shiler C. Ueber Muskelspindeln und intramuskulare nervenendigungen bei schlangen und froschen. Arch Mikros Anat Entwickl. 1895;46:709–723. [Google Scholar]
- 17.Wong MT, Lim AY, Coninck CD, et al. Functional units within the latissimus dorsi muscle based on Sihler technique. Ann Plast Surg. 2007;59:152–155. doi: 10.1097/01.sap.0000252731.60748.cb. [DOI] [PubMed] [Google Scholar]
- 18.Gülekon N, Peker T, Turgut HB, et al. Qualitative comparison of anatomical microdissection, Sihler’s staining and computerized reconstruction methods for visualizing intramuscular nerve branches. Surg Radiol Anat. 2007;29:373–378. doi: 10.1007/s00276-007-0225-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Lim AY, Lim IJ, et al. Innervation of the face studied using modifications to Sihler’s technique in a primate model. Plast Reconstr Surg. 2008;121:1188–1205. doi: 10.1097/01.prs.0000305563.77782.35. [DOI] [PubMed] [Google Scholar]
- 20.Mu L, Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat. 2010;23:777–791. doi: 10.1002/ca.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peker T, Gülekon N, Turgut BH, et al. Observation of the relationship between the shape of skeletal muscles and their nerve distribution patterns: a transparent and microanatomic study. Plast Reconstr Surg. 2006;117:165–176. doi: 10.1097/01.prs.0000186539.80555.27. [DOI] [PubMed] [Google Scholar]
- 22.Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec. 2000;258:406–420. doi: 10.1002/(SICI)1097-0185(20000401)258:4<406::AID-AR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Kumar VP, Shen Y, et al. Modified Sihler’s technique for studying the distribution of intramuscular nerve branches in mammalian skeletal muscle. Anat Rec. 1997;247:137–144. doi: 10.1002/(SICI)1097-0185(199701)247:1<137::AID-AR16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]


