Abstract
Gain-of-function D816V point mutation within the kinase domain of the transmembrane receptor KIT is found in the great majority of patients with systemic mastocytosis (SM) and is attractive therapeutic target. Twenty patients with SM were enrolled during 2003–2005 in phase II clinical trial with imatinib mesylate (400mg daily), a KIT inhibitor. Median time on therapy was 9 months (range, 0.5 – 44+). Only 1 patient, with D816V KIT mutation-negative FIP1L1-PDGFRα-negative SM-HES, achieved complete remission (now lasting for 44 months). Six other patients reported symptomatic improvement, including 2 with D816V KIT mutation-positive SM (one reported improvement in diarrhea and the other in fatigue). Other patients had no benefit. Imatinib was relatively well tolerated. Our study confirms that imatinib therapy does not result in appreciable clinical activity in patients with D816V mutation-positive SM, but may result in a significant benefit in occasional patient with D816V mutation-negative SM.
Introduction
Mastocytosis is a myeloproliferative neoplasm that results from the clonal expansion of the mast cell (MC) progenitor. Although virtually any organ can be involved, MCs tend to focally infiltrate the bone marrow, skeletal system, spleen, liver, and lymph nodes [1]. The factor primarily responsible for the neoplastic transformation of MC in systemic mastocytosis (SM) and their uncontrolled growth is believed to be a gain-of-function somatic point mutation within the KIT gene that encodes for the KIT receptor. KIT is a 145 kDa transmembrane class III receptor tyrosine kinase structurally characterized by 5 extracellular immunoglobulin-like repeats, a transmembrane domain, a juxtamembrane domain, and a split tyrosine kinase domain. KIT mutations can be found in the great majority of patients with SM and lead to ligand-independent constitutive activation of KIT signaling [1]. In healthy state, KIT has been shown to play an important role in hematopoiesis, gametogenesis, melanogenesis and gastrointestinal function [2]. In addition, KIT serves as a receptor on MC for stem cell factor, and is required for human MC growth, differentiation, and functional activation. The first evidence of a mutation in the KIT gene was reported by Furitsu et al in 1993 [3]. The KIT D816V mutation, which maps to the tyrosine kinase domain of KIT in exon 17 and results in the substitution of asparaginase by valine, has been reported in the majority of patients with SM [1]. Mutant KIT kinase is amenable to targeted therapies with small-molecule inhibitors [4]. Up to 20% of patients with SM present also with eosinophilia. In some of those cases, the eosinophilia may be clonal and harbor a fusion transcript involving the FIP1-like-1 and the platelet derived growth factor receptor-alpha genes (FIP1L1-PDGFRα) [1,2].
Imatinib mesylate (Gleevec®) is an ATP-competitive, orally bioavailable, inhibitor of various protein tyrosine kinases such as ABL1, PDGFR, ARG, and KIT [4]. Imatinib has demonstrated significant activity against neoplastic MCs carrying wild type KIT or sporadic KIT mutant isoforms in SM, such as KIT F522C, but not common KIT D816V mutant [5]. The U.S. Food & Drug Administration (FDA) approved in October of 2006 the use of imatinib for adult patients with aggressive systemic mastocytosis (ASM) without the KIT D816V mutation, or with unknown KIT mutational status, at a dose of 400 mg daily. In ASM associated with eosinophilia, the recommended starting dose is 100 mg daily due to exquisite sensitivity of PDGFRα to imatinib [4]. Although imatinib should not have clinical activity in SM patients with KIT D816V mutation, one study reported modest clinical activity (possibly compounded by the concomitant use of prednisone) [6]. Here, we present the results of a prospective open-label phase II study conducted in 20 patients with SM at our institution between 2003 and 2005, before imatinib’s approval.
Materials and Methods
Study design and patients
The primary objective was to determine the clinical activity of imatinib in patients with SM, regardless of the SM subtype and the KIT mutational status. The protocol was approved by the Institutional review board of The University of Texas M.D. Anderson Cancer Center. We obtained written informed consent, according to institutional guidelines and the declaration of Helsinki. Inclusion criteria were as follows: i) Diagnosis of indolent SM (ISM), ASM or SM with associated hematologic non-mast cell disease (SM-AHNMD); ii) age older than 18 years; iii) both serum bilirubin and creatinine <2 mg/dL; iv) Eastern Collaborative Oncology Group performance status <3; v) adequate cardiac status (New York Heart Association grade <3); and vi) life expectancy >12 weeks. Patients with ISM had to have uncontrolled symptoms related to the disease, despite optimal supportive care, in order to participate. Women with the potential to become pregnant were required to practice birth control, and both women and men were required to continue some form of birth control for the duration of the trial and at least 3 months after the last dose of imatinib.
Treatment
Imatinib mesylate (Gleevec; Novartis, Basel, Switzerland) was administered orally at a dose of 400 mg daily. The dose was increased up to a maximum of 800 mg (400 mg twice daily), in 200 mg monthly increments starting after 1 month of treatment, if there was no evidence of response and no toxicity. Patients who developed toxicity on 400 mg daily, had their dose reduced to 300 mg daily, lowest acceptable dose. Treatment was interrupted for clinically significant grade 2 non-hematologic toxicity and held until toxicity had resolved to grade ≤1, then resumed at the same dose. If grade 2 non-hematologic toxicity recurred, imatinib was again held until resolution of the toxicity to grade ≤1, and then resumed at lower dose. For grade 3–4 non-hematologic toxicity, imatinib was held until the toxicity resolved to grade ≤ 1 and then resumed at a reduced dose. For grade 3–4 drug-related hematologic toxicity therapy was held until resolution of the toxicity to grade ≤1; if recovery occurred within 2 weeks, therapy was resumed at the same dose but if this took more than 2 weeks, therapy was resumed at a reduced dose. If myelosuppression recurred, imatinib was resumed at a reduced dose, no matter how long recovery had taken. Patients received imatinib therapy indefinitely, until disease progression, or until the occurrence of unacceptable toxicity. No other therapies were permitted during the study period to treat SM except for hematopoietic growth factors at the investigator’s discretion. Patients were allowed to continue to use medications for the relief of SM-associated symptoms (e.g. H1 and H2 blockers, but not prednisone).
Follow up and response assessment
Complete blood counts and basic biochemistry panels with liver function tests and serum tryptase were assessed at diagnosis and every 2 to 6 weeks for the first 6 months on the study and every 3 months thereafter. A bone marrow biopsy was performed every 3 to 6 months during the study and always included morphologic evaluation, histochemistry, and flow cytometry test. The KIT D816V and F1L1P1-PDGFRα (in those patients with eosinophilia) mutational status was determined in patients prior to study entry by PCR and direct sequencing. We were unable to test D816V mutation-negative patient samples for the presence of alternative KIT mutation. The response criteria used in this study for patients with ASM was previously published [7]. Response criteria for patients with ISM and SM-AHNMD are descriptive only, since response criteria for these subtypes have not been established.
Results
Patients and disease characteristics
Between March 2003 and May 2005, twenty patients were enrolled into the study (Table 1). Bone marrow biopsy was diagnostic of involvement with mastocytosis in all 20 patients, with typical clusters of spindle shaped MC. Ten patients had bone marrow MC infiltration of ≤5%, nine patients had 6–30%, and one had 80% MC infiltration, as determined further by the histochemistry technique using staining for tryptase and CD25 on biopsy slides. Involvement with abnormal MC was also documented with flow cytometry evaluation for aberrant co-expression of CD117, CD25, and CD2 on MC. Four patients had ASM, 11 ISM and 5 SM-AHNMD (chronic myelomonocytic leukemia with eosinophilia in 2, hypereosinophilic syndrome in 2, and plasma cell dyscrasia in 1). Four patients with hypereosinophilia were negative for the F1P1L1-PDGFRα fusion gene. The median hemoglobin level for 4 patients with ASM was 11.4 (range 9.8–14.4 g/dL), white blood cell count 3.9 (range 2.5–31.7 × 103/μl) and platelet count 152 (range 44–625 × 103/μl). Blood tryptase level was >200 ng/mL in 3 patients, 100–200 ng/mL in 1, 21–99 ng/mL in 7, and <20 ng/ml in 4 patients. Tryptase level was not assessed in 5 patients at the start of the therapy.
Table 1.
Baseline characteristics of patients (n=20)
| Age, years (median and range) | 52 (23–75) |
|---|---|
| Males | 10 |
| Type of SM: ISM ASM SM-AHNMD |
11 4 5 |
| KIT D816V positive | 13 |
| Cytogenetic abnormality | 0 |
| CD25 positive | 17 (of 18 evaluable) |
| Elevated tryptase (>20ng/mL) | 11 (of 15 evaluable) |
| Previously treated (other than steroids) | 5 |
Five patients received prior therapy for SM: cyclosporine and PUVA in 1 case each, and imatinib in 3 cases (all before coming to M.D. Anderson; one received imatinib 400 mg daily for 2 years with symptomatic improvement but stopped therapy due to insurance issues; 2 received imatinib 100 mg daily without response).
Efficacy
Median time on imatinib therapy was 9 months (range, 0.5 – 44+). One patient with SM-AHNMD achieved an objective response. This 48 year old male presented to M.D. Anderson Cancer Center complaining of a skin rash with pruritus for past 3 years, fatigue, and having hypereosinophilia. Upon evaluation the patient was found to be anemic (hemoglobin 9.8 g/dL), with normal WBC count but high eosinophil percentage (29%); tryptase was elevated (31 ng/mL). Bone marrow biopsy revealed MC infiltrate, and aspirate showed 29% eosinophils. Karyotype was normal; testing for KIT D816V mutation and the F1P1L1-PDGFRα rearrangement was negative. The patient was diagnosed with SM-HES. After 3 months of receiving imatinib 400 mg daily, patient’s CBC and bone marrow normalized and symptoms resolved. Tryptase level decreased to 1 ng/mL. This patient remains in complete remission after more than 44 months on imatinib therapy.
Six patients with ISM reported improvement in SM-related symptoms while on imatinib therapy, including decreased frequency of allergic reactions and episodes of hypotension, decreased flushing, itching, shortness of breath and frequency of diarrhea, and improvement in fatigue. Duration of improvement lasted between 9 and 36 months; all patients have stopped therapy due to a loss in response (recurrence of symptoms). Blood tryptase levels and bone marrow percent of MC in these patients has been recorded over time and all available results (including before and after imatinib therapy) are presented in Figure 2. Among them two patients were KIT-D816V positive: the first patient was 70 year old female who reported improvement in fatigue that lasted 14 months; blood tryptase level decreased while on therapy (Figure 1, panel D). The second patient was 39 year old female who had previously been exposed to imatinib therapy (400 mg daily for 2 years) with improvement in diarrhea; she again reported improvement in diarrhea while on therapy, lasting 9 months; no other benefits have been noted (Figure 1, panel E). Two patients previously exposed to low dose imatinib (100 mg a day) did not respond to higher doses. Other patients had no clinical benefit from imatinib therapy.
Figure 1.
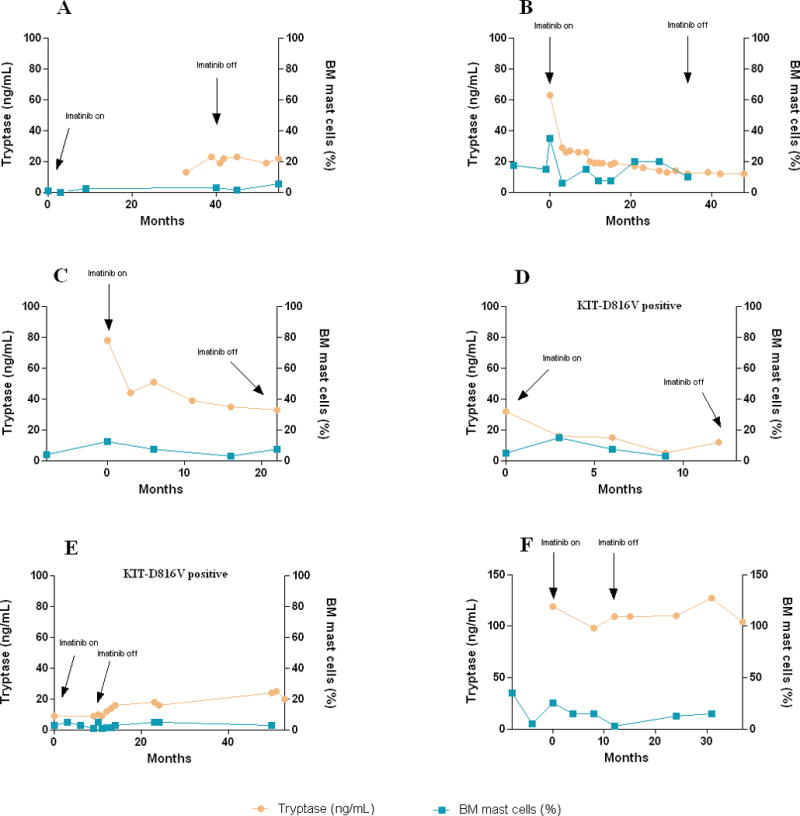
Serum tryptase levels and bone marrow (BM) mast cell percentage in patients with indolent systemic mastocytosis reporting symptomatic improvement while on imatinib therapy. Available data is presented, including pre- and post-therapy measurements.
Safety and tolerability
All patients received an initial imatinib dose of 400 mg once daily. The dose was not modified in 7 patients (decision rendered by the treating physician), it was reduced 1 dose level due to toxicity in 7 patients, and it was increased in 6 patients due to a lack of a response (in 5 to 800 mg and in 1 to 600 mg). Treatment related adverse events for all patients on all dose levels are summarized in Table 2. There was no significant difference in tolerance of 400 vs. 800 mg imatinib dose. Imatinib was discontinued within the first month of therapy in 1 patient due to grade 4 neutropenia and thrombocytopenia, and after 5 weeks of therapy in another patient due to grade 3 skin rash.
Table 2.
Number and grade of adverse events during treatment with imatinib
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Anemia | 5 | 7 | – | – |
| Low platelets | – | – | 1 | 1 |
| Neutropenia | – | – | 1 | 1 |
| Ascites | – | – | 1 | – |
| Diarrhea | 3 | 2 | 1 | – |
| Dyspepsia | 1 | – | – | – |
| Edema | 2 | – | 1 | – |
| Fatigue | 1 | 1 | 1 | – |
| Fever | 1 | – | – | – |
| Headache | 1 | – | – | – |
| Nausea/Vomiting | 3 | – | – | – |
| Pain | 2 | 2 | 1 | – |
| Rash | 4 | 1 | 1 | – |
Discussion
New molecular targets such as the gain-of-function mutations in the KIT gene represent potential treatment opportunities for patients SM [2]. Other potential targets for therapy in SM are the surface marker CD25, which is aberrantly expressed on the surface of neoplastic MC, or the nuclear factor kB (NFkB) [4]. Since the initial description of the KIT D816V mutation, several other sporadic KIT mutations have been described in patients with SM [1]. The discovery that the small molecule tyrosine kinase inhibitor imatinib mesylate was a potent inhibitor of KIT kinase led researchers to hypothesize that this agent might be clinically useful in patients with SM. Early clinical data have been contradictory, with some authors reporting no activity of the drug in this setting, and some others claiming that imatinib therapy was associated with clinical responses [5,6,8–11]. It is now well established that imatinib does not have direct effect on the KIT mutation present in the majority of SM patients (D816V KIT mutation), but that it may affect other, sporadic mutations (e.g. F522C KIT mutation) [1]. This led to the approval of imatinib by FDA in 2006 as therapy for adult patients with ASM that do not have D816V mutation.
We have started our study several years earlier, in 2003, with a hope to observe significant reduction in “tumor burden” in patients (as judged by tryptase level in blood and MC percentage in bone marrow, the 2 parameters used as a part of response criteria for ASM), not just possible symptomatic improvements. In our study, imatinib therapy resulted in one patient, with D816V mutation-negative SM-HES, experiencing complete resolution of signs and symptoms of the disease, with normalization of bone marrow and blood abnormalities. Similar successful outcomes have been reported previously in patients with SM-HES [9], and may be the result of the presence of yet unknown kinase sensitive to imatinib. We have also recorded long-term symptomatic improvement in 6 ISM patients, regardless of the presence or absence of the KIT D816V mutation. However, a major limitation of the present study is the difficulty in evaluating symptomatic improvement reported by patients, when the study was not randomized and placebo controlled, and did not employ established quality-of-life assessment. It is possible therefore that “placebo effect” significantly contributed to the observed improvements (e.g. improved fatigue in one patient with D816V-mutation positive ISM). In these 6 patients we did not observe uniform and consistent (to account for known variability in findings in samples obtained at different time points from same patient while not on therapy) improvement in the bone marrow MC percentage, but have recorded significant decrease in blood tryptase level in 2 patients (Figure 1, panels B and C). Interestingly, despite the recurrence of symptoms and cessation of therapy, tryptase levels did not rebound to initial high levels. In general, imatinib therapy was associated with a mild toxicity profile.
In summary, our study confirms that imatinib therapy does not result in appreciable clinical activity in patients with D816V mutation-positive SM, but may result in a significant benefit in occasional patient with D816V mutation-negative SM.
Bibliography
- 1.Horny HP, Sotlar K, Valent P. Mastocytosis: state of the art. Pathobiology. 2007;74:121–132. doi: 10.1159/000101711. [DOI] [PubMed] [Google Scholar]
- 2.Patnaik MM, Tefferi A, Pardanani A. Kit: molecule of interest for the diagnosis and treatment of mastocytosis and other neoplastic disorders. Curr Cancer Drug Target. 2007;7:492–503. doi: 10.2174/156800907781386614. [DOI] [PubMed] [Google Scholar]
- 3.Furitsu T, Tsujimura T, Tono T, et al. Identifications of mutations in the coding sequence of the proto-oncogen c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintas-Cardama A, Aribi A, Cortes J, et al. Novel Approaches in the Treatment of Systemic Mastocytosis. Cancer. 2006;107:1429–1439. doi: 10.1002/cncr.22187. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Zeng S, Metcalfe DD, et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- 6.Droogendijk HJ, Kluin-Nelemans HJ, van Doormaal JJ, et al. Imatinib Mesylate in the Treatment of Systemic Mastocytosis: a phase II trial. Cancer. 2006;107:345–351. doi: 10.1002/cncr.21996. [DOI] [PubMed] [Google Scholar]
- 7.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. 2001;25(7):603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 8.Piccaluga PP, Rondoni M, Paolini S, et al. Imatinib mesylate in the treatment of hematologic malignancies. Expert Opin Biol Ther. 2007;7:1597–1611. doi: 10.1517/14712598.7.10.1597. [DOI] [PubMed] [Google Scholar]
- 9.Pardanani A, Elliot M, Reeder T, et al. Imatinib for systemic mast cell disease. Lancet. 2003;362:535–537. doi: 10.1016/s0140-6736(03)14115-3. [DOI] [PubMed] [Google Scholar]
- 10.Akin C, Fumo G, Yavuz AS, et al. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–3225. doi: 10.1182/blood-2003-11-3816. [DOI] [PubMed] [Google Scholar]
- 11.Frost MJ, Ferrao PT, Hughes TP, et al. Juxtamembrane mutant V560Gkit is more sensitive to imatinib (STI571) compared with wild-type c-kit whereas the kinase mutant D816Vkit is resistant. Mol Cancer Ther. 2002;1:1115–1124. [PubMed] [Google Scholar]


