Abstract
Context:
Low-dose GH (LGH) therapy has been reported to improve insulin sensitivity in GH-deficient adults; however, the mechanism is unclear.
Hypothesis:
Effects of LGH therapy on insulin sensitivity are mediated through changes in cortisol metabolism and ectopic fat accumulation.
Design and Setting:
This was a double-blind, placebo-controlled, parallel, 3-month study.
Participants and Intervention:
Seventeen GH-deficient adults were randomized to receive either daily LGH or placebo injections. Fasting blood samples were collected at baseline, and months 1 and 3, whereas hyperinsulinemic-euglycemic clamps, magnetic resonance spectroscopy scans, 24-hour cortisol production rates (CPRs), and sc abdominal fat biopsies were performed at baseline and month 3.
Main Outcome Measures:
Clamp glucose infusion rate, intramyocellular, extramyocellular, and intrahepatic lipid content, 24-hour CPRs, adipocyte size, and adipocyte 11β-hydroxysteroid dehydrogenase activity in adults with GH deficiency were evaluated.
Results:
At month 1, LGH did not alter fasting levels of glucose, insulin, C-peptide, free fatty acid, adiponectin, total IGF-1, IGF-1 bioactivity, IGF-2, IGF binding protein (IGFBP)-2, or IGF-1 to IGFBP-3 molar ratio. At month 3, LGH increased clamp glucose infusion rates (P < .01) and IGF-1 to IGFBP-3 molar ratio (P < .05), but fasting glucose, insulin, C-peptide, free fatty acid, adiponectin, IGF-1 bioactivity, IGF-2, IGFBP-2, 24-hour CPRs, adipocyte size, adipocyte 11β-hydroxysteroid dehydrogenase activity, intrahepatic lipid, extramyocellular, or intramyocellular were unchanged. In the placebo group, all within-group parameters from months 1 and 3 compared with baseline were unchanged.
Conclusions:
Short-term LGH therapy improves insulin sensitivity without inducing basal lipolysis and had no effect on cortisol metabolism and ectopic fat accumulation in GH-deficient adults. This may reflect an LGH-induced increase in IGF-1 to IGFBP-3 molar ratio exerting insulin-like effects through the abundant muscle IGF-1 receptors, but this hypothesis requires confirmation with further studies.
The clinical features of adult GH deficiency (GHD) share many similarities to patients with chronic hypercortisolism (eg, Cushing's disease), including central obesity and insulin resistance (1). Although previous studies have demonstrated that GH replacement in adults with GHD using doses exceeding 0.3 mg/d decreases fat mass and increases lean body mass, insulin sensitivity remains unchanged (2, 3), improved (4, 5), or worsened (6, 7). Conversely, we (8, 9) and others (10) have found that low doses of GH therapy (0.1–0.3 mg/d) improved insulin sensitivity without modifying body composition; however, the mechanism remains unclear.
In Cushing's syndrome, the development of central obesity and insulin resistance is related to increased cortisol production rates (CPRs) and tissue-specific changes in cortisol levels, independent of underlying total adiposity (11, 12). Previous studies have also reported the GH-inhibitory effects on 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) activity and expression (13, 14), a tissue-specific enzyme that converts inactive cortisone to active cortisol (15). By contrast, in adults with GHD, 11β-HSD1 activity is increased (16). This has prompted us to speculate that increased CPRs and conversion of adipocyte cortisone to cortisol might play a role in the expression of central obesity and insulin resistance in adults with GHD.
Ectopic fat accumulation [ie, intrahepatic (IHL) and intramyocellular lipid (IMCL)] is now acknowledged as an important factor associated with insulin resistance (17). To accurately assess ectopic fat accumulation, proton magnetic resonance spectroscopy (MRS) has been used to noninvasively quantify IHL (18), extramyocellular lipid (EMCL) and IMCL (19). These measurements are physiologically relevant because IHL has been shown to be a better predictor of metabolic risk than visceral adiposity in obese individuals (20).
Therefore, the purpose of this study was to investigate the mechanism/s by which low-dose GH (LGH) therapy might improve insulin sensitivity in adults with GHD. To this end, we measured circulating cortisol levels, adipocyte cortisol metabolism, and ectopic fat accumulation because we hypothesized that that short-term LGH therapy would improve insulin sensitivity by modulating overnight CPRs and adipocyte 11β-HSD1 activity. We also anticipated that the overall altered phenotype would remain unchanged but that reductions in IHL and IMCL might be observed after 3 months of LGH therapy.
Patients and Methods
Patients
Seventeen nondiabetic adults with GHD attending the Oregon Health and Science University (OHSU) Endocrinology Out-Patient Clinic were recruited into the study. Baseline characteristics are shown in Table 1. The diagnosis of adult GHD was confirmed with either a single or combination of insulin tolerance test, GHRH-arginine test, and glucagon test. The diagnoses of the patients' pituitary diseases were nonfunctioning pituitary adenoma (n = 5), congenital hypopituitarism (n = 3), Cushing's disease (n = 2), traumatic brain injury (n = 2), craniopharyngioma (n = 1), hypothalamic dysgerminoma (n=1), lymphocytic hypophysitis (n = 1), optic glioma with previous cranial radiotherapy (n = 1), and whole-body radiotherapy for childhood leukemia (n = 1). In addition to GHD, 12 patients had other pituitary hormone deficiencies and received stable hormone replacement therapy in the form of levothyroxine, hydrocortisone, desmopressin, and sex steroids (injectable and transdermal T replacement in males; transdermal estrogen replacement in females) for at least 3 months before study entry. Two patients in the LGH group and two patients in the placebo group had secondary adrenal insufficiency and received hydrocortisone replacement therapy at divided doses with total doses ranging from 15 to 20 mg/d. The adequacy of hormone replacement therapy during the study was assessed at months 1 and 3. None of the patients received GH therapy within 6 months prior to study entry. The Institutional Review Board at OHSU approved the study, and written informed consent was obtained from each subject before study entry.
Table 1.
Anthropometric and Biochemical Parameters of Patients at Baseline and Months 1 and 3
| LGH (n = 8) |
Placebo (n = 9) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 1 | Month 3 | δMonth 3 − Baseline | Baseline | Month 1 | Month 3 | δMonth 3 − Baseline | |
| Gender, male/female | 3/5 | 4/5 | ||||||
| Age, y | 40.3 (3.7) | 38.1 (4.3) | ||||||
| Waist circumference, cm | 111.2 (6.8) | 113.3 (6.2) | 111.0 (5.7) | −0.2 (2.4) | 107.2 (9.3) | 108.8 (8.0) | 110.7 (9.1) | 2.9 (1.5) |
| BMI, kg/m2 | 35.7 (3.9) | 35.8 (3.9) | 35.9 (3.9) | 0.2 (0.2) | 34.2 (3.6) | 34.4 (3.6) | 35.0 (3.7) | 0.8 (0.4) |
| Body weight, kg | 104.4 (14.4) | 104.9 (14.4) | 100.1 (11.0) | −4.3 (4.8) | 99.0 (12.6) | 99.6 (12.9) | 101.1 (13.0) | 2.0 (1.2) |
| Glucose, mg/dL | 79.7 (5.2) | 80.1 (4.9) | 76.5 (3.3) | −3.2 (3.7) | 77.8 (4.5) | 76.2 (4.4) | 83.0 (4.8) | 5.2 (4.0) |
| Insulin, mIU/L | 15.7 (4.8) | 16.5 (4.4) | 13.9 (4.8) | −3.8 (2.6)a | 6.8 (1.1) | 10.6 (2.7) | 9.6 (2.3) | 2.8 (1.4) |
| C-peptide, ng/mL | 3.1 (0.8) | 3.9 (1.1) | 3.2 (1.1) | 0.1 (0.4) | 2.2 (0.4) | 2.7 (0.5) | 2.9 (0.5) | 0.7 (0.2) |
| FFA, mmol/L | 0.67 (0.08) | 0.65 (0.07) | 0.56 (0.09) | −0.24 (0.11) | 0.61 (0.07) | 0.66 (0.06) | 0.59 (0.09) | −0.02 (0.09) |
| Adiponectin, mg/L | 9.4 (2.1) | 10.1 (2.3) | 9.9 (2.0) | −0.9 (0.9) | 9.2 (1.0) | 9.8 (1.2) | 10.2 (1.1) | 1.0 (0.8) |
| IGF-1, μg/L | 86.1 (15.6) | 100.0 (14.3) | 110.4 (21.2) | 24.3 (13.1) | 75.8 (12.4) | 92.9 (16.1) | 95.8 (15.4) | 20.0 (8.7) |
| IGF-1 KIRA, μg/L | 0.52 (0.13) | 0.41 (0.07) | 0.44 (0.09) | −0.08 (0.09) | 0.71 (0.16) | 0.82 (0.15) | 0.94 (0.21) | 0.23 (0.13) |
| IGF-2, μg/L | 409.6 (39.6) | 455.8 (38.9) | 381.7 (24.8) | −8.0 (16.7) | 415.9 (40.6) | 424.6 (48.6) | 415.8 (40.6) | −0.1 (17.4) |
| IGFBP-2, μg/L | 145.0 (27.2) | 184.5 (43.3) | 163.0 (43.2) | −2.8 (16.3) | 144.2 (47.0) | 140.9 (31.9) | 103.3 (16.6) | −40.9 (34.1) |
| IGFBP-3, mg/L | 4.13 (0.43) | 4.48 (0.38) | 3.91 (0.41) | −0.22 (0.20)a | 3.70 (0.49) | 4.20 (0.53) | 4.07 (0.53) | 0.37 (0.19) |
| IGF-1 to IGFBP-3 molar ratio, % | 7.44 (0.96) | 8.22 (0.72) | 10.42 (1.76)b | 2.98 (1.24) | 7.50 (0.90) | 8.01 (1.15) | 8.61 (1.24) | 1.12 (0.82) |
| IGF-2 to IGFBP-3 molar ratio, % | 38.4 (0.9) | 39.4 (1.2) | 40.2 (1.6) | 1.5 (2.2) | 46.4 (3.3) | 40.1 (1.5) | 40.8 (2.0) | −5.5 (2.3) |
| IGF-1 + IGF-2 to IGFBP-3 molar ratio, % | 45.9 (1.0) | 47.6 (1.2) | 49.0 (1.9) | 3.4 (2.0) | 53.9 (3.2) | 48.1 (1.8) | 49.4 (1.8) | −4.4 (2.8) |
Data are presented as mean (SE), except for data on gender. Bold values indicate statistical significance of the data.
P < .05 vs placebo δmonth 3 − baseline.
P < .05 vs LGH baseline.
Methods
Study design
In this 3-month, double-blind, parallel study, eligible patients after completing the initial screening assessment visit were randomized to receive sc daily LGH (0.1 mg/d) or placebo injections for 3 months using the randomization scheme generated by the website for randomization (http://www.randomization.com). The patients were taught to self-administer the GH or placebo injections daily between 8:00 and 9:00 pm. Compliance was assessed by asking the patients whether they had missed any injections and by measuring the volume of GH in the returned vials at months 1 and 3 visit assessments. The patients were then provided further supplies of GH cartridges and were asked to return any half-used or unused GH cartridges at month 3. Fasting blood samples were collected at baseline and months 1 and 3, whereas 3-hour hyperinsulinemic-euglycemic clamps, MRS, dual-energy X-ray absorptiometry (DEXA), 24-hour CPR studies, and sc abdominal fat biopsies were performed at baseline and month 3.
Study procedures at baseline and month 3
One-step, 3-hour hyperinsulinemic-euglycemic clamp
Patients were admitted to the Oregon Clinical and Translational Research Institute (OCTRI) at OHSU at 8:00 am after fasting for 10 hours overnight. For the four patients with adrenal insufficiency, hydrocortisone tablets (15–20 mg) were ingested on the day before, whereas the morning dose was taken just before undergoing the clamp. At the start of each clamp, three peripheral iv lines (one to obtain blood samples in a heated sleeve, and the other two for infusions) were placed in the antecubital fossa and dorsum of the hand. An iv insulin bolus (7.0 mIU/kg) was administered followed by an iv insulin infusion of 1.5 mIU/kg·min. Blood glucose levels were measured at 5-minute intervals and maintained at x mg/dL (where x was the mean fasting blood glucose level from 8:30 to 09:00 am minus 10 mg/dL) using a variable rate of 20% dextrose infusion.
Twenty-four-hour CPRs
Patients remained at OCTRI in the evening to undertake the 24-hour CPR studies. For those patients on hydrocortisone replacement, their evening hydrocortisone dosing and for the whole duration of this part of the study was omitted. Starting at 2:00 am, two peripheral iv lines were started, one for infusion of deuterium-labeled cortisol isotope and the other for blood sampling. The continuous infusion of deuterium-labeled cortisol (54.6 nmol/h) was administered over the next 30 hours (until 8:00 am of the final study day), and blood samples (5 mL) were drawn every 30 minutes from the second iv line during the final 24 hours of the deuterated cortisol infusion. The total amount of deuterated analog infused over 24 hours (1.31 μmol) was less than 5% of the normal endogenous 24-hour CPR. Plasma was separated, and 50 μL from each sample was combined into a 24-hour pooled sample for mass spectral analysis. The samples were then stored at −80°C, and sample preparation and analyses using gas chromatography mass spectrometry and liquid chromatography mass spectrometry techniques were performed later, as previously described (21).
Subcutaneous fat biopsy and adipocyte 11β-HSD activity analysis
Subcutaneous fat tissue biopsies were collected by liposuction from the periumbilical region. After local anesthesia with lidocaine was administered, isotonic saline was injected through the liposuction cannula and biopsies obtained by applying vacuum. Adipose tissue biopsy samples were washed with isotonic saline to remove blood and frozen at −80°C for later measurement of 11β-HSD activity. Parallel samples were rinsed in DMEM containing F12 salts, 2.5 mM L-glutamine, and 15 mM HEPES and blotted on a Telfa pad. The tissue was then apportioned, weighted (50–100 mg), and placed in 1.0 mL DME-F12 (without serum) containing 1 μCi 3H-steroid (cortisol or cortisone) in triplicate. A control for metabolism in the absence of tissue was prepared in parallel. Tissue and no tissue control samples were incubated at 37°C with 5% CO2 over 24 hours. After incubation, carrier steroids were added and the medium was extracted with ethyl acetate. An aliquot of extracts containing labeled and unlabeled steroids (15 μL) were separated by thin-layer chromatography on LK6DF plates. Separation of steroids was monitored using UV fluorescence cochromatographed with each sample.
Radioactivity was determined after transfer of thin-layer chromatography sections to a vial for liquid scintillation counting. The tissue (10–15 mg) was digested at 37°C with 200 μL of collagenase buffer (2.0 mg/mL) to liberate the adipocytes. A digital image of the adipocyte suspension was obtained by photo-microscopy, and cell diameter was measured using computerized software (Image J; National Institutes of Health, Bethesda, Maryland). The mean adipocyte diameter of at least 200 cells was used to calculate cell volume, and data were normalized to time of incubation, cell volume, and amount of tissue per tube. The adipocytes were then incubated with 3H-steroid (cortisol or cortisone) and extracted with ethyl acetate before unlabeled steroid was added as carrier. A portion of the extracts (15 μL) was applied to the preabsorbent area and chromatographed in chloroform-ethanol (92:8 vol/vol). After chromatography, unlabeled steroids were detected by UV activated fluorescence, and radioactivity in the corresponding silica gel sections was determined by liquid scintillation counting.
MRS and DEXA studies
The MRS studies were performed at the OHSU Advanced Imaging Research Center using a Siemens Magnetom Tim Trio 3 Tesla (Siemens Medical Solutions) whole-body system. Initially, magnetic resonance imaging of the abdomen for visceral and sc fat was obtained, as previously described (22). A single slice at the level on the umbilicus was used for analysis of the visceral and sc fat. Then measurements of IHL, EMCL, and IMCL were performed using image-guided, 1H-localized MRS after a high-resolution T1-weighted spin-echo image (23). Volumes of interest within right leg muscle were centered over the midsoleus muscle (12 mm3). Similarly, volumes of interest within the liver were located away from major vascular structures, typically within the right lobe. Localized proton spectra within muscle were collected using a PRESS sequence with the following parameters: repetition time = 5 seconds, time to echo = 30 milliseconds, 64 averages, no water suppression, and 1024 data points greater than 2000 kHz spectral width. Liver spectra were collected using the same sequence and parameters except larger voxel size (30 mm3) for a method of signal averaging to correct for liver motion due to patient respiration. IHL, EMCL, and IMCL were calculated by taking the ratio of the integrated lipid peak at 1.3 ppm to the integrated water peak at 4.7 ppm. Both water and lipid peaks were corrected for relaxation time effects.
The DEXA (Hologic QDR 4500 densitometer; Hologic) studies were performed at OCTRI to measure total fat mass, lean body mass, and percentage total fat.
Assays
Blood glucose levels were measured using a YSI model 2300 (Yellow Springs Instruments). Plasma insulin levels were measured by ELISA (Mercodia), and serum C-peptide levels by an automated chemiluminescence assay (Immulite 1000; Siemens Healthcare Diagnostics). Plasma free fatty acid (FFA) levels were measured by a commercially available calorimetric assay (Zen-Bio). Serum adiponectin levels were measured by an in-house time-resolved immunofluorometric assay based on commercially available reagents obtained from R&D Systems. The assay has been previously validated (24) and has been shown to detect all major molecular forms of adiponectin (25). The assay has an intra- and interassay coefficient of variation (CV) of 5% and 10%.
Serum IGF-1 and IGF binding protein (IGFBP)-3 levels were measured by commercial immunoassays (Immunodiagnostic Systems) using the automated iSYS platform, as previously described (26). In vitro IGF-1 bioactivity was measured by an in-house IGF-1 receptor kinase receptor activation (KIRA) assay, as previously described (27). The KIRA assay is a cell-based bioassay based on human embryonic kidney-293 cells overexpressing the human IGF-1 receptor. The assay detects the ability of serum to phosphorylate the IGF-1 receptor in vitro (28). The intra- and interassay CV of the bioassay was 10% and 15%, respectively. Serum IGF-2 was measured by time-resolved immunofluorometric assay (29). The assay has been calibrated against the international IGF-2 reference preparation World Health Organization 96/538, and the detection antibody was a goat polyclonal antibody (Sigma-Aldrich), which prior to use has been labeled with europium according to the manufacturer (PerkinElmer Life Sciences). Serum IGFBP-2 was measured by an in-house time-resolved immunofluorometric assay, as previously described (30), and has an intra- and interassay CV of 5% and 12%.
Calculations
Serum IGF-1, IGF-2, and IGFBP-3 levels are quoted in micrograms per liter in Table 1, but the calculation of the IGF-1 to IGFBP-3, IGF-2 to IGFBP-3, and IGF-1 + IGF-2 to IGFBP-3 molar ratios were performed after conversion into nanomoles per liter (IGF-1: micrograms per liter × 0.131 = nanomoles per liter; IGF-2: micrograms per liter × 0.134 = nanomoles per liter; IGFBP-3: micrograms per liter × 0.0348 = nanomoles per liter). To illustrate the relative abundance of circulating IGF-1, IGF-2, and IGFBP-3 molecules, the resulting molar ratio was expressed as a percentage (26). The glucose infusion rate (GIR) divided by lean body weight obtained by DEXA measurements (M-value) to calculate insulin sensitivity was determined during the final 30 minutes of the hyperinsulinemic-euglycemic clamp from the mean rate of infusion of 20% dextrose required to maintain euglycemia per kilogram body weight per minute.
Statistical analysis
All statistical analyses were performed using SPSS for Windows (version 20). The primary outcome of interest was within-group (month 1 vs baseline and month 3 vs baseline) and between-group (LGH δmonth 3 − baseline vs placebo δmonth 3 − baseline) differences. Distributions of residuals were examined for normality by graphical methods. For within-group and between-group comparisons that were normally distributed, the Student's paired and unpaired t test was used, respectively. Where the residuals were not normally distributed, nonparametric methods such as the Wilcoxon signed-rank test and the Mann-Whitney test were used, where appropriate. Data are reported as mean (SE), unless otherwise stated, and values of P < .05 defined statistical significance.
Results
Patient characteristics at baseline
All 17 patients completed the study. The patients in the 2 groups were well matched at baseline, with no differences with regard to gender, age, waist circumference, body mass index (BMI), or body weight (Table 1).
Biochemical parameters
Fasting blood data (Table 1)
Compared with baseline, there were no differences for within- and between-group comparisons at months 1 and 3 for patients treated with LGH and placebo with regard to waist circumference, BMI, body weight, or fasting levels of glucose, C-peptide, FFA, adiponectin, total IGF-1, IGF-1 KIRA, IGF-2, or IGFBP-2. Although there were no within-group changes for fasting insulin and IGFBP-3 levels for both groups, between-group differences of these levels were observed between month 3 compared with baseline (both P < .05). Compared with baseline, LGH therapy at month 3 increased the IGF-1 to IGFBP-3 molar ratio (P < .05), whereas the changes in IGF-2 to IGFBP-3 and IGF-1 + IGF-2 to IGFBP-3 molar ratios between month 3 compared with baseline tended to differ (P = .06). Conversely, there were no differences in the within-group comparisons from months 1 and 3 compared with baseline in the placebo group.
Three-hour hyperinsulinemic-euglycemic clamp
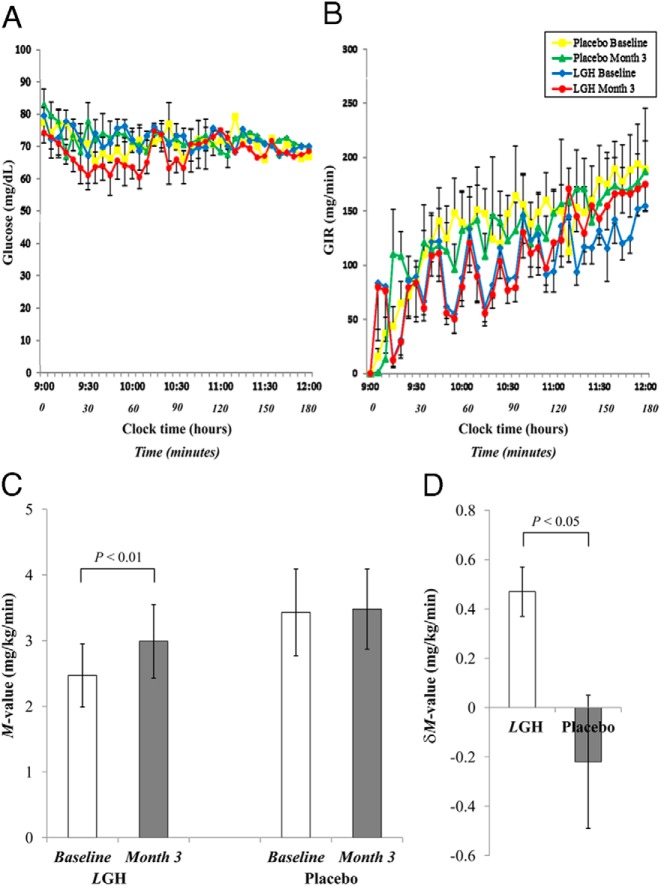
Steady state levels of glucose (Figure 1A) and GIR (Figure 1B) were achieved in the final 30 minutes of the clamp. In the LGH group, the M-value was significantly higher at month 3 compared with baseline (P < .01), but no changes were observed in the placebo group (Figure 1C). There was also a significant difference in δM-value between month 3 compared with baseline when comparing the two groups (P < .05) (Figure 1D). Conversely, there was no correlation between δIGF-1 to IGFBP-3 molar ratio with δM-value in either group.
Figure 1.
Glucose levels (A), GIR plotted against time (B), M-value (GIR adjusted for lean body weight) at baseline and month 3 (C), and δM-value (δmonth 3 − baseline) (D) for patients treated by LGH and placebo during the 3-hour hyperinsulinemic-euglycemic clamps at baseline and month 3. Data are presented as mean ± SE.
Cortisol metabolism, body composition, and ectopic fat assessments (Table 2)
Table 2.
Twenty-Four CPR, Adipocyte Size, Adipocyte 11β-HSD Activity, and DEXA and MRS Parameters of Patients at Baseline and Months 1 and 3
| LGH (n = 8) |
Placebo (n = 9) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Month 3 | δMonth 3 − Baseline | Baseline | Month 3 | δMonth 3 − Baseline | |
| Twenty-four-hour CPR, mg/d | 36.3 (9.5) | 35.9 (10.7) | −0.4 (3.4) | 25.9 (5.3) | 23.0 (5.2) | −2.9 (2.6) |
| Adipocyte size | ||||||
| Adipocyte diameter, μm | 102.0 (4.4) | 103.0 (1.5) | 1.0 (4.3) | 93.9 (5.7) | 96.4 (5.1) | 2.5 (6.4) |
| Adipocyte volume, cm3 | 56.7 (6.9) | 57.3 (2.4) | 0.6 (6.5) | 45.7 (8.0) | 48.8 (7.6) | 3.0 (10.0) |
| Adipocyte 11β-HSD activity, pmol/mg·h × 10−3 | ||||||
| Conversion of cortisone to cortisol | 3.2 (0.5) | 5.6 (1.2) | 2.4 (1.4) | 10.1 (2.5) | 5.6 (0.6) | −4.5 (2.6) |
| Conversion of cortisol to cortisone | 2.2 (0.5) | 3.4 (1.1) | 1.8 (1.6) | 1.9 (0.2) | 2.4 (0.3) | 0.4 (0.5) |
| DEXA studies | ||||||
| Total fat mass, kg | 38.9 (4.9) | 38.4 (5.4) | 0.47 (0.73) | 38.4 (6.6) | 37.3 (7.7) | 0.49 (0.80) |
| Total lean body mass, kg | 58.0 (7.1) | 56.3 (8.0) | 0 (1.11) | 57.7 (5.9) | 59.1 (7.0) | 1.57 (0.59) |
| Total fat, % | 39.2 (3.2) | 39.7 (3.5) | 0.43 (0.46) | 36.9 (3.1) | 35.5 (3.3) | −0.39 (0.42) |
| MRS studies, % of water peak | ||||||
| IHL | 11.8 (6.5) | 10.7 (9.3) | 2.9 (2.7) | 12.5 (6.3) | 11.4 (5.5) | −1.1 (1.1) |
| EMCL | 4.7 (1.6) | 5.8 (2.3) | 0.7 (0.6) | 2.8 (0.4) | 3.4 (0.9) | 0.7 (0.4) |
| IMCL | 2.0 (0.6) | 1.7 (0.4) | 0.1 (0.4) | 1.2 (0.2) | 0.9 (0.2) | −0.2 (0.1) |
Data are presented as mean (SE). Comparisons of all data within groups (month 3 vs baseline) and between groups (LGH vs placebo of δmonth 3 − baseline) were all not significant.
There were no within- or between-group differences for 24-hour CPR, adipocyte diameter, adipocyte volume, and adipocyte 11β-HSD activity, as evidenced by the comparable adipocyte interconversion of cortisone to cortisol levels between month 3 compared with baseline in both groups. In addition, DEXA and MRS studies revealed no within- or between-group differences between month 3 compared with baseline for total fat mass, total lean body mass, percentage total fat, IHL, EMCL, or IMCL in both groups. Furthermore, there was no correlation between IHL and IMCL in either group.
Discussion
The present study was undertaken to further elucidate the mechanism(s) of the insulin-sensitizing effects of LGH therapy in adults with GHD by testing the hypothesis that these effects are mediated through modulation of cortisol metabolism and ectopic fat accumulation. This study is an extension from our previous 12-month study (9) but used a placebo-controlled design, shorter duration of therapy to minimize potential changes in visceral adiposity, and novel techniques to analyze IGF-1 bioactivity, CPR, adipocyte size, adipocyte cortisone to cortisol interconversion, and ectopic fat accumulation.
This study demonstrated similar improvements in insulin sensitivity, increased IGF-1 to IGFBP-3 molar ratio (hereafter referred to as bioavailable IGF-1), and no changes in total fat, lean body mass, and basal lipolysis after 3 months compared with our previous observations after 7 days (8) and 12 months (9) of LGH therapy. Although this study did not confirm our hypothesis, it yielded several important findings. First, 3-month LGH therapy did not alter circulating cortisol levels, adipocyte size, adipocyte cortisol metabolism, and ectopic fat accumulation. Second, biochemical parameters of the IGF system (IGF-1 bioactivity, IGF-2, and IGFBP-2) and adiponectin levels were also unchanged.
We previously demonstrated that IGF-1 and IGFBP-3 levels and IGF-1 to IGFBP-3 molar ratio begins to rise from day 3 onward with LGH therapy and that by day 7, direct acute insulinotropic effects on pancreatic β-cells without changes in insulin sensitivity were observed (8). Conversely, Krusenstjerna-Hafstrom et al (31) demonstrated the induction of insulin resistance that rapidly reversed 5 hours after discontinuation of GH exposure in adults with GHD, but the GH dose used was higher compared with that in our study. Nonetheless, in contrast to the findings from our previous studies in which reductions in fasting glucose levels were demonstrated after 12 months of LGH therapy (9) and persisting after 6 months of discontinuing therapy (32), the present study did not demonstrate any lowering of fasting glucose and FFA levels, indicating that hepatic insulin sensitivity and basal lipolysis were unchanged, respectively.
A potential explanation of the discrepancy between our present and previous (8, 9, 32) findings and those by Krusenstjerna-Hafstrom et al (31) is that GH therapy may induce time-course changes in β-cell function and insulin sensitivity that are dependent on the dose and timing of GH administration in relationship to the time as to when insulin sensitivity is assessed. The lack of reduction in fasting glucose levels could also be due to the greater BMI and total adiposity of the subjects in this study, whom were more likely to have underlying hepatic steatosis (33) and greater degrees of hepatic insulin resistance (34). Furthermore, because the patients in this study were mainly obese, it is also possible that the increased IGF-1 to IGFBP-3 molar ratio generated by LGH therapy reflects a greater GH responsiveness in obesity that induces larger increments of IGF-1 relative to IGFBP-3 (35).
In the present study, the in vivo activity of the IGF system was assessed by two distinct methods, ie, by the KIRA bioassay and by calculating the molar ratio between IGF-1 and IGFBP-3. The KIRA bioassay is designed to measure the ability of serum to phosphorylate (ie, activate) the IGF-1 receptor in vitro. Thus, the KIRA bioassay integrates the contribution of free, unbound IGF-1 and dissociable IGF-1, ie, IGF-1 loosely bound to proteolyzed IGFBPs, and takes into account of the short-term metabolic changes in IGFBP-1 levels (28). The bioassay also detects the contribution of IGF-2, which in its pure form has an affinity of 12% to that of IGF-1 (27). Conversely, the IGF-1 to IGFBP-3 molar ratio is an indirect measurement and likely to be affected by changes in IGF-2 and other IGFBPs and by IGFBP-3 proteolysis, as virtually all IGFBP-3 immunoassays comeasures IGFBP-3 fragments to some extent, irrespective of the IGF binding ability of the fragments. Therefore, the signal reported by the KIRA bioassay is distinct from that obtained by calculating the ratio between the immunoassay-determined IGF-1 and IGFBP-3 levels. We acknowledge that the IGF-1 to IGFBP-3 molar ratio has been studied to a limited extent (36) and requires further validation as a marker of IGF-1 bioavailability (37). Given the small subject numbers in the present study, the observed discrepancy between IGF-1 KIRA levels and the IGF-1 to IGFBP-3 molar ratio may represent a chance finding, biological variability, and/or method-specific assay variation.
Alternatively, it is also possible that increased IGF-1 bioavailability may enhance muscle insulin signaling via cross talk with abundant muscle IGF-1 receptors. Specifically, the increased coexpression of muscle IGF-1 and insulin receptors permits the generation of IGF-1/insulin hybrid receptors (38) that are activated by IGF-1 but not insulin, leading to IGF-1-induced autophosphorylation of insulin receptor hemireceptor components of the receptor hybrid, resulting in insulin-like intracellular signaling. Further studies are needed using muscle biopsy samples to examine myocyte IGF-1 and insulin receptor signaling in adults with GHD to confirm this hypothesis.
The effects of cortisol depend on CPR and tissue-specific responses to 11β-HSD1 activity in converting inactive cortisone to active cortisol (39), with adipocyte 11β-HSD1 activity correlating with visceral adiposity and insulin resistance (40). Current evidence implies that ectopic fat accumulation contributes to the association of adiposity with insulin resistance (41). Proton MRS was recently developed to quantify ectopic fat in various organs and is now considered the gold standard method in noninvasive triglyceride assessment in liver and muscle (41). Adiponectin is a hormone that modulates glucose regulation and fatty acid oxidation and inversely correlates with total adiposity (42). Because CPR, adipocyte 11β-HSD1 activity, IMCL, and IHL were unchanged after LGH therapy in the present study, these observations may be explained by the low GH dose used over a short duration, whereas the lack of change in adiponectin levels is consistent with the unchanged adipocyte size, total fat mass, and ectopic fat patterning.
Strengths of our study include the comprehensive assessment of circulating and adipocyte cortisol metabolism and liver and muscle triglyceride content using novel biochemical and imaging techniques. However, the study also has some limitations. First, the sample size was relatively small because of the extensive investigations and was not adequately powered to detect subtle changes in the IGF system. Second, the IGF-1 to IGFBP-3 molar ratio may not accurately reflect IGF-1 bioavailability but rather an altered relationship between IGF-1 and IGF-2 levels within the two groups. Third, 11β-HSD activity may be higher in human visceral compared with sc adipose tissue. Analyzing 11β-HSD activity in visceral adipose tissue samples would have been informative in providing further in-depth insight of adipocyte cortisol metabolism, but collecting visceral adipocyte samples is invasive and technically challenging. Therefore, it remains plausible that LGH therapy could still modify cortisol metabolism in visceral adipocytes, although unlikely, given that there were no notable changes in adiponectin levels and total fat mass.
In summary, short-term LGH therapy improves insulin sensitivity, most likely at the muscle, without modifying circulating and adipocyte cortisol metabolism, and ectopic fat accumulation in adults with GHD. The exact mechanism remains unclear but potentially may be related to the LGH-induced increase in bioavailable IGF-1 exerting insulin-like effects through the abundant muscle IGF-1 receptors. From a clinical standpoint, in contrast to higher GH doses (13), our data also imply that glucocorticoid replacement doses in patients with concurrent ACTH deficiency do not require adjusting to compensate for reduced 11β-HSD1 activity when LGH therapy is initiated. Future glucocorticoid dose adjustments may still be necessary but requires confirmation with long-term studies.
Acknowledgments
We thank Pfizer Inc for kindly providing the recombinant human GH and placebo injections used in this study. We are also grateful to David Brandon and Ken Newcomb for performing the measurements of adipocyte size and 11β-HSD activity analyses and Lone Kvist, Elsebeth Hornemann, Lisa Buus, Kirsten Nyborg Rasmussen, and Susanne Sorensen for the measurements of IGF-1, IGF-1 bioactivity, IGF-2, IGFBP-2, and IGFBP-3.
This study had a clinical trials registry number of NCT00517062.
This work was supported by Pfizer, Inc and National Institutes of Health Grant R01 DK068146 (to J.Q.P.) and National Center for Advancing Translational Sciences Grant UL1RR024140. K.C.J.Y. was supported by investigator-initiated research grants from Pfizer, Inc and from the Growth Hormone Research Society.
Disclosure Summary: K.C.J.Y. has received research grants from Pfizer, Novo Nordisk, Eli Lilly, and Versartis and has served on the advisory boards for Pfizer, Novo Nordisk, and Corcept Therapeutics. C.T.R. has received research grants from Merck and Co and Takeda Pharmaceuticals North America. J.Q.P. has served on the advisory boards for Novo Nordisk and Corcept Therapeutics and is an investigator in a clinical trial funded by Sanofi.
Footnotes
- BMI
- body mass index
- CPR
- cortisol production rate
- DEXA
- dual-energy x-ray absorptiometry
- EMCL
- extramyocellular lipid
- FFA
- free fatty acid
- GHD
- GH deficiency
- GIR
- glucose infusion rate
- 11β-HSD1
- 11β-hydroxysteroid dehydrogenase type 1
- IGFBP
- IGF binding protein
- IHL
- intrahepatic lipid
- IMCL
- intramyocellular lipid
- KIRA
- kinase receptor activation
- LGH
- low-dose GH
- MRS
- magnetic resonance spectroscopy
- OCTRI
- Oregon Clinical and Translational Research Institute
- OHSU
- Oregon Health and Science University.
References
- 1. Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing's disease: clinical and health-related quality of life aspects. Eur J Endocrinol. 2012;167(3):311–326 [DOI] [PubMed] [Google Scholar]
- 2. Beauregard C, Utz AL, Schaub AE, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93(6):2063–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roemmler J, Kuenkler M, Schneider HJ, Dieterle C, Schopohl J. Comparison of glucose and lipid metabolism and bone mineralization in patients with growth hormone deficiency with and without long-term growth hormone replacement. Metabolism. 2010;59(3):350–358 [DOI] [PubMed] [Google Scholar]
- 4. Colao A, Di Somma C, Spiezia S, et al. Growth hormone treatment on atherosclerosis: results of a 5-year open, prospective, controlled study in male patients with severe growth hormone deficiency. J Clin Endocrinol Metab. 2008;93(9):3416–3424 [DOI] [PubMed] [Google Scholar]
- 5. Sato T, Katabami T, Furukawa K, et al. Intracellular lipid content of liver and skeletal muscle in patients with adult growth hormone deficiency without diabetes mellitus. Obes Res Clin Pract. 2012;6(4):e263–e346 [DOI] [PubMed] [Google Scholar]
- 6. Bramnert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, Groop L. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab. 2003;88(4):1455–1463 [DOI] [PubMed] [Google Scholar]
- 7. Rosenfalck AM, Maghsoudi S, Fisker S, et al. The effect of 30 months of low-dose replacement therapy with recombinant human growth hormone (rhGH) on insulin and C-peptide kinetics, insulin secretion, insulin sensitivity, glucose effectiveness, and body composition in GH-deficient adults. J Clin Endocrinol Metab. 2000;85(11):4173–4181 [DOI] [PubMed] [Google Scholar]
- 8. Yuen K, Cook D, Ong K, et al. The metabolic effects of short-term administration of physiological versus high doses of GH therapy in GH deficient adults. Clin Endocrinol (Oxf). 2002;57(3):333–341 [DOI] [PubMed] [Google Scholar]
- 9. Yuen KC, Frystyk J, White DK, et al. Improvement in insulin sensitivity without concomitant changes in body composition and cardiovascular risk markers following fixed administration of a very low growth hormone (GH) dose in adults with severe GH deficiency. Clin Endocrinol (Oxf). 2005;63(4):428–436 [DOI] [PubMed] [Google Scholar]
- 10. Arafat AM, Mohlig M, Weickert MO, Schofl C, Spranger J, Pfeiffer AF. Improved insulin sensitivity, preserved beta cell function and improved whole-body glucose metabolism after low-dose growth hormone replacement therapy in adults with severe growth hormone deficiency: a pilot study. Diabetologia. 2010;53(7):1304–1313 [DOI] [PubMed] [Google Scholar]
- 11. Chanson P, Salenave S. Metabolic syndrome in Cushing's syndrome. Neuroendocrinology. 2010;92(suppl 1):96–101 [DOI] [PubMed] [Google Scholar]
- 12. Krikorian A, Khan M. Is metabolic syndrome a mild form of Cushing's syndrome? Rev Endocr Metab Disord. 2010;11(2):141–145 [DOI] [PubMed] [Google Scholar]
- 13. Giavoli C, Libe R, Corbetta S, et al. Effect of recombinant human growth hormone (GH) replacement on the hypothalamic-pituitary-adrenal axis in adult GH-deficient patients. J Clin Endocrinol Metab. 2004;89(11):5397–5401 [DOI] [PubMed] [Google Scholar]
- 14. Paulsen SK, Pedersen SB, Jorgensen JO, et al. Growth hormone (GH) substitution in GH-deficient patients inhibits 11β-hydroxysteroid dehydrogenase type 1 messenger ribonucleic acid expression in adipose tissue. J Clin Endocrinol Metab. 2006;91(3):1093–1098 [DOI] [PubMed] [Google Scholar]
- 15. Tomlinson JW, Walker EA, Bujalska IJ, et al. 11β-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25(5):831–866 [DOI] [PubMed] [Google Scholar]
- 16. Gelding SV, Taylor NF, Wood PJ, et al. The effect of growth hormone replacement therapy on cortisol-cortisone interconversion in hypopituitary adults: evidence for growth hormone modulation of extrarenal 11β-hydroxysteroid dehydrogenase activity. Clin Endocrinol (Oxf). 1998;48(2):153–162 [DOI] [PubMed] [Google Scholar]
- 17. Lettner A, Roden M. Ectopic fat and insulin resistance. Curr Diab Rep. 2008;8(3):185–191 [DOI] [PubMed] [Google Scholar]
- 18. Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54(1):122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol (1985). 1999;87(6):2068–2072 [DOI] [PubMed] [Google Scholar]
- 20. Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106(36):15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klopfenstein BJ, Purnell JQ, Brandon DD, Isabelle LM, DeBarber AE. Determination of cortisol production rates with contemporary liquid chromatography-mass spectrometry to measure cortisol-d(3) dilution after infusion of deuterated tracer. Clin Biochem. 2011;44(5–6):430–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol. 2012;85(1018):e826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276(5 Pt 1):E977–E989 [DOI] [PubMed] [Google Scholar]
- 24. Frystyk J, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia. 2005;48(9):1911–1918 [DOI] [PubMed] [Google Scholar]
- 25. Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab. 2007;92(5):1857–1862 [DOI] [PubMed] [Google Scholar]
- 26. Friedrich N, Wolthers OD, Arafat AM, et al. Age and sex specific reference intervals across life-span for insulin-like growth factor binding protein 3 (IGFBP-3) and the IGF-I/IGFBP-3 ratio measured by new automated chemiluminescence assays. J Clin Endocrinol Metab. 2014;jc20133060 [DOI] [PubMed] [Google Scholar]
- 27. Chen JW, Ledet T, Orskov H, et al. A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum. Am J Physiol Endocrinol Metab. 2003;284(6):E1149–E1155 [DOI] [PubMed] [Google Scholar]
- 28. Frystyk J. Quantification of the GH/IGF-axis components: lessons from human studies. Domest Anim Endocrinol. 2012;43(2):186–197 [DOI] [PubMed] [Google Scholar]
- 29. Frystyk J, Dinesen B, Orskov H. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul. 1995;5(4):169–176 [PubMed] [Google Scholar]
- 30. Reinhard M, Frystyk J, Jespersen B, et al. Effect of hyperinsulinemia during hemodialysis on the insulin-like growth factor system and inflammatory biomarkers: a randomized open-label crossover study. BMC Nephrol. 2013;14(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krusenstjerna-Hafstrom T, Clasen BF, Moller N, et al. Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J Clin Endocrinol Metab. 2011;96(8):2548–2557 [DOI] [PubMed] [Google Scholar]
- 32. Yuen KC, Dunger DB. Persisting effects on fasting glucose levels and insulin sensitivity after 6 months of discontinuation of a very low-dose GH therapy in adults with severe GH deficiency. Clin Endocrinol (Oxf). 2006;64(5):549–555 [DOI] [PubMed] [Google Scholar]
- 33. Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev. 2004;5(1):27–42 [DOI] [PubMed] [Google Scholar]
- 34. Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–32353 [DOI] [PubMed] [Google Scholar]
- 35. Yuen KC, Cook DM, Rumbaugh EE, Cook MB, Dunger DB. Individual IGF-I responsiveness to a fixed regimen of low-dose growth hormone replacement is increased with less variability in obese compared to non-obese adults with severe growth hormone deficiency. Horm Res. 2006;65(1):6–13 [DOI] [PubMed] [Google Scholar]
- 36. Frystyk J. Free insulin-like growth factors—measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res. 2004;14(5):337–375 [DOI] [PubMed] [Google Scholar]
- 37. Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14(5):329–341 [DOI] [PubMed] [Google Scholar]
- 38. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623 [DOI] [PubMed] [Google Scholar]
- 39. Rask E, Olsson T, Soderberg S, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86(3):1418–1421 [DOI] [PubMed] [Google Scholar]
- 40. Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11β-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296(2):E351–E357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169(3):166–176 [DOI] [PubMed] [Google Scholar]
- 42. Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med (Berl). 2002;80(11):696–702 [DOI] [PubMed] [Google Scholar]