Abstract
Context:
Pelvic organ prolapse (POP) increases in prevalence with age; recurrence after surgical repair is common.
Objective:
The objective of the study was to determine the effects of local estrogen treatment on connective tissue synthesis and breakdown in the vaginal wall of postmenopausal women planning surgical repair of POP.
Design:
This was a randomized trial.
Setting:
The study was conducted at an academic tertiary medical center.
Patients or Other Participants:
Postmenopausal women with a uterus and symptomatic anterior and/or apical prolapse at stage 2 or greater participated in the study.
Intervention:
Estrogen (Premarin) or placebo cream for 6 weeks preoperatively was the intervention.
Main Outcome Measures:
Full-thickness anterior apical vaginal wall biopsies were obtained at the time of hysterectomy and analyzed for mucosa and muscularis thickness, connective tissue synthesis, and degradation. Serum levels of estrone and 17β-estradiol were analyzed at baseline and the day of surgery using highly sensitive liquid chromatography-tandem mass spectrometry.
Results:
Fifteen women per group (n = 30 total) were randomized; 13 per group underwent surgery. Among drug-adherent participants (n = 8 estrogen, n = 13 placebo), epithelial and muscularis thickness was increased 1.8- and 2.7-fold (P = .002 and P =.088, respectively) by estrogen. Collagen types 1α1 and 1α2 mRNA increased 6.0- and 1.8-fold in the vaginal muscularis (P < .05 for both); collagen type Ia protein increased 9-fold in the muscularis (P = .012), whereas collagen III was not changed significantly. MMP-12 (human macrophage elastase) mRNA was suppressed in the vaginal mucosa from estrogen-treated participants (P = .011), and matrix metalloprotease-9 activity was decreased 6-fold in the mucosa and 4-fold in the muscularis (P = .02). Consistent with menopausal norms, serum estrone and 17β-estradiol were low and did not differ among the two groups.
Conclusions:
Vaginal estrogen application for 6 weeks preoperatively increased synthesis of mature collagen, decreased degradative enzyme activity, and increased thickness of the vaginal wall, suggesting this intervention improves both the substrate for suture placement at the time of surgical repair and maintenance of connective tissue integrity of the pelvic floor.
Pelvic organ prolapse (POP) is the most common reason for hysterectomy among postmenopausal women and is an indication for more than 338 000 inpatient surgical procedures each year (1). With advancing age, both the incidence and prevalence of POP increases (2). Whereas it is difficult to separate the effects of declining estrogen levels in menopause from aging in general, it is clear that pelvic organs and their surrounding muscular and connective tissue support are estrogen responsive and that epidemiological studies indicate that menopause is a major risk factor for development of POP with symptoms and severity increasing after menopause (3, 4). No doubt, estrogens (17β-estradiol or conjugated equine estrogens) improve the health of the vaginal epithelial layer (5, 6), but it is unclear whether hormones alter connective tissue support of the pelvic viscera.
Surgical repair is the mainstay treatment for symptomatic prolapse, but despite a continuous evolution in available surgical techniques, reoperation for recurrent prolapse is common (7). Fialkow et al (8) described a reoperation rate of 3.7 per 100 women-years, and others report 17% of patients needing repeat operations within 10 years, which likely represents an underestimate of the true rate (9). Any agent or therapy that could potentially augment the strength or longevity of surgical prolapse repairs deserves further investigation. The objective of this study was to determine the effects of local conjugated equine estrogen (Premarin) treatment on the vaginal muscularis and, by extension, connective tissues of the pelvic floor in postmenopausal women planning surgical repair of POP. Outcomes of interest generally sorted either to the side of matrix synthesis (ie, mRNA levels for various fribrillar collagens types 1 and 3, tropoelastin, lysyl-oxidase, and protease inhibitors; protein concentrations of collagen I and III; and total collagen content) or matrix degradation (ie, mRNA levels of proteases important in collagen degradation and quantitative measurements of gelatinase activity). Histologically, we investigated how these changes at the level of the extracellular matrix translated to the vaginal muscularis and epithelium, the thickness and substance of which could impact upon quality of surgical repair.
Materials and Methods
This was a double-blind, randomized controlled trial conducted at the University of Texas Southwestern Medical Center and Parkland Health and Hospital System (Dallas, Texas) with enrollment completed in 2012. The study was approved by the Institutional Review Board of University of Texas Southwestern, and all participants gave written, informed consent to participate. Eligible women were between 1 and 10 years beyond their last menses, aged 40–70 years, with symptomatic uterine and/or anterior vaginal wall prolapse of stage 2 or greater (ie, bulge extending to 1 cm above the hymen or beyond) desiring surgical repair and amenable to a total hysterectomy. Participants could have no estrogen replacement by any route in the prior month and needed to be physically capable of applying a vaginal cream. Exclusions to participation were body mass index (BMI) greater than 35 kg/m2, prior surgical repair including anterior colporrhaphy or prior hysterectomy, corticosteroid therapy in the prior month, history of connective tissue disease (eg, Ehlers-Danlos or Marfan syndromes), prior vaginal radiation, concurrent use of steroid cream (eg, treatment of lichen sclerosis), current tobacco use, history of vaginal infection in the preceding month, and contraindications to estrogen replacement therapy (ie, history of spontaneous deep vein thrombosis, estrogen responsive malignancy, unexplained vaginal bleeding).
A computer-generated randomization table (blocks of four) was used to allocate participants to either vaginal conjugated estrogen cream (Premarin; Pfizer) or placebo cream. A pharmacy with expertise in drug compounding (Drug Crafters) retubed commercially available Premarin cream (0.625 mg drug per 1 g cream) and its placebo match into identical unbranded white tubes. The Investigational Drug Service pharmacy of Parkland Hospital maintained the randomization scheme and was the only party unblinded to drug allocation. Participants were instructed to apply 1 g of cream via applicator intravaginally nightly for 2 weeks and then twice weekly until surgery. A total of 6 (minimum 4, maximum 8) weeks of treatment was required before surgery. Previous studies comparing local Premarin cream to placebo have demonstrated objective improvements in signs of urogenital atrophy (vaginal maturation index, vaginal pH) achieved after 3–4 weeks of application (10, 11), and Premarin cream was also used in one other study of preoperative vaginal preparation using local estrogen (12). The dose and frequency of Premarin selected for this study has been reported in other trials and was effective in improving objective and subjective quality measures of the vaginal epithelium (13).
A thorough history and physical examination was completed at the time of surgery scheduling. Total hysterectomies could be either vaginal or abdominal as part of a planned prolapse repair. At the time of surgery, a sample of vaginal wall was excised. After removal of the uterus and before closure of the cuff, a full-thickness triangular-shaped biopsy (∼1.5 cm parallel to the vaginal cuff and 0.5–2 cm deep) was taken from the anterior margin of the vaginal apex using Metzenbaum scissors. The samples were marked with suture at the time of surgery to clarify orientation so that histologic preparations would be oriented uniformly to determine vaginal wall thickness. Tissues were rinsed three times in PBS and a full-thickness portion stored in neutral buffer formalin (10%) and later processed for histology. Cross-sections (5 μm) of the formalin-fixed, paraffin-embedded tissues were obtained at 0.1 mm intervals and stained with hematoxylin and eosin and Masson's trichrome. Thickness of the vaginal epithelium, lamina propria, and muscularis was determined as previously described by examiners blinded to treatment allocation (14). The remainder of the specimen had muscularis separated from mucosa using a dissecting microscope, and both tissues were snap frozen in liquid nitrogen prior to storage at −80°C; there was no additional histological confirmation of separation of muscularis and mucosa.
Protein extraction and immunoblot analysis
Immunoblot analysis was completed in four patients in each group with sufficient tissue for protein analysis. Frozen vaginal tissue was pulverized with a liquid nitrogen-chilled mortar and pestle. Tissue powder was homogenized in basic buffer plus protease inhibitors (PIs) and centrifuged. The supernatant was removed and homogenization step repeated. The remaining tissue pellet was extracted with urea buffer at 4°C overnight. The samples were then centrifuged and the supernatant removed. Protein concentrations were determined using a bicinchroninic acid protein assay with standard curves of bovine serum albumin used for comparison. Those samples with adequate tissue (ie, 10 μg protein/lane) were applied to 4%–20% gradient polyacrylamide gels (Bio-Rad Laboratories), separated by electrophoresis and transferred to nitrocellulose membranes. Identical gels were run side by side and Commassie blue stained for protein loading comparison among samples. After protein transfer, membranes were treated with blocking buffer [Tris buffered saline and Tween 20 (TBS-T) with 2.5% nonfat milk] for 1 hour. Then the blot was treated with primary antibody to either human collagen I α1 (R&D Systems) or Collagen III α1 (Abcam; ab83829) for 90 minutes at 37°C. Membranes were serially washed with TBS-T, followed by treatment with secondary antibody (1:8000) at room temperature × 1 hour. Membranes were serially washed with TBS-T and subsequently incubated with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer) for 2 minutes. Signal strength was captured using a Fujifilm FLA 5100 image capture system. Protein band density was calculated using Image J software (ImageJ 1.46r; National Institutes of Health, Bethesda, Maryland).
Real-Time PCR
Quantitative PCR was used to determine the relative levels of mRNAs in vaginal tissues as described previously (15). Briefly, cDNA synthesis was carried out with 2 μg of total RNA. The reverse transcription product from 25 ng RNA was used as template, and reaction volumes (30 μL) contained Master Mix (Applied Biosystems). SYBR Green was used for amplicon detection. Two housekeeping genes (GAPDH and h36B4) were used to normalize expression of target genes with similar results. Results using h36B4 are presented. A preprogrammed dissociation protocol was used after amplification to ensure that all samples exhibited a single amplicon. Levels of mRNA were determined using the ddCt method (Applied Biosystems) and expressed relative to an external calibrator present on each plate. Primers used in this study are listed in Supplemental Table 1.
Hydroxyproline assay
Collagen solubility measurements were used as an index of collagen structure within tissue (16). Weights of vaginal tissues were determined before and after lyophilization. Lyophilized tissue was homogenized with 1M NaOH with PI at 4°C for 24 hours and centrifuged, and the supernatant was saved as fraction A (newly synthesized, non-cross-linked collagen fraction). The remaining residue was washed with water + PI and extracted with 0.5 M acetic acid + PI at 4°C for 24 hours with rotation. Samples were centrifuged and the supernatant saved as fraction B (denatured mature collagen). The remaining tissue pellet was saved as fraction C (mature, cross-linked collagen). Collagen content in each fraction was determined by measurement of hydroxyproline content by the chloramine (13)-T method after overnight hydrolysis in 6 M HCl at 100°C. Hydroxyproline values were converted to collagen (×7.6 constant) and normalized to tissue wet weight relative to the average thickness of the muscularis or epithelium in the placebo controls.
Gelatin zymography
Gelatin zymography was used to assess both the pro- and active forms of matrix metalloprotease (MMP)-9 and -2 in vaginal tissues as described elsewhere (17). Clear zones of lysis against a dark background indicated enzyme activity was quantified using a Fuji LAS 3000 image analysis system. Conditions of zymography and analysis were quantitative because enzyme activity was linear with the time of incubation and protein loading, and samples for each experiment were applied to the same gel to account for intergel variation.
Estradiol and estrone assays and assessment of adherence
To determine systemic absorption of estrogen during the 6-week preoperative period of cream application, serum levels of estrone (E1) and 17β-estradiol (E2) were assessed at baseline and on the morning of surgery using a highly sensitive liquid chromatography-tandem mass spectrometry assay. The chromatographic separation was performed on 150 × 2.0 (inner diameter) mm Phenomenex Synergi 4μMax-RP columns as previously described (18). The assay had previously been validated and, for E1, critical analyte concentration was 1.2 pg/mL with a detection limit of 3.5 pg/mL and a practical detection range of 3.5–600 pg/mL; functional assay sensitivity for E1 was less than 11.9 pg/mL. The intraassay coefficient of variation (CV) for E1 ranged from 9% at a concentration of 11.5 pg/mL to 2% at 365 pg/mL, whereas interassay CVs were between 12% (11.9 pg/mL) and 4% (368 pg/mL). For E2, the critical concentration was 1.7 pg/mL with a lower detection limit of 2.8 pg/mL; the detection range was 2.8–600 pg/mL, and the functional sensitivity was 6.3 pg/mL. The intraassay CV ranged from 15% at a concentration of 6.6 pg/mL to 2% at 357 pg/mL; interassay CVs were between 20% (6.3 pg/mL) and 5% (352 pg/mL). Hysterectomy specimens were examined by a blinded pathologist for endometrial and myometrial thickness and pathology.
Adherence to therapy was assessed by comparing tube weights at the time of surgery to the anticipated weight if used according to protocol. Participants were also given a diary to complete indicating the nights of cream application. Those with greater than a 30% discrepancy from the anticipated amount of cream (ie, use of less than 70% of the prescribed amount) or those reporting early cessation of treatment were deemed nonadherent and assigned this designation before unmasking drug assignment.
Statistical analysis
Based on our prior laboratory experience in specimens prepared from premenopausal women and data driven from animal studies similarly comparing histologic thickness of the vaginal muscularis (micrometers) and total collagen content (micrograms per vagina) in ovariectomized animals receiving estrogen vs vehicle replacement (19), we estimated that full-thickness vaginal wall biopsies from 10 women would be required in each arm of this study to demonstrate significant differences in protein content and protease activity (ie, the primary end points). Presuming some patients would be nonadherent and/or reschedule or cancel surgery to outside the acceptable 4- to 8-week window of preoperative treatment, 15 women were recruited per arm (total n = 30). Given the pilot nature and small sample size, plans were made to compare treatment arms according to those adherent with medication (ie, per protocol), in addition to an intent-to-treat analysis. Continuous data were analyzed using Student's t test or the Mann-Whitney U test, and proportions were compared using a χ2 or Fisher's exact test, as appropriate (SigmaStat version 4).
Results
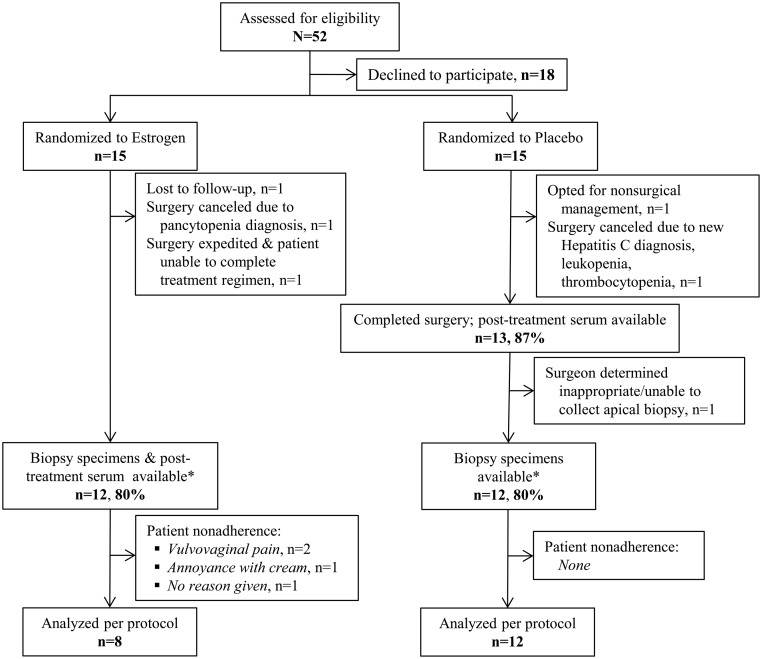
A total of 52 women were screened for eligibility in order to identify 30 participants to enroll, consent to study participation, and be randomized (Figure 1). The primary reason for declining study participation was a desire to proceed with surgery without waiting 4–8 weeks; few voiced concern about use of local estrogen. Ultimately, 13 women in each group underwent surgery and 12 participants in each group had vaginal tissue biopsies available for analysis. After excluding patients nonadherent with vaginal cream, eight participants remained in the estrogen group and 12 in the placebo group. Reasons cited for nonadherence were vulvovaginal irritation/pain with application (n = 2) and annoyance with having to remember to use the cream, which was perceived as messy (n = 1). There were no statistically significant baseline demographic or perioperative differences between the groups (Table 1). Slightly more than half of all participants were Hispanic white.
Figure 1.
Study recruitment and follow-up. *, Number available for intention-to-treat analysis.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients Receiving Placebo or Estrogen
| Patient Characteristic | Placebo (n = 15) | Estrogen (n = 15) |
|---|---|---|
| Age, y | 55.1 ± 5.4 | 58.9 ± 5.1 |
| Ethnicity/race | ||
| Non-Hispanic white | 7 (46.7) | 2 (13.3) |
| Non-Hispanic black | 1 (6.7) | 3 (20.0) |
| Hispanic white | 7 (46.7) | 10 (66.7) |
| Parity | 3 (2, 4) | 4 (3, 6) |
| BMI, kg/m2 | 29.2 ± 5.4 | 31.7 ± 4.1 |
| Anterior vaginal wall prolapse stage | 2 (2, 3) | 2 (2, 3) |
| Route of hysterectomya | ||
| Abdominal | 3 (23.1) | 5 (38.5) |
| Vaginal | 10 (76.9) | 8 (61.5) |
| Estimated blood loss, mL | 285 ± 134 | 285 ± 270 |
Data are mean ± SD, n (percentage), or median (interquartile interval).
Fewer participants than at baseline completed surgery (n = 13 for both groups).
Histological changes and collagen synthesis
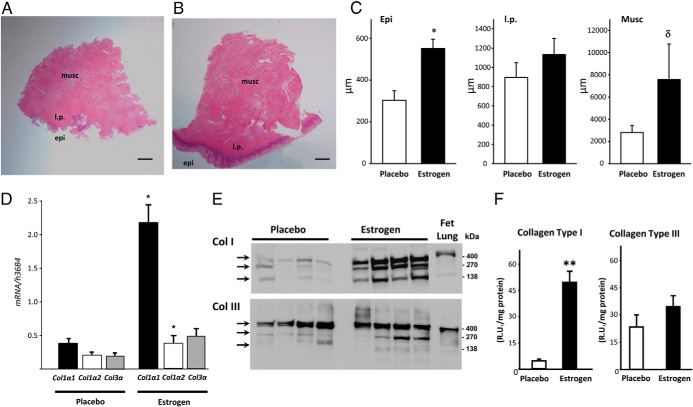
On histological assessment, both epithelial/mucosal and muscularis layers appeared thicker among samples from women treated with conjugated equine estrogens (Figure 2, A–C). Analyzed per protocol (estrogen = 8; placebo = 12), epithelial thickness was increased 1.8-fold in the estrogen group [t(18) = 3.70, P = .002], and albeit not statistically significant, muscularis thickness was increased 2.7-fold [t(18) = 1.78, P = .088]. The thickness of the lamina propria appeared similar between the two groups.
Figure 2.
Effect of vaginal estrogen on synthesis activity in the vaginal wall. Hematoxylin and eosin stains are shown; a representative cross-section of vaginal wall from postmenopausal women with prolapse treated with placebo (A) or vaginal estrogen (B) is also shown. Epi, epithelium; l.p., lamina propria; Musc, muscularis. Bar, 1 mm. C, Thickness (micrometers) of epithelium, lamina propria, and muscularis of vaginal tissues from women treated with placebo or estrogen. Data represent mean ± SEM of 12 placebo and eight estrogen. *, P = .002; δ, P = .088. D, Levels of Col1α1, Col 1α2, and Col 3α mRNA in tissues from women treated with placebo (n = 12) or estrogen (n = 8). Data represent mean ± SEM. *, P < .05 compared with the same gene in the placebo group. Immunoblot analysis (E) and densitometry (F) of collagen types I and III (n = 4 per group). Monomeric (140 kDa), dimeric (270 kDa), and trimeric (∼400 kDa) forms of collagen I or collagen III are indicated by arrows. The human fetal lung represents positive control. **, P = .012.
To determine whether the trend observed in estrogen-induced increase in muscularis thickness was associated with increased collagen synthesis, relative levels of collagen types 1α1, 1α2, and 3α mRNA were quantified in muscularis samples from both groups. Col1α1 was increased 6-fold in vaginal muscularis from estrogen [U = 0, n1 = 8, n2 = 12, P < .001], whereas Col1α2 and Col3α were increased 1.8-, and 2.5-fold, respectively [t(18) = 2.22, P = .037; U = 23, n1 = 8, n2 = 12, p = 0.059] (Figure 2D). Relative levels of mRNA encoding other extracellular matrix-related genes (tropoelastin, LOX, and LOXL1) were similar in tissues from estrogen- and placebo-treated women, and TGFβ1, a major regulator of LOX gene expression, was also similar between the two groups (Supplemental Table 2). Immunoblot analysis demonstrated that collagen type Ia monomers, dimers, and trimers were all increased in the muscularis from women treated with estrogen; total collagen type I was increased 9-fold [t(6) = 3.54, P = .012] (Figure 2, E and F). Collagen I is typically identified in ligamentous tissue with well-organized fibers. In contrast, differences in collagen III protein, which is the collagen predominant in loose areolar tissue, were not significant [t(6) = 1.20, P = .28]. Histological measurements, mRNA levels, and collagen content were not significantly different if analyzed strictly by intention to treat. Whereas total collagen content (as determined by hydroxyproline assays) was similar in the vaginal mucosal layer from both groups, collagen content trended toward increased thickness in the muscularis of estrogen patients [3.2-fold, t(18) = 1.69, P = .10, Table 2]. These studies therefore suggest that collagen type I is preferentially enhanced by vaginal estrogen treatment and that the increase in collagen content is localized to the underlying fibromuscular layer.
Table 2.
Collagen Content in Vaginal Mucosa and Muscularis From Cream-Adherent Women Treated With Placebo or Estrogen
| Placebo (n = 12) | Estrogen (n = 8) | Fold Difference | P Value | |
|---|---|---|---|---|
| Vaginal mucosa | ||||
| Collagen (immature soluble) | 3.6 (1.0) | 4.1 (1.18) | 1.1 | .77 |
| Collagen (denatured) | 1.7 (0.4, 6.8) | 0.8 (0.0, 2.7) | 0.5 | .42 |
| Collagen (mature cross-linked) | 105.7 (26.4) | 118.4 (29.4) | 1.1 | .77 |
| Total collagen | 112.9 (26.7) | 124.6 (31.4) | 1.1 | .79 |
| Mature collagen, %) | 92.3 | 95.3 | 1.0 | |
| Vaginal muscularis | ||||
| Collagen (immature soluble) | 5.8 (2.9, 16.0) | 20.6 (4.9, 26.2) | 3.5 | .17 |
| Collagen (denatured) | 2.8 (1.3, 7.9) | 10.6 (2.5, 16.8) | 3.7 | .17 |
| Collagen (mature cross-linked) | 78.0 (25.8) | 260.7 (139.7) | 3.3 | .13 |
| Total collagen | 95.0 (29.9) | 302.6 (146.1) | 3.2 | .10a |
| Mature collagen, % | 82.4 | 82.5 | 1.0 | |
Data are mean (SE) or median (interquartile interval) and presented as milligrams collagen fractions per milligrams epithelium (vaginal mucosa) or muscularis (vaginal muscularis) wet weight relative to average thickness of epithelium (vaginal mucosa) or muscularis (vaginal muscularis) in the placebo controls. Total collagen values are a summation of collagen fraction means and may not equal the sum of column values as medians are reported for nonparametric data.
t(18) = 1.69 (P = .10).
Collagenase and elastolytic activity
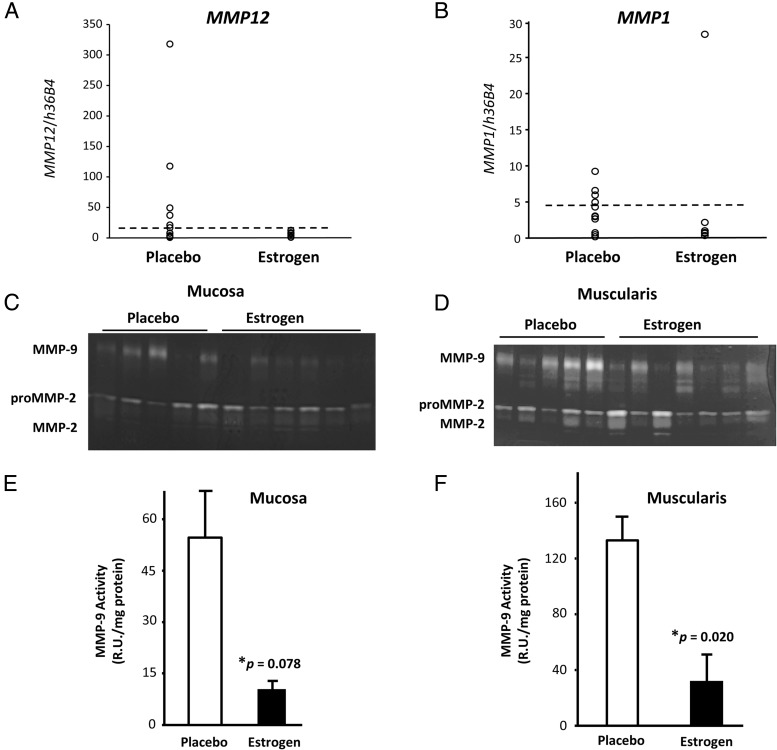
Because vaginal epithelium is known to be a rich source for protease inhibitors (20), the mucosa was evaluated for the expression of three serine protease inhibitors (elafin, secretory leukocyte protease inhibitor, and α1-antitrypsin) and two MMP inhibitors (TIMP1 and TIMP2). These inhibitors were similar between estrogen and placebo (Supplemental Table 2). Likewise, mRNA levels of certain proteases were similar between the two groups. Specifically, a serine protease recently found to be highly expressed in vaginal epithelium (PRSS3), a cysteine elastase (cathepsin K), and MMP2 were all similar between the two groups. Interestingly, however, MMP1 and MMP12 (two proteases important for collagen degradation) were decreased in tissues from women treated with estrogen compared with placebo (Figure 3, A and B). Expression of MMP12, otherwise known as human macrophage elastase, was within normal limits in eight of eight women treated with estrogen (5.8 ± 1.5 R.U. per h36b4) but increased (ie, > 95% confidence interval of normal premenopausal controls) in 5 of 12 placebo (P = .035). MMP1, another major interstitial collagenase, was low and within normal limits in six of seven mucosal samples from estrogen. In placebo-treated women, however, MMP1 gene expression was highly variable. Although differences were not statistically significant for MMP1, the overall similar pattern between MMP12 and MMP1 suggests that vaginal estrogen may suppress these collagenases in the vaginal wall.
Figure 3.
Effect of vaginal estrogen on degradative activity in the vaginal wall. A, In the vaginal mucosa, MMP12 (human macrophage elastase) was increased in 5 of 12 placebo (greater than 95% confidence interval of normal premenopausal controls) but within normal limits in eight of eight women treated with estrogen (5.8 ± 1.5, P = .035). Dashed line represents 95% confidence interval of data from 15 premenopausal controls without prolapse. B, MMP1 mRNA was suppressed in six of seven mucosal samples from estrogen but more variable in women treated with placebo. Dashed line represents 75th percentile of placebo controls. Representative gelatin zymograms of vaginal mucosa (C) and muscularis (D) from women treated with placebo or estrogen are shown. Quantitative analysis of MMP9 activity in mucosa (E) (n = 8, placebo; n = 7 estrogen) or muscularis (F) (n = 5, placebo; n = 7, estrogen) is also shown. Bars represent mean ± SEM.
The most striking effects of vaginal estrogen were found using quantitative gelatin zymography to analyze levels of pro- and active forms of gelatinases A and B (ie, MMP2 and MMP9). Because it is difficult to assess differences between pro- and active forms of MMP9 due to similarities in molecular size (∼ 5 kDa), MMP9 activity was assessed as total MMP9 (Figure 3, C–F). Consistent with mRNA levels, pro- and active MMP2 were similar between the two groups. MMP9, on the other hand, was down-regulated 6.3-fold in the mucosa [t(13) = 1.91, P = .078] and 3.6-fold in the muscularis [t(10) = 2.76, P = .020] from estrogen-treated women (Figure 3, E and F).
Adherence and safety
As detailed above and in Figure 1, although all 13 of 13 placebo patients making it to the operating room were adherent with their medication, 4 of 13 Premarin cream users were nonadherent (P = .030). At surgery, there were no differences in endometrial thickness [1.8 ± 1.2 vs 1.4 ± 0.7 mm, t(20) = 0.95, P = .35] or myometrial thickness [1.7 ± 0.6 vs 2.2 ± 1.6 cm, t(20) = 0.97, P = .34] for estrogen vs placebo, respectively. There were no cases of proliferative, secretory, or hyperplastic endometrium. Although serum E1 increased modestly in both groups (Table 3), serum E1 and E2 remained within the normal range for postmenopausal women (E1, 7–40 pg/mL; E2, <10 pg/mL) and were not different between groups. Preoperative BMI for this cohort showed a positive correlation with baseline serum E1 (R2 = 0.382, P = .037), but not E2, levels. At the 6-month follow-up visit (or last available, if less than 6 mo), median (interquartile range) postoperative anterior compartment stage was 0.5 (0.5, 0.8) vs 1 (0, 1.3) (P = .56), in estrogen vs placebo, respectively, and change from preoperative POP Quantification System values was similar in both groups 6 months after surgery. It should be emphasized, however, that this study was not intended or powered to examine anatomic end points, and the study drug was not administered postoperatively.
Table 3.
Serum Estrone and 17β-Estradiol Before/After Six Weeks of Placebo or Estrogen Cream
| Placebo Group |
Estrogen Group |
Pretherapy Comparison |
Posttherapy Comparison |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before (n = 15) | After (n = 13) | P Valuea | Before (n = 15) | After (n = 12) | P Valuea | df | t | P Valueb | df | t | P Valuec | |
| E1 | 18.5 ± 3.9 | 24.9 ± 6.5 | .06 | 18.0 ± 2.3 | 22.8 ± 3.5 | .05 | 28 | 0.11 | .91 | 23 | 0.28 | .78 |
| E2 | 8.4 ± 1.6 | 11.6 ± 2.7 | .24 | 11.1 ± 3.7 | 7.8 ± 1.3 | .49 | 28 | 0.67 | .51 | 23 | 1.23 | .24 |
| (n = 15) | (n = 13) | P Valuea | (n = 8) | (n = 8) | P Valuea | P Valueb | P Valuec | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1d | 18.5 ± 3.9 | 24.9 ± 6.5 | .06 | 19.8 ± 2.9 | 27.7 ± 4.2 | .05 | 21 | 0.22 | .81 | 19 | 0.31 | .76 |
| E2d | 8.4 ± 1.6 | 11.6 ± 2.7 | .24 | 13.1 ± 5.0 | 9.3 ± 1.6 | .31 | 21 | 1.12 | .32 | 19 | 0.62 | .56 |
Abbreviations: df, degrees of freedom; t, t statistic. Data are mean ± SEM (picograms per milliliter).
Within-group paired t test.
Between-group t test before therapy.
Between-group t test after therapy.
Data exclude nonadherent patients (ie, per protocol only participants).
Discussion
In this cohort of postmenopausal women with symptomatic prolapse of stage 2 or greater, application of preoperative local vaginal estrogen for approximately 6 weeks resulted in significantly increased thickness of the vaginal epithelium and appeared to increase the thickness of the muscularis (2.7-fold, P = .088). Estrogen-induced increases in collagen type I mRNA and protein (typical of well organized fibers and associated ligamentous tissue) were disproportionate to that of collagen type III (more common in loose areolar tissue), resulting in increased type I-enriched collagen content of the muscularis. Furthermore, results indicate that preoperative treatment with local estrogen in drug-adherent patients lead to the down-regulation of collagenases MMP9 and MMP12 in the vaginal wall with no change in expression of several protease inhibitors. Taken together, our findings from this trial suggest that 6 weeks of preoperative vaginal estrogen in postmenopausal women with prolapse may improve the substrate for suture placement at the time of repair while mitigating surgical induction of several degradative enzymes.
In the current study, with the application of vaginal estrogen, it is reassuring that even using highly sensitive assays, systemic E1 and E2 remained well within typical postmenopausal levels with no significant differences between groups. Although we did not assay for other biologically active equine estrogens in serum, we found no uterotropic effects, ie, no cases of secretory or hyperplastic endometrium and no differences in endometrial thickness between groups. Other studies of local Premarin have shown objective improvements in the vaginal maturation index and decreasing vaginal pH while also generally demonstrating steady-state systemic estradiol concentrations that are within or slightly above the normal postmenopausal reference range (13, 21).
The 69% adherence rate observed among the Premarin-treated women is similar to another recent trial using preoperative estrogen cream in prolapse patients (12). Adherence to prescription medications is often poor in general but is particularly worse when studies involve older participants (≥65 y), asymptomatic conditions (as in this scenario of pretreatment for surgery), polypharmacy (four or more chronic medications), and labor-intensive therapies such as vaginal application of a cream (22). Cream users commonly express bother with messiness when filling and inserting the applicator, needing to wash the applicator, and leakage of the cream after application (23). Similar bother with cream application was voiced by one of this study's nonadherent participants.
There are several limitations to this study. The small sample size increases the likelihood of sampling error. Although there was not a statistically significant difference in proportion of races and ethnicities between group assignments, there appears to be more non-Hispanic whites and fewer blacks in the placebo group compared with the estrogen group but more Hispanic whites in the estrogen group. Non-Hispanic white race/ethnicity may be an independent risk for having one or more pelvic disorders (24). Given the importance of defining adherent vs nonadherent participants, it is a strength that this was defined a priori and designations made before unblinding occurred. Among this small cohort, interesting patient heterogeneity was observed in many of the molecular findings (eg, see immunoblot and zymography images, Figures 2E and 3, C–F, respectively), and yet significant differences were still identified in mucosa thickness, collagen content, and MMP degradative activity.
This trial is strengthened by its randomized, double-blind design to test the hypothesis that local estrogen alters histological morphology and biochemical composition of the vaginal wall. It was not intended or powered to identify differences in clinical end points such as prolapse recurrence or postoperative patient-reported outcomes. Our data among the estrogen-adherent participants compared with placebo-treated controls, however, suggest that local conjugated equine estrogens have beneficial effects on connective tissues of the vagina and beg further study in larger, randomized trials.
The end point for this study occurred at the time of surgery. It is unclear whether the down-regulation of collagenase activities would continue postoperatively long enough to protect or lessen the likelihood of progressive connective tissue degradation and prolapse recurrence. Previously, using a murine model of menopause, we demonstrated a burst in pro- and active MMP9 activity for 1–7 days after withdrawal of estrogen; Lox and Loxl1 mRNA were simultaneously decreased as were overall protein content and mature elastic fibers, indicating that remodeling occurs rapidly with withdrawal of estrogen (Drewes, P. G., and R. A. Word, unpublished data). Thus, the optimal design of a clinical study examining the potential for benefit with perioperative use of local estrogen augmenting POP surgery may require not only an expansion in number of participants but also continuation of estrogen therapy postoperatively, at least until mature scar formation is complete.
The findings here have important clinical implications regarding use of local estrogen, which may serve as an adjunct for native tissue transvaginal pelvic reconstructive surgery. In summary, this trial highlights potential benefits of preoperative local estrogen treatment in postmenopausal women with prolapse. A thicker epithelium and muscularis was coupled with preferential increases in collagen type I relative to type III in the vaginal muscularis. Furthermore, the down-regulation of proteases important for collagen and elastic fiber breakdown may mitigate the surgical induction of these degradative enzymes and thereby improve long-term maintenance of connective tissue integrity of the pelvic floor.
Acknowledgments
We acknowledge the support and assistance in patient recruitment by Drs Clifford Wai, Marlene Corton, Sunil Balgobin, T. Ignacio Montoya, Sujatha Pathi, and Amanda B. White and by E. Kelly Moore, RN, Shanna Atnip, WHNP, and Pamela Martinez, WHNP. Mr Patrick Keller, MS, provided technical laboratory support.
This study had the clinical trial registration (www.clinicaltrials.gov) with the number of NCT 01778985.
This work was supported by the American Urogynecologic Society Foundation's Astellas Research Award and National Institutes of Health Grant AG028048.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- E1
- estrone
- E2
- 17β-estradiol
- MMP
- matrix metalloprotease
- PI
- protease inhibitor
- POP
- pelvic organ prolapse
- TBS-T
- Tris-buffered saline and Tween 20.
References
- 1. Popovic JR, Kozak LJ. National hospital discharge survey: annual summary, 1998. Vital and Health Stat. 2000;13:1–194 [PubMed] [Google Scholar]
- 2. Hunskaar S, Burgio K, Clark A. Epidemiology of urinary and fecal incontinence and pelvic organ prolapse. In: Abrams P, Cordozo L, Khoury S, Wein A, eds. Third International Consultation on Incontinence. 1st ed Paris: Health Publication Ltd.; 2005 [Google Scholar]
- 3. Swift S, Woodman P, O'Boyle A, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192:795–806 [DOI] [PubMed] [Google Scholar]
- 4. Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104:489–497 [DOI] [PubMed] [Google Scholar]
- 5. Cooke PS, Uchima FD, Fujii DK, Bern HA, Cunha GR. Restoration of normal morphology and estrogen responsiveness in cultured vaginal and uterine epithelia transplanted with stroma. Proc Natl Acad Sci USA. 1986;83:2109–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bercovici B, Uretzki G, Palti Y. The effects of estrogens on cytology and vascularization of the vaginal epithelium in climacteric women. Am J Obstet Gynecol. 1972;113:98–103 [DOI] [PubMed] [Google Scholar]
- 7. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet Gynecol. 2009;113:367–373 [DOI] [PubMed] [Google Scholar]
- 8. Fialkow MF, Newton KM, Weiss NS. Incidence of recurrent pelvic organ prolapse 10 years following primary surgical management: a retrospective cohort study. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1483–1487 [DOI] [PubMed] [Google Scholar]
- 9. Denman MA, Gregory WT, Boyles SH, Smith V, Edwards SR, Clark AL. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2008;198:555 e551–e555 [DOI] [PubMed] [Google Scholar]
- 10. Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause. 2009;16:719–727 [DOI] [PubMed] [Google Scholar]
- 11. Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16:735–741 [DOI] [PubMed] [Google Scholar]
- 12. Vaccaro CM, Mutema GK, Fellner AN, et al. Histologic and cytologic effects of vaginal estrogen in women with pelvic organ prolapse: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2013;19:34–39 [DOI] [PubMed] [Google Scholar]
- 13. Manonai J, Theppisai U, Suthutvoravut S, Udomsubpayakul U, Chittacharoen A. The effect of estradiol vaginal tablet and conjugated estrogen cream on urogenital symptoms in postmenopausal women: a comparative study. J Obstet Gynaecol Res. 2001;27:255–260 [DOI] [PubMed] [Google Scholar]
- 14. Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187:1501–1508; discussion 1508–1509 [DOI] [PubMed] [Google Scholar]
- 15. Havelock JC, Keller P, Muleba N, et al. Human myometrial gene expression before and during parturition. Biol Reprod. 2005;72:707–719 [DOI] [PubMed] [Google Scholar]
- 16. Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82:33–64 [DOI] [PubMed] [Google Scholar]
- 17. Wieslander CK, Marinis SI, Drewes PG, Keller PW, Acevedo JF, Word RA. Regulation of elastolytic proteases in the mouse vagina during pregnancy, parturition, and puerperium. Biol Reprod. 2008;78:521–528 [DOI] [PubMed] [Google Scholar]
- 18. Nelson RE, Grebe SK, O'Kane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384 [DOI] [PubMed] [Google Scholar]
- 19. Balgobin S, Montoya TI, Shi H, et al. Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs. Biol Reprod. 2013;89:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slayden OD, Hettrich K, Carroll RS, Otto LN, Clark AL, Brenner RM. Estrogen enhances cystatin C expression in the macaque vagina. J Clin Endocrinol Metab. 2004;89:883–891 [DOI] [PubMed] [Google Scholar]
- 21. Dorr MB, Nelson AL, Mayer PR, et al. Plasma estrogen concentrations after oral and vaginal estrogen administration in women with atrophic vaginitis. Fertil Steril. 2010;94:2365–2368 [DOI] [PubMed] [Google Scholar]
- 22. Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–2571 [DOI] [PubMed] [Google Scholar]
- 23. Minkin MJ, Maamari R, Reiter S. Improved compliance and patient satisfaction with estradiol vaginal tablets in postmenopausal women previously treated with another local estrogen therapy. Int J Womens Health. 2013;5:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol. 2014;123:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]