Abstract
Context:
The optimal circulating concentration of 25(OH) vitamin D is controversial.
Objective:
The aim was to investigate if FGF-23 and 24,25(OH)2D can guide cholecalciferol replacement.
Design:
Oral cholecalciferol (10,000 IU weekly) administered to subjects with 25(OH)D levels < 20 ηg/mL and eGFR > 60 mL/min/1.73 m2 (n = 25), chronic kidney disease (CKD) (n = 27), or end stage renal disease (ESRD) (n = 14).
Setting:
The study was conducted at the Veterans Affairs clinics.
Main Outcome Measure:
Serum FGF-23, PTH, 25(OH)D, 1,25(OH)2D, 24,25(OH)2D, calcium, and phosphorous concentrations, and urinary excretion of calcium and phosphorus at baseline and after 8 weeks of treatment.
Results:
Cholecalciferol treatment increased concentrations of serum 25(OH)D by (19.3 ± 8 ηg/mL, P = .001; 12.2 ± 9 ηg/mL, P = .0001) and 24,25(OH)2D (1.14 ± 0.89 ηg/mL, P = .0024; 1.0 ± 0.72 ηg/mL P = .0002), and reduced serum PTH (−11 ± 21 pg/mL, P = .0292; −42 ± 68 pg/mL, P = .0494) in normal and CKD subjects, respectively. Cholecalciferol increased serum FGF-23 levels only in normal subjects (44 ± 57 ηg/mL, P = .01). Increments in serum 25(OH)D positively correlated with serum FGF-23 and 24,25(OH)2D and negatively correlated with PTH. In ESRD, cholecalciferol administration increased 25(OH)D by (16.6 ± 6.6 ηg/mL P ≤ .05) without changing 24,25(OH)2D, FGF-23 or PTH levels.
Conclusion:
Modest elevations of serum 25(OH)D levels after cholecalciferol treatment are sufficient to induce compensatory degradative pathways in patients with sufficient renal reserves, suggesting that optimal circulating 25(OH)D levels are approximately 20 ηg/mL. In addition, catabolism of 25(OH)D may also contribute to the low circulating vitamin D levels in CKD, since elevations of FGF-23 in CKD are associated with increased 24,25(OH)2D after cholecalciferol administration.
Vitamin D plays a key role in bone and mineral metabolism and innate and adaptive immune responses (1). Circulating serum 25-hydroxyvitamin D (25(OH)D) concentrations are used for diagnosing vitamin D deficiency (2, 3). Vitamin D deficiency is estimated to occur in 36–57% (4) of the general population and in 50–86% of patients with chronic kidney disease (CKD) (5).
The optimal circulating concentration of 25(OH)D is uncertain (1). The Institute of Medicine (IOM) recommends 25(OH)D levels between 16 ηg/mL (40 ηM) and 20 ηg/mL (50 ηM) (6). Since most individuals have 25(OH)D levels above 20 ηg/mL, further vitamin D supplementation may not be needed and could have adverse effects if the serum 25(OH)D exceeds 50 ηg/mL (125 ηM) (6). On the other hand, the Endocrine Society guidelines define vitamin D deficiency as levels below 20 ηg/mL (50 ηM), recommend a target 30 ηg/mL (75 ηM), but suggest levels up to 80 ηg/mL (200 ηM) have minimal risks (7). Both recommendations have sparked controversy as either being too low or too high (6, 8). In CKD, vitamin D insufficiency is also defined as a serum 25(OH)D of <30 ηg/mL (75 ηM) (9), although the mechanisms responsible for low circulating 25(OH)D in CKD differs from the general population (10–13). The lack of a consensus regarding the optimal circuiting 25(OH)D concentration confounds recommendations regarding 25(OH)D repletion in both the general and CKD populations (9).
Synthetic and degradative enzymatic pathways regulate vitamin D metabolism in response to several factors, including PTH, fibroblast growth factor (FGF-23), and 1,25-dihydroxyvitamin D (1,25(OH)2D) (14). In the kidney proximal tubule, Cyp27B1 (renal cytochrome P450 enzyme 1-α hydroxylase) regulates the conversion of 25(OH)D to the active 1,25(OH)2D, whereas, Cyp24A1 (renal cytochrome P450 enzyme 24-α hydroxylase) controls the catabolism of 25(OH)D and 1,25(OH)2D to inactive 24,25-dihydroxyvitamin D (24,25(OH)2D) and 1,24,25(OH)3D, respectively (14–21). Vitamin D deficiency results in compensatory increases in the PTH that stimulates Cyp27B1 and suppresses Cyp24A1 to optimize the production of 1,25(OH)2D. On the other hand, excess 1,25(OH)2D stimulates FGF-23, a hormone produced by bone that lowers 1,25(OH)2D by suppression of Cyp27B1-regulated synthetic and stimulation of Cyp24A1-regulated degradative pathways in the kidney (14, 22–24).
Reciprocal changes in these compensatory pathways (ie, a decrease in PTH-mediated synthetic and increase in FGF-23-mediated degradative pathways) might define a threshold for low vitamin D status. To test this possibility, we measured changes in 25(OH)D and counter-regulatory pathways, including FGF-23 and PTH, and 24,25(OH)2D levels, in a prospective cohort of vitamin D deficient subjects with normal renal function or with varying degrees of renal insufficiency before and after treatment with cholecalciferol.
Subjects and Methods
Study population
This is a prospective study of patients with vitamin D deficiency with 25(OH)D < 20 ηg/mL (50 ηM), identified between April 2009 and July 2010 from the outpatient clinics at the Veterans Affairs Medical Center (VAMC) in Memphis, TN. The study was approved by the Institutional Review Board (IRB) of VAMC, Memphis, TN. Inclusion criteria included: 1. Men and women over the age of 18 years, 2. Serum 25(OH) D levels < 20 ηg/mL (50 ηM). Exclusion criteria included: 1. Estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 (except for the 14 end-stage renal disease (ESRD) subjects), 2. Cirrhosis, sarcoidosis, lymphoma, malabsorption syndrome, solid organ transplant, or primary hyperparathyroidism, 3. The chronic use of medications known to alter vitamin D metabolism, including rifampin, corticosteroids, antiepileptics, 4. The concurrent use of phosphate binders, active forms of vitamin D, and calcimimetics (except in the ESRD group).
We identified 52 patients, 25 subjects with normal renal function (eGFR > 60 mL/min 1.73 m2) and 27 subjects with CKD (eGFR ≤ 60 mL/min 1.73 m2) who had serum 25(OH)D levels of less than 20 ηg/mL. Four patients in the CKD group had nephrotic range proteinuria (urine protein creatinine ratio > 3 g/g). We also assessed the levels of 25(OH)D and 24,25(OH)2D in a cohort of vitamin D deficient anuric ESRD hemodialysis patients before and after cholecalciferol supplementation (n = 14). Informed consent was obtained from all participants.
Demographic data (age, gender, and race), clinical characteristics (body weight, height, presence or absence of CKD, hypertension, and diabetes mellitus were obtained from a review of the medical records. All patients received 10 000 U of oral cholecalciferol per week for 8 weeks. Serum levels of FGF-23, 25(OH)D, 1,25 (OH)2D, 24,25(OH)2)D, PTH, calcium, phosphorus, and creatinine were obtained before treatment and after 8 weeks of weekly cholecalciferol supplementation. We did not measure serum 1,25(OH)2D or urinary parameters in subjects with ESRD. All participants completed the protocol. Patients were followed on a monthly basis to ensure adherence, and to monitor for adverse effects.
Procedures, assays, and calculations
Serum and urine calcium, phosphorus, and creatinine were assessed in the hospital's core laboratory using an autoanalyzer (Ortho Clinical 5600). Serum albumin levels were measured by the bromocresol green assay and serum creatinine levels by isotope dilution mass spectrometry traceable Jaffé method from Roche. The eGFR was calculated according to the 4-variable MDRD formula. CKD was defined as an eGFR < 60 mL/min/1.73 m2. Body mass index (BMI) was calculated by the person's weight in kilograms, divided by height in square meters. Urinary protein excretion was measured as a ratio of spot urine protein-creatinine ratio or as spot urine albumin-creatinine ratio (ACR) [ACR = UPC(1.054) × 0.596]. Serum FGF-23 levels were measured using the FGF-23 ELISA kit (Kainos Laboratories). Serum 24,25(OH)2D was measured by radioimmunoassay (Heartland Assays LLC) according to previously published procedures (25, 26). Serum 25(OH)D levels were measured by immunochemiluminometric assay using the DiaSorin Liaison instrument. 1,25(OH)2D was measured by a column chromatography radioimmunosassay (LABCORP).
Statistical analysis
Continuous variables were presented as means and SD, categorical variables as percentages, unless otherwise specified. Normally distributed continuous variables were compared using unpaired t test for unpaired data and paired t test. Non-normally distributed continuous variables were compared using Mann-Whitney U test for unpaired data and Wilcoxan Sign Rank test for paired data. A multivariate regression model was used with logFGF-23 as dependent variable and the following predictor variables: age, race, DM, BMI, eGFR, urine ACR, serum calcium, phosphorus, FGF-23, PTH, 25(OH)D, 1,25(OH)2D, and urinary fractional excretion of calcium and phosphorus (FECa and FEPO4). Since the CKD group was older and included more persons with diabetes, an additional analysis was performed in a subset of participants: 14 CKD subjects were matched in a 1:1 basis with 14 subjects with normal renal function. Matching variables were age (within 5 years), gender, race, and DM. All these variables have been reported to affect vitamin D status in humans (4, 27). To evaluate the factors affecting the post-treatment 24,25(OH)2D level, multivariate analyses were done using the following predictor variables: age, race, DM, BMI, eGFR, urine ACR, pre and post-treatment serum calcium, phosphorous, FGF-23, PTH, 25(OH)D, 1,25(OH)2D, and FECa, and FEPO4. An additional regression model was performed with logFGF-23 as the dependent variable and the following predictor variables: age, race, DM, BMI, eGFR, urine ACR, serum calcium, phosphorus, PTH, 25(OH)D, 1,25(OH)2D, and urinary fractional excretion of calcium and phosphorus (FECa, and FEPO4). Identical regression analyses were done separately for the CKD and normal renal function groups. Only variables with P value <.10 were included in the multivariable stepwise regression analyses. All tests were two-sided and P value <.05 was considered significant unless otherwise specified. Statistical analysis was conducted using SAS version 9.2 (SAS Institute Inc).
Results
Demographic, clinical, and baseline laboratory characteristics of patients
All participants were male. The average eGFR and urine protein/creatinine ratio in non-CKD patients (hereafter referred to as normal subjects) were 88 mL/min/1.73 m2 and 0.12 g/g, and in CKD subjects were 36 mL/min/1.73 m2 and 1.64 g/g, respectively. Normal and CKD subjects had similar racial distribution, whereas, all of the ESRD patients were African American (Table 1 and Supplemental Table 1). The BMI was also similar in normal and CKD patients, but this parameter was lower in patients with ESRD. A 2.5-fold greater number of patients with diabetes mellitus were present in the CKD group compared to normal and ESRD subjects (Table 1). As per the entry criteria, pretreatment serums 25(OH)D were low in all three groups (Table 2). Pretreatment 1,25(OH)2D, albumin, calcium, and phosphorus concentrations were similar between CKD and non-CKD subjects. Also as expected, patients with CKD had significantly higher pretreatment FGF-23, PTH, and fractional excretion of phosphorous (FEPO4) and significant 50% lower serum 24,25(OH)2D levels (Table 2). Patients with ESRD had even greater baseline elevations in serum FGF-23 and PTH concentrations, similar reductions in 24,25(OH)2D concentrations, and increased serum phosphorus and serum alkaline phosphatase compared to the other groups.
Table 1.
Pretreatment Demographic and Laboratory Test Results
| eGFR ≥ 60 (mL/min/1.73 m2) (n = 25) | eGFR < 60 (mL/min/1.73 m2) (n = 27) | ESRD (n = 14) | |
|---|---|---|---|
| African American | 20 (80%) | 17 (65%) | 14 (100%) |
| Diabetes mellitus | 6 (24%) | 17 (63%) | 5 (36%) |
| Age (y) | 60.36 ± 6.56 | 66.44 ± 9.95 | 63.28 ± 15.21 |
| Body mass index (kg/m2) | 28.69 ± 5.05 | 29.68 ± 9.95 | 27.05 ± 4.76 |
| eGFR (mL/min/1.73 m2) | 88.04 ± 17.61 | 36.11 ± 9.45 | — |
| Urine protein/creatinine (g/g) | 0.12 ± 0.06 | 1.64 ± 0.80 | — |
Categorical variables are presented as percentage and continuous variables as mean ± sd.
Table 2.
Serum and Urine Values Before and After Cholecalciferol Treatment in Patients With Normal Renal Function, CKD, and ESRD
| Normal Renal Function (n = 25) |
CKD (n = 27) |
ESRD (n = 14) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | P Value | Pre-Treatment | Post-Treatment | P Value | Pre-Treatment | Post-Treatment | P Value | |
| Serum | |||||||||
| 25(OH)D (ng/mL) | 12 ± 4.1 | 31.1 ± 8.3 | .0001 | 14.5 ± 4.4 | 27.8 ± 5.9 | .0001 | 12.8 ± 5.2 | 29.4 ± 7.9 | .0001 |
| FGF23 (ng/mL) | 74.9 ± 32.3 | 119.9 ± 37.7 | .0001 | 169.7 ± 110 | 191.1 ± 116.9 | .4706 | 2137 ± 2632 | 2306 ± 2664 | .2734 |
| 1,25(OH)2D (pg/mL) | 42.2 ± 22.7 | 59.0 ± 26.2 | .0071 | 45.3 ± 19.7 | 47.2 ± 19.6 | .5448 | — | — | — |
| 24,25(OH)2D (ng/mL) | 1.2 ± 1 | 2.3 ± 0.6 | .0024 | 0.6 ± 0.5 | 1.6 ± 0.8 | .0005 | 0.4 ± 0.4 | 0.4 ± 0.1 | .7042 |
| PTH (pg/mL) | 54.8 ± 24.1 | 43.8 ± 27.3 | .0292 | 153.1 ± 92.6 | 124 ± 71.9 | .0113 | 781 ± 679 | 753 ± 625 | .6698 |
| PO4 (mg/dL) | 3.3 ± 0.5 | 3.7 ± 0.7 | .3027 | 3.7 ± 0.7 | 3.8 ± 0.8 | .3410 | 4.6 ± 0.8 | 4.9 ± 1.1 | .5508 |
| Ca (mg/dL) | 9.6 ± 0.2 | 9.5 ± 0.3 | .2065 | 9.4 ± 0.4 | 9.5 ± 0.4 | .1395 | 9 ± 0.6 | 8.8 ± 0.7 | .3276 |
| Alk. phos (U/liter) | 83.5 ± 23.9 | 80.7 ± 20.6 | .1794 | 94.7 ± 24.1 | 93.5 ± 25.8 | .4872 | 163 ± 113 | 156 ± 110 | .4354 |
| Albumin (g/liter) | 4.2 ± 0.2 | 4.3 ± 0.2 | .3202 | 4.0 ± 0.3 | 4.2 ± 0.36 | .0527 | 3.9 ± 0.3 | 3.9 ± 0.4 | .8152 |
| Urine | |||||||||
| FEPO4 (%) | 10.9 ± 4.1 | 11.9 ± 6.2 | 1.0000 | 26.9 ± 10.5 | 27.3 ± 9.8 | .8701 | — | — | — |
| FECa (%) | 0.6 ± 0.6 | 0.7 ± 0.7 | .4871 | 0.8 ± 1.4 | 0.6 ± 0.9 | .1395 | — | — | — |
Abbreviations: 24,25(OH)2D, 24,25-dihydroxyvitamin D; PO4, phosphorus; Ca, calcium; Alk. Phos, alkaline phosphatase; FEPO4, fractional excretion of phosphorous; FECa, fractional excretion of calcium. Paired t test serum PO4, Paired t test used for serum PO4 in patients with normal renal function, and serum 25(OH)D in ESRD patients. Wilcoxon signed rank test for the rest of the variables.
Effects of cholecalciferol on serum and urine biochemical parameters in normals
In normal subjects, cholecalciferol treatment resulted in significant 160% increase in serum 25(OH)D (Table 2). The mean post-treatment 25(OH)D was 31.1 ± 8.3 ηg/mL, values above the IOM recommendation but lower than the higher targets proposed by other groups (8, 9). We found evidence for Cyp27B1 conversion of 25(OH)D to 1,25(OH)2D, as evidence by a 40% increment in circulating 1,25(OH)2D concentrations that was accompanied by a significant decrease in serum PTH concentrations following cholechalciferol treatment. More importantly, this modest increment of serum 25(OH)D was also associated with a significant increase in serum FGF-23 and a twofold increase in serum 24,25(OH)2D, consistent with stimulation of FGF-23-dependent vitamin D catabolic pathways. There were no changes in serum concentrations of calcium or phosphorus or changes in the mean fractional excretion of calcium (FECa) or phosphorus (FEPO4) after administration of cholecalciferol to subjects with normal renal function (Table 2).
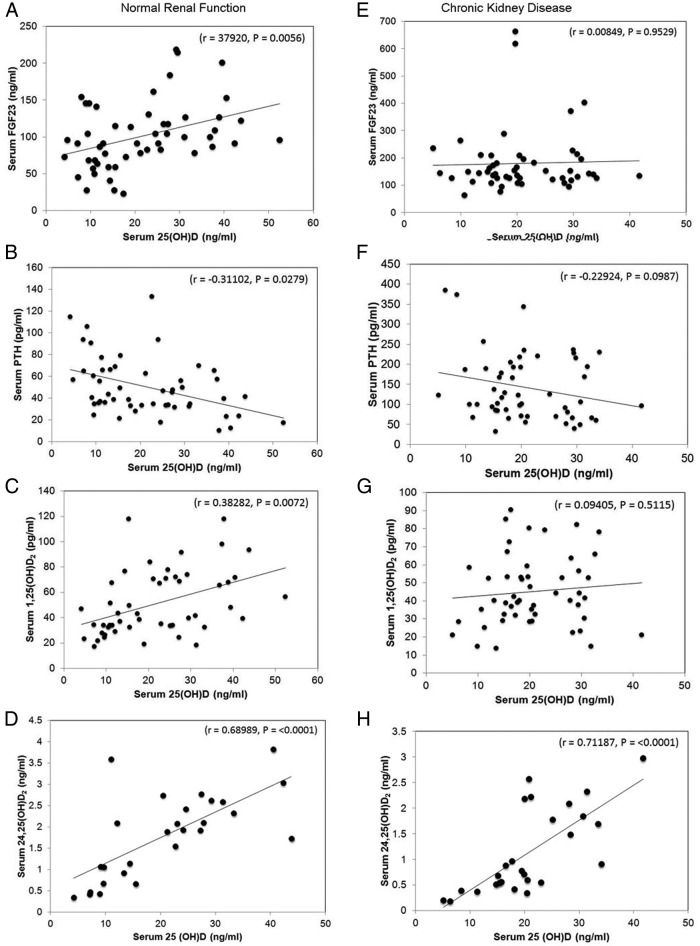
Using values from both before and after treatment, we found that serum 25(OH)D concentrations were positively correlated with serum FGF-23 (r = 0.38, P < .005) (Figure 1 A) and inversely correlated with PTH (r = −0.31, P < .03) (Figure 1B). Serum 25(OH)D was also positively correlated with 1,25(OH)2D (r = 0.38, P < .007) (Figure 1C) and 24,25(OH)2D (r = 0.69, P < .001) (Figure 1D) in normal subjects with normal renal function. Post-treatment FEPO4 did not show any significant correlation with either pre- or post-treatment FGF-23, possibly because the phosphaturic effects of FGF-23 were offset by the reductions in PTH. Post-treatment FEPO4, however, did correlate inversely with estimated GFR (r = −0.42304, P = .0364) and post-treatment 25(OH)D level (r = −0.46198, P = .0201). In addition, post-treatment FECa showed significant positive correlation with pretreatment 1,25(OH)2D (r = −0.49386, P = .0166).
Figure 1.
Effects of cholecalciferol treatment on serum parameters. Respective correlation between serum 25(OH)D and FGF-23, PTH, 1,25(OH)2D, and 24,25(OH)2D in subjects without (A, B, C, D) and with (E, F, G, H) CKD. Serum 25(OH)D was positively correlated with FGF-23 in subjects with normal renal function (A), but not in those with CKD (E). Serum 25(OH)D was significantly negatively correlated with PTH (B) and positively correlated with 1,25(OH)2D (C) in subjects with normal renal function, but not in subjects with CKD (F and G). 25(OH)D was positively correlated with 24,25(OH)2D in both subjects with normal renal function (D) and with CKD (H). Values represent serum concentrations obtained before and after treatment with cholecalciferol in subjects with normal renal function (n = 25) and CKD (n = 27), except for 24,25(OH)2D values, which were available only in the matched CKD (n = 14) and normal renal function (n = 14) cohort.
Effects of cholecalciferol on serum and urine biochemical parameters in CKD
In patients with CKD, cholecalciferol treatment also resulted in a significant increase in serum 25(OH)D concentrations, achieving a mean post-treatment value of 27.8 ± 5.93 ηg/mL, an increment that was significantly less (12.2 ± 9 ηg/mL P = .026) than that achieved in normal subjects (19.13 ± 8.25 ηg/mL). Cholecalciferol treatment had no effect on serum calcium or phosphorus concentrations in patients with CKD. FGF-23 levels, which were increased prior to treatment, were nonsignificantly increased (12%) following cholecalciferol administration in CKD. There was a small (19%), but significant, decrease in serum PTH concentrations, in spite of the fact that there was no demonstrable change in serum 1,25(OH)2D or calcium following cholecalciferol treatment in patients with CKD (Table 2). Nevertheless, cholecalciferol treatment resulted in a significant increase in serum 24,25(OH)2D in patients with CKD, consistent with FGF-23 effects to stimulate Cyp24A1 activity in the presence of adequate substrate (25(OH)D). The rise in 24,25(OH)2D, but not 1,25(OH)2D, suggests that Cyp27B1 activity may be suppressed in CKD, also possibly due to the actions of FGF-23. The FEPO4 was 2.5-fold higher in CKD compared to controls. There was no significant change in FECa or FEPO4 in CKD subjects pre- and post-treatment (Table 2).
In contrast to normal subjects, we found no correlation between serum 25(OH)D and FGF-23 (Figure 1E), PTH (Figure 1F), or 1,25(OH)2D (Figure 1G) in CKD patients, however, we did find a direct and significant relationship between 25(OH)D and 24,25(OH)2D (r = 0.71, P < .0001) (Figure 1H). Post-treatment FEPO4 also correlated positively with post-treatment FGF-23 (r = 0.50198, P = .0090) and PTH (r = 0.43438, P = .0236) and negatively with eGFR (r = −0.58066, P = .0015).
Because of the differences in baseline characteristics between normal and CKD groups, we also performed a subanalysis of a matched cohort of patients with CKD (n = 14) and non-CKD (n = 14), to validate the analysis performed in the entire group of 52 subjects. The findings in the matched CKD (n = 14) and normal subject (n = 14) cohort demonstrated the same findings as seen in the overall group (see Supplemental Tables 2–5).
In ESRD patients, cholecalciferol treatment resulted in significant increases in 25(OH)D to a mean of 29.4 ± 7.9 ηg/mL (Table 2). Similar to CKD subjects, 24,25(OH)2D levels were also reduced below that observed in controls in ESRD patients prior to treatment. In contrast to subjects with normal renal function and CKD, serum 24,25(OH)D2 levels in ESRD patients showed no significant change after vitamin D supplementation (Table 2). Serum FGF-23 and PTH concentrations were markedly elevated in ESRD and also did not significantly change following cholecalciferol supplementation. These findings suggest that insufficient total renal Cyp27B1 and Cyp24A1 activity was present in ESRD to metabolize 25(OH)D. Also, neither serum 25(OH)D nor PTH correlated with FGF-23 levels in subjects with ESRD, indicating that impaired renal function alters the ability of cholecalciferol and 25(OH)D to regulate FGF-23.
Determinants of FGF-23 and 24,25(OH)2 D
By univariate analysis, we found that pretreatment FGF-23, PTH, eGFR, 24,25(OH)2D, as well as post-treatment 25(OH)D, PTH, and calcium were associated with post-treatment 24,25(OH)2D levels (Supplemental Table 6). In multivariable analysis, the final model predicting post-treatment serum 24,25(OH)2D levels consisted of only pretreatment PTH, eGFR, post-treatment 25(OH)D and calcium without any interaction terms (Table 3). These variables together explained 72% of the variations in post-treatment serum 24,25(OH)2D level (Radj2 = 0.7269), and the overall relationship was significant F(4, 27) = 18.83, P = < .0001. With other variables held constant, post-treatment serum 24,25(OH)2D level is inversely associated with pretreatment PTH (t23 = −3.18, P = .0007) and directly associated with eGFR (t23 = 2.02, P = .0549), post-treatment 25(OH)D (t23 = 2.45, P = .0221), and post-treatment serum calcium levels (t23 = 3.66, P = .0013).
Table 3.
Multivariable Analysis of Post-Treatment Serum 24,25(OH)2D
| Parameter Estimate | Standard Error | P Value | |
|---|---|---|---|
| Pre-treatment serum PTH (pg/mL) | −0.00362 | 0.00092 | .0007 |
| Pre-treatment eGFR (mL/min/1.73 m2) | 0.00662 | 0.00327 | .0549 |
| Post-treatment serum 25(OH)D (ng/mL) | 0.02843 | 0.01158 | .0221 |
| Post-treatment serum calcium (mg/dL) | 0.96104 | 0.26229 | .0013 |
We also investigated factors that correlated with serum FGF-23 using this data set. In univariate regression analysis, serum FGF-23 levels were correlated with serum 25(OH)D, eGFR, FEPO4, serum PTH, and serum PO4. In the final multivariable model, serum FGF-23 level was directly associated with serum 25(OH)D (t104 = 2.5, P = .0143), and FEPO4 (t104 = 2.63, P = .0100), and inversely associated with eGFR (t104 = −3.05, P = .0029), but not with serum phosphorus or PTH. The model explained 40% of the variation in FGF-23 levels (Radj2 = 0.4034). Separate multiple regression analysis of FGF-23 showed significant effects of eGFR and phosphorus on FGF-23 in CKD patients, but no significant predictors for FGF-23 were identified in patients with normal renal function (Supplemental Tables 7–9).
Discussion
We found that repletion of 25(OH)D with cholecalciferol stimulated compensatory degradative pathways, as measured by increased serum FGF-23 and 24,25(OH)2D levels in subjects with normal renal function. Serum 25(OH)D was positively correlated with both FGF-23 and 24,25(OH)2D in these subjects. In addition, cholecalciferol treatment suppressed serum PTH levels in association with increased circulating 1,25(OH)2D concentrations. Thus, cholecalciferol treatment shifts vitamin D metabolism toward net degradation through increments in FGF-23 and reductions in PTH to respectively stimulate Cyp24A1 (28) and inhibit Cyp27B1 activity in the kidney (14, 17, 18, 22–24). Early in vitro studies showed that low phosphorus concentrations in the media stimulates 1,25(OH)2D and high concentrations stimulate 24,25(OH)2D. However, in the current studies we did not observe any change in serum phosphorus concentrations. Most importantly, and surprisingly, these compensatory changes were observed at post-treatment 25(OH)D levels below 30 ηg/mL. Indeed, increments in FGF-23 and 24,25(OH)2D concentrations occurred as serum 25(OH)D increased from 12 (30 ηM) to 31 ηg/mL (80 ηM). Thus, raising serum 25(OH)D levels even above the recommended threshold of 20 ηg/mL (6, 29) activates FGF-23 mediated degradative pathways. Thus, our data support the IOM guidelines, which recommend 25(OH)D levels of 20 ηg/mL to indicate adequate levels necessary to achieve bone health in the general population (6).
Our findings also provide new insights into the mechanisms of decreased 25(OH) and 1,25(OH)2D levels in CKD. It is widely thought that low circulating 1,25(OH)2D and 25(OH)D concentrations in CKD are, respectively, due to reduced synthesis caused by loss of functioning renal tissue and superimposed nutritional vitamin D deficiency. The observations that FGF-23 stimulates Cyp24A1 and inhibits Cyp27B1 activity, and the fact that FGF-23 is elevated early in the course of CKD (15, 30), creates an alternative possibility that FG-F23 mediated catabolism contributes to low circulating levels of both 25(OH)D and 1,25(OH)2D in CKD (10–12, 31). We found that after administration of cholecalciferol to subjects with CKD, the serum 24,25(OH)2D increased to a greater degree than 1,25(OH)2D, indicating that catabolism predominates over synthesis in the setting of CKD and elevations of FGF-23. The presence of increased 25(OH)D catabolism in CKD is also supported by the observation that the magnitude of the change in serum 25 (OH) D levels after cholicalciferol therapy was significantly lower in CKD patients in comparison to subjects normal kidney function. Increased degradation of 25(OH)D in CKD due to FGF-23 mediated increased Cyp24A1 activity would predict the need for a higher dose of cholecalciferol treatment to raise serum 25(OH)D levels (10–12). Even though we did not measure vitamin D binding protein (VDBP), the lack of different responses in protenuric patients or in subjects of different race, indirectly suggests that alterations in VDBP do not account for the smaller increments in 25(OH)D levels in CKD. We believe the current data represent the first demonstration that FGF-23 mediated degradation of vitamin D, which has previously been demonstrated in animal models, also has clinical relevance in humans.
CKD patients with high FGF-23 levels might be expected to have high circulating 24,25(OH)2D levels prior to cholecalciferol treatment (13). However, 24,25(OH)2D levels are low in subjects with CKD (15, 31). The current data show that increasing 25(OH)D provides substrate for Cyp24A1 and Cyp27B1 enzyme hydroxylation in CKD leading to preferential increments in serum 24,25(OH)D compared to 1,25(OH)2D, consistent with elevated FGF-23 preferentially activating CYP24A1 causing 24,25(OH)2D synthesis to increase with no increase in 1,25(OH)2D (27). In contrast, following cholecalciferol treatment in subjects with ESRD, we found no increase in serum 24,25(OH)2D levels, suggesting loss of Cyp24A1 activity in advanced renal disease. Thus, depending on the degree of renal insufficiency increased degradation due to excess FGF-23, decreased production due to loss of renal mass and nutritional deficiency may contribute to abnormalities in vitamin D metabolism (13).
Once weekly dosing of cholecalciferol resulted in elevations of FGF-23. Whether vitamin D replacement strategies that limit FGF-23 elevations might be developed is not clear. Since elevations of FGF-23, even in the normal range, are associated with increased mortality in subjects without demonstrable kidney disease and FGF-23 is strongly correlated with LVH in patients with CKD, possibly due to direct effects of FGF-23 on the myocardium to increase LVH (32, 33), long-term vitamin D therapy that leads to sustained increments in FGF-23 might be viewed with caution. Further studies are needed to determine if elevated FGF-23 might offset the beneficial effect of vitamin D replacement (34).
The changes in 25(OH)D noted in our study tended to be greater than changes reported in several prior studies, particularly in the subjects with normal renal function. The precise reason for this difference is not clear, but may be related to several factors, such as differences in route of administration, frequency of administration, type of vitamin D product (vitamin D2 vs D3), possible differences in adherence rates, and differences in the study populations 25(OH)D (35–38).
To our knowledge, this is the first study to compare 24,25(OH)2D levels in vitamin D deficient cohorts with normal renal function, CKD, and ESRD before and after cholecalciferol therapy. Nevertheless, our study has several limitations. The sample size is small and the patient population was all male. Therefore, our findings need to be confirmed in a larger and more heterogenous population. The ESRD group, however, while small, was composed of prevalent anuric hemodialysis patients (ie, lacking residual kidney function). Inclusion of ESRD with greater degrees of residual renal function and Cyp24A1 and Cyp27B1 enzyme activity may show FGF-23 effects to also increase 24, 25(OH)2D in ESRD after cholecalciferol treatment. The predominance of African Americans in our population could also limit generalizability, although there is no reason to believe that vitamin D does not stimulate FGF-23 in all ethnic groups. In addition, the study groups were not matched, although the analysis of a subset of patients well matched for factors known to alter vitamin D metabolism, confirmed our findings. Serial measurements of vitamin D metabolites during correction of vitamin D deficiency and alternate markers of CKD-MBD were also not measured. Different relationships may have been identified if examined earlier during the course of vitamin D repletion.
In summary, our study found that cholecalciferol-mediated elevations in serum 25(OH)D levels induces compensatory changes in counter regulatory hormone FGF-23 and the degradative metabolite 24,25(OH)2D in subjects with normal renal function and CKD.
Acknowledgments
This study was supported by National Institutes of Health grant 5RO1AR045955-15.
Clinical Trial Registration Number: NCT01528176.
Disclosure Summary: Dr Quarles is a consultant and speaker for Amgen. No other author has any interest to disclose.
Footnotes
- ACR
- albumin-creatinine ratio
- BMI
- body mass index
- CKD
- chronic kidney disease
- eGFR
- estimated glomerular filtration rate
- ESRD
- end-stage renal disease
- FGF-23
- fibroblast growth factor
- IOM
- Institute of Medicine
- 1,25(OH)2 D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D.
References
- 1. Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutrition. 2005;135:317–322 [DOI] [PubMed] [Google Scholar]
- 2. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806 [DOI] [PubMed] [Google Scholar]
- 3. Vieth R, McCarten K, Norwich KH. Role of 25-hydroxyvitamin D3 dose in determining rat 1,25-dihydroxyvitamin D3 production. Am J Physiol. 1990;258:E780–E789 [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clinic proceedings. Mayo Clinic. 2006;81:353–373 [DOI] [PubMed] [Google Scholar]
- 5. Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38 [DOI] [PubMed] [Google Scholar]
- 6. Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrin Metab. 2012;97:1146–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrin Metab. 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 8. Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–457 [DOI] [PubMed] [Google Scholar]
- 9. National Kidney F. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201 [PubMed] [Google Scholar]
- 10. Garcia-Lopes MG, Pillar R, Kamimura MA, et al. Cholecalciferol supplementation in chronic kidney disease: restoration of vitamin D status and impact on parathyroid hormone. Ann Nutr Metab. 2012;61:74–82 [DOI] [PubMed] [Google Scholar]
- 11. Marckmann P, Agerskov H, Thineshkumar S, et al. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant. 2012;27:3523–3531 [DOI] [PubMed] [Google Scholar]
- 12. Alvarez JA, Law J, Coakley KE, et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012;96:672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quarles LD. The bone and beyond: ‘Dem bones’ are made for more than walking. Nat Med. 2011;17:428–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435 [DOI] [PubMed] [Google Scholar]
- 15. Dai B, David V, Alshayeb HM, et al. Assessment of 24,25(OH)2D levels does not support FGF23-mediated catabolism of vitamin D metabolites. Kidney Int. 2012;82:1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086 [DOI] [PubMed] [Google Scholar]
- 18. Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095 [DOI] [PubMed] [Google Scholar]
- 19. Tomiyama K, Maeda R, Urakawa I, et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci USA. 2010;107:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, DeLuca HF. Human 25-hydroxyvitamin D3–24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–8472 [DOI] [PubMed] [Google Scholar]
- 21. Hoenderop JG, Chon H, Gkika D, et al. Regulation of gene expression by dietary Ca2+ in kidneys of 25-hydroxyvitamin D3-1 alpha-hydroxylase knockout mice. Kidney Int. 2004;65:531–539 [DOI] [PubMed] [Google Scholar]
- 22. Perwad F, Portale AA. Vitamin D metabolism in the kidney: regulation by phosphorus and fibroblast growth factor 23. Mol Cell Endocrinol. 2011;347:17–24 [DOI] [PubMed] [Google Scholar]
- 23. Takeyama K, Kato S. The vitamin D3 1alpha-hydroxylase gene and its regulation by active vitamin D3. Biosci Biotech Biochem. 2011;75:208–213 [DOI] [PubMed] [Google Scholar]
- 24. Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18 [DOI] [PubMed] [Google Scholar]
- 25. Horst RL, Littledike ET, Gray RW, Napoli JL. Impaired 24,25-dihydroxyvitamin D production in anephric human and pig. J Clin Invest. 1981;67:274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horst RL, Shepard RM, Jorgensen NA, DeLuca HF. The determination of 24,25-dihydroxyvitamin D and 25,26-dihydroxyvitamin D in plasma from normal and nephrectomized man. J Lab Clin Med. 1979;93:277–285 [PubMed] [Google Scholar]
- 27. Alshayeb HM, Wall BM, Showkat A, Mangold T, Quarles LD. Chronic kidney disease and diabetes mellitus predict resistance to vitamin D replacement therapy. Am J Med Sci. 2013;345:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lips P. Vitamin D physiology. Progr Biophys Mol Biol. 2006;92:4–8 [DOI] [PubMed] [Google Scholar]
- 29. Khosla S. What do we tell our patients about calcium and vitamin D supplementation? J Clin Endocrin Metab. 2011;96:69–71 [DOI] [PubMed] [Google Scholar]
- 30. Helvig CF, Cuerrier D, Hosfield CM, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78:463–472 [DOI] [PubMed] [Google Scholar]
- 31. Bosworth CR, Levin G, Robinson-Cohen C, et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012;82:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Int Med. 2010;152:640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr., Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shapses SA, Manson JE. Vitamin D and prevention of cardiovascular disease and diabetes: why the evidence falls short. JAMA. 2011;305:2565–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shab-Bidar S, Bours SP, Geusens PP, van der Velde RY, Janssen MJ, van den Bergh JP. Suboptimal effect of different vitamin D3 supplementations and doses adapted to baseline serum 25(OH)D on achieved 25(OH)D levels in patients with a recent fracture: a prospective observational study. Eur J Endocrinol. 2013;169:597–604 [DOI] [PubMed] [Google Scholar]
- 36. Gravesen E, Hofman-Bang J, Lewin E, Olgaard K. Ergocalciferol treatment and aspects of mineral homeostasis in patients with chronic kidney disease stage 4–5. Scand J Clin Lab Invest. 2013;73:107–116 [DOI] [PubMed] [Google Scholar]
- 37. Tepper S, Shahar DR, Geva D, Ish-Shalom S. Predictors of serum 25(Oh)D increase following bimonthly supplementation with 100,000IU vitamin D in healthy, men aged 25–65 years [published online December 12, 2013]. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 38. Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98:973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]