Abstract
Context:
In hypoparathyroidism, quality of life (QOL) is compromised as compared to normal subjects. We previously reported our results showing an association with recombinant human PTH(1–84) therapy in hypoparathyroidism and improvement in QOL measures for 1 year.
Objective:
We tested the hypothesis that PTH(1–84) therapy in hypoparathyroidism through 5 years would be associated with continued improvement in QOL measures.
Design:
Sixty-nine hypoparathyroid subjects received open-label PTH(1–84). Before and during therapy, subjects completed the RAND 36-Item Short Form (SF-36) Health Survey, a measure of health-related QOL covering eight domains of physical and mental health.
Results:
At baseline, subjects scored significantly lower than the normative reference range in all 8 domains (T-scores −1.4 to −0.9; P < .001 for all). With PTH therapy, intention-to-treat analysis showed significant improvement in the overall score at 2 months that persisted through 5 years (386 ± 19 to 482 ± 25; P < .0001). The mental component summary score improved at 2 months and was sustained through 5 years (199 ± 11 to 246 ± 14; P = .001), as did all four individual mental health domains and T-scores (vitality, social functioning, role emotional, mental health). The physical component summary score improved at 2 months and was sustained through 5 years (187 ± 10 to 237 ± 13; P < .0001), as did 3 physical health domains and T-scores (physical functioning, role physical, general health).
Conclusions:
PTH(1–84) therapy is not only associated with improvement in biochemical and skeletal indices, previously well-documented, but also in mental and physical health as determined by the SF-36 metric.
Hypoparathyroidism is a rare disease characterized by hypocalcemia and absent or deficient parathyroid hormone. It is the only classic endocrine deficiency disorder for which the missing hormone, PTH, is not yet an approved therapy. Standard treatment consists of calcium and active vitamin D supplementation, but with this standard approach, serum calcium is often difficult to manage and there are attendant concerns about long-term complications from large doses of these supplements (1–4). PTH treatment, an attractive alternative, reduces supplemental calcium and vitamin D requirements and improves skeletal parameters (5–12).
Complaints of cognitive dysfunction in hypoparathyroid patients are common. Our group and others have shown that hypoparathyroidism is associated with reduced quality of life (QOL) (13–15). We previously reported our results showing an association with recombinant human (rh) PTH(1–84) in hypoparathyroidism and improvement in QOL measures through 1 year of therapy (14). We now extend these results investigating the effect of PTH(1–84) therapy on QOL in hypoparathyroid subjects through 5 years.
Materials and Methods
Study design
We conducted an open-label uncontrolled study of rh PTH(1–84) (NPS Pharmaceuticals, Bedminster, NJ). The earliest subjects enrolled were initially treated with a dose of 100 μg every other day (7). Alternative dosing regimens of 25, 50, and 75 μg daily were made available during the study period. The PTH dose and calcium and active vitamin D supplementation regimen were titrated for each subject based on biochemical parameters. The original trial period was set at 2 years, however, an extension protocol was initiated and subjects were allowed to remain in the study indefinitely.
Subjects
The diagnosis of hypoparathyroidism in men and women was established by supplemental calcium and/or active vitamin D requirements to maintain serum calcium in the intended low-normal range with a low or insufficient PTH concentration. Hypoparathyroidism was present for at least 1 year in order to define a chronic hypoparathyroid state. All subjects had to be on stable regimens of supplemental calcium and/or vitamin D intake for at least 6 months before enrollment. Subjects were excluded if they (a) had been treated with a bisphosphonate within 5 years or for more than 6 months' duration at any time (b) were women within 5 years of menopause (c) were using any of the following medications: estrogens, progestins, raloxifene, calcitonin, systemic corticosteroids, fluoride, lithium, statins, loop diuretics, or methotrexate. The following potentially confounding disorders were also exclusionary criteria: Paget's disease of the bone, diabetes mellitus, chronic liver or renal disease, acromegaly, Cushing's syndrome, rheumatoid arthritis (RA), or multiple myeloma.
Patients were recruited from the Metabolic Bone Diseases Unit of Columbia University Medical Center and from the Hypoparathyroidism Association. The study was approved by the Institutional Review Board of Columbia University Medical Center. All subjects gave written informed consent. All subjects from our prior analysis are included in this report, in addition to subjects that enrolled after its publication (14).
Biochemical evaluation
The average of two or three pretreatment serum calcium values was used for the baseline calcium value. Blood sampling was performed 24 h after the last PTH injection for daily dosing or 48 h after injection for every other day dosing. Biochemistries were measured by automated techniques. The normal ranges for the assays are provided in Table 2 below.
Table 2.
Baseline Biochemistries of the Hypoparathyroid Population
| Mean ± se (n = 69) | Range (Median) | Normal Range | |
|---|---|---|---|
| Serum calcium (mg/dL)a | 8.7 ± 0.1 | 6.6–10.4 (8.8) | 8.6–10.2 |
| PTH (pg/mL) | 2 ± 0.6 | <3–24.5 (<3) | 10–65 |
| Creatinine (mg/dL) | 0.93 ± 0.03 | 0.60–1.80 (0.90) | 0.50–1.30 |
| Phosphate (mg/dL) | 4.4 ± 0.8 | 3.0–6.7 (4.3) | 2.5–4.5 |
| Total alkaline phosphatase activity (U/L) | 62 ± 2 | 35–106 (60) | 33–96 |
| Urinary calcium excretion (mg/day) | 255 ± 14 | 37–564 (240) | 50–250b |
| 25-hydroxyvitamin D (ng/mL) | 51 ± 9 | 12–571 (35) | 30–100 |
| 1,25-dihydroxyvitamin D (pg/mL) [n = 53] | 35 ± 2 | 9–145 (30) | 15–60 |
Serum calcium concentration was typically normal as a result of calcium and vitamin D supplementation.
For men, 50–300 mg/day.
Quality of life evaluation
The RAND 36-Item Short Form (SF-36) Health Survey (version 1.0) was developed as part of the Medical Outcomes Study (16, 17). It is one of the most widely used measures of health-related QOL and has been applied in various populations (16–21). It consists of 36 items covering 8 domains of physical and mental health: physical functioning (PF), role limitations caused by physical health problems (RF), bodily pain (BP), perception of general health (GH), vitality (VT), social functioning (SF), role limitations due to emotional health problems (RE), and mental health (MH). Scores are placed on a scale of 0–100. Higher scores indicate more favorable physical functioning and psychological well-being. The eight domains may be further grouped into two summary measures: the physical component summary (PCS) comprised of PF, RF, BP, and GH and the mental component summary (MCS) comprised of VT, SF, RE, and MH (16, 17). Subjects were measured at baseline and following treatment with PTH(1–84) at months 2, 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60. We have baseline data from all subjects and data from at least one follow-up for all but two subjects. Follow-up data were not available from subjects after discontinuation from the protocol.
Statistical analysis
A linear mixed model for repeated measures approach was applied with a single fixed effect of time and baseline level of the outcome entered as a continuous covariate. The autoregressive covariance structure [AR(1)] was determined prior to inferential testing to provide the best covariance model fit across all of the outcomes to be tested. This analysis assesses the reliability of the within-subject change from baseline (SAS Proc MIXED, Version 9.2; SAS Institute). Our primary analysis investigated the change in RAND SF-36 scores from baseline through 5 years of therapy with PTH(1–84). T-scores were calculated from the normal ranges for healthy US men and women using the same instrument (16, 17). As secondary analyses, we investigated changes at other time points; proportions of subjects that continued PTH(1–84) therapy at defined T-score categories at baseline and annual time points; whether there were differences in scores due to age of onset, duration of hypoparathyroidism or etiology of hypoparathyroidism; and whether there were differences at 1 year in scores between subjects who discontinued therapy vs those who remain in the study. We compared baseline characteristics for subjects who discontinued therapy before 5 years to subjects who continued treatment using t-tests for continuous variables and Χ2 for discrete variables. The reported analyses are intention-to-treat, save for the proportions of subjects who continued PTH therapy at defined T-score categories and the comparison between subjects who discontinued therapy vs those who remain in the study. Data are reported as model-estimated means and standard errors, and differences between baseline and subsequent times were tested by simultaneous confidence intervals. P-values <0.05 were used to establish significance.
Results
Baseline characteristics and adherence to treatment
Table 1 shows the baseline characteristics of the 69 hypoparathyroid subjects. The mean age was 46 ± 2 years (range 18–72 y) and 80% were female, consistent with the demographics of the disease. The two major etiologies of hypoparathyroidism were surgical and idiopathic. The mean duration of hypoparathyroidism was 12 ± 1 year (range 1–46 years).
Table 1.
Baseline Characteristics of the Hypoparathyroid Population
| Mean ± se (n = 69) | Range (Median) | |
|---|---|---|
| Age (y) | 46 ± 2 | 18–72 (45) |
| Sex | Female 55 | |
| Male 14 | ||
| Etiology | Postoperative 42 | |
| Idiopathic 26 | ||
| DiGeorge 1 | ||
| Duration of hypoparathyroidism (years) | 12 ± 1 | 1–46 (7) |
| Elemental calcium supplement dose (g/d) | 2.55 ± 0.2 | 0–11.0 (1.9) |
| Calcitriol supplement dose (μg/d) | 0.69 ± 0.07 | 0–3.0 (0.5) |
| Daily parent vitamin D dose (IU/d) | 4882 ± 1,837 | 0–100 000 (400) |
The data over time are available from baseline (n = 69) and months 2, 6, 12 (n = 58), 18, 24 (n = 42), 30, 36 (n = 33), 42, 48 (n = 26), 54 and 60 (n = 25) of treatment. Twenty-seven subjects discontinued the intervention within the 5 year period (mean 21 ± 3 months) due to adverse events [depression, renal cancer, renal failure, vestibular neuritis, loss of bone density at the 1/3 radius, dizziness, gastrointestinal illness, headache; n = 8], withdrawn consent (n = 6), loss to follow-up (n = 5), nephrolithiasis (n = 2), unrelated health issues (n = 2), logistics of travel (n = 2), and recovery of parathyroid hormone function (n = 2). Some subjects have not yet reached 5 years of therapy (n = 17). Subjects who continued PTH were maintained on doses of 25 μg daily (n = 7), 50 μg daily (n = 16), 75 μg daily (n = 11), 100 μg daily (n = 7), and 100 μg every other day (n = 1). The average decrease in calcium supplementation was 41 ± 8% and the average decrease in active vitamin D supplementation was 58 ± 7%.
Biochemical evaluation
Baseline biochemistries are shown in Table 2. Most subjects maintained serum calcium values of at least 8.0 mg/dL throughout the study period (78.3% at baseline, ranging from 62.1 to 86.4% throughout the 5 years). The proportion of subjects with values within the reference range (8.6–10.2 mg/dL) was lower (59.4% at baseline, ranging from 34.6 to 59.1% throughout the 5 years). Hypercalcemia was rare (2.1% of values).
Quality of life evaluation
The results of the RAND SF-36 survey at baseline and following treatment with PTH(1–84) at months 2, 6, 12, 24, 36, 48, and 60 are shown in Table 3. Intention-to-treat analysis showed an improvement in the overall score at 2 months (386 ± 19 to 468 ± 21; P < .0001) that persisted through 5 years (482 ± 25; P < .0001). The MCS score improved at 2 months and remained significantly improved through 5 years (199 ± 11 to 246 ± 14; P = .001), as did each of the four individual mental health domains (VT, SF, RE, MH). The PCS score improved at 2 months and remained significantly improved through 5 years (187 ± 10 to 237 ± 13; P < .0001), as did three of the physical health domains (PF, RF, GH). Using T-scores, 7 of 8 domains improved early with sustained effects at 5 years: VT, −1.4 to −0.9 (P = .001); SF, −1.1 to −0.7 (P = .003); RE, −0.9 to −0.4 (P = .011); MH, −0.9 to −0.4 (P = .001); PF, −0.9 to −0.3 (P < .0001); RF, −1.2 to −0.5 (P = .002); GH, −1.3 to −0.9 (P = .001) (Figure 1).
Table 3.
RAND 36-Item Health Survey Total, Component, and Individual Domain Scores at Baseline and Through 5 year of PTH(1–84) Therapy
| Time | n | Total | MCS | VT | SF | RE | MH | PCS | PF | RF | BP | GH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 69 | 386 ± 19 | 199 ± 11 | 32 ± 3 | 57 ± 3 | 51 ± 5 | 59 ± 2 | 187 ± 10 | 62 ± 3 | 40 ± 5 | 43 ± 2 | 45 ± 3 |
| 2 months | 51 | 468 ± 21c | 248 ± 11c | 46 ± 3c | 70 ± 3c | 63 ± 5a | 69 ± 2c | 221 ± 11c | 72 ± 3b | 52 ± 5a | 46 ± 2 | 55 ± 3c |
| 6 months | 57 | 482 ± 20c | 250 ± 11c | 45 ± 3c | 70 ± 3c | 68 ± 5b | 69 ± 2c | 233 ± 11c | 74 ± 3c | 59 ± 5c | 46 ± 2 | 54 ± 3c |
| 1 y | 58 | 462 ± 20c | 235 ± 11c | 44 ± 3c | 67 ± 3c | 57 ± 5 | 69 ± 2c | 227 ± 11c | 74 ± 3c | 53 ± 5a | 47 ± 2 | 54 ± 3c |
| 2 y | 42 | 472 ± 22c | 241 ± 12c | 45 ± 3c | 68 ± 4b | 61 ± 6 | 68 ± 2c | 233 ± 11c | 73 ± 3c | 57 ± 6b | 50 ± 2a | 54 ± 3c |
| 3 y | 33 | 440 ± 23b | 226 ± 13a | 41 ± 3b | 63 ± 4 | 57 ± 6 | 65 ± 3b | 215 ± 12b | 68 ± 4 | 53 ± 6 | 41 ± 3 | 53 ± 3b |
| 4 y | 26 | 448 ± 25b | 225 ± 14a | 43 ± 3c | 65 ± 4a | 55 ± 7 | 63 ± 3 | 224 ± 13b | 70 ± 4a | 61 ± 7b | 44 ± 3 | 51 ± 3a |
| 5 y | 25 | 482 ± 25c | 246 ± 14c | 42 ± 3b | 68 ± 4b | 69 ± 7a | 68 ± 3b | 237 ± 13c | 76 ± 4c | 62 ± 7b | 45 ± 3 | 54 ± 3b |
Values are mean ± se.
P < .05 compared to baseline.
P < .01 compared to baseline.
P < .001 compared to baseline.
Figure 1.
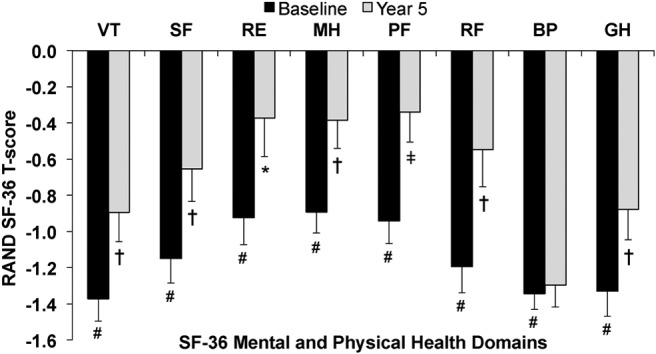
RAND 36-item health survey domain T-scores at baseline and 5 years of PTH(1–84) therapy. Values are mean ± SE. #, P < .001 compared to normal population. *, P < .05 compared to baseline. †, P < .01 compared to baseline. ‡, P < .001 compared to baseline.
Secondary analyses
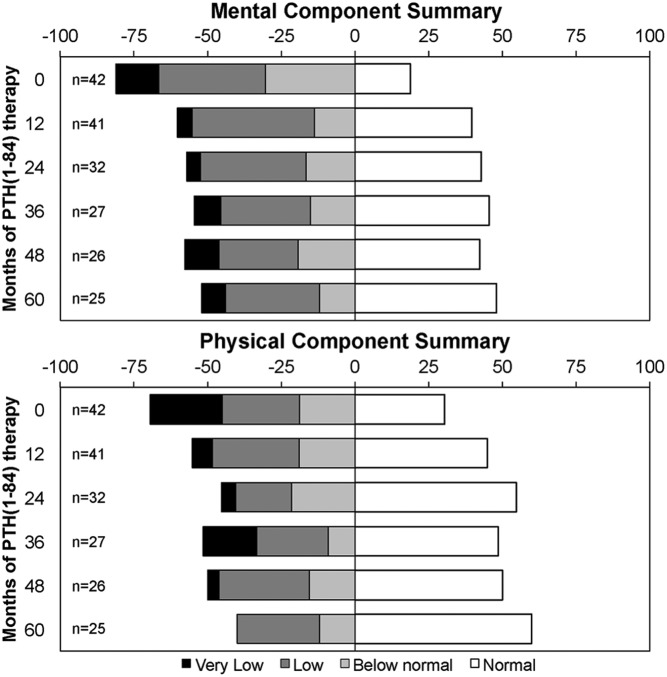
Figure 2 shows the proportions of subjects who continued PTH(1–84) therapy (n = 42) with RAND SF-36 T-scores for the MCS and PCS scores in the normal, below normal, low, and very low range at baseline and months 12, 24, 36, 48, and 60 of PTH(1–84) therapy. Normal was defined as a T-score ≥ 0, below normal as −1 SD ≥ T-score < 0, low as −2 SD ≥ T-score < −1, and very low as T-score < −2 SD. Subjects with full 5-year data and subjects who have not yet reached the 5-year time point but remain in the study protocol were included. The proportion of subjects with normal T-scores was lowest at baseline and increased at the other time points (23.8% at baseline to 48.0% at 5 years for the MCS and 33.3% at baseline to 60.0% at 5 years for the PCS). In addition, there were 21.4% of subjects with T-scores in the very low range for the PCS at baseline and 0.0% in the very low range at 5 years.
Figure 2.
Proportions of subjects that continued PTH(1–84) therapy with RAND SF-36 T-scores in the normal, below normal, low, and very low range at baseline and months 12, 24, 36, 48, and 60 of PTH(1–84) therapy. Normal: T-score ≥ 0, below normal: −1 SD ≥ T-score < 0, low: −2 SD ≥ T-score < −1, very low: T-score < −2 SD.
Age of onset, duration of hypoparathyroidism, and etiology of hypoparathyroidism were not significant predictors of the total or component summary scores or individual domains. There were no differences between subjects who continued or discontinued therapy during the study period with respect to baseline age, gender, etiology, duration of hypoparathyroidism, calcium or 1,25-dihydroxyvitamin D supplementation, or serum or urine calcium. As compared to those who continued therapy, the 27 subjects who discontinued PTH before 5 years had significantly lower values for the total (392 ± 35 vs 503 ± 25 at 1 y; P = .011) and MCS score (203 ± 19 vs 255 ± 13 at 1 year; P = .024) as well as RP (34 ± 9 vs 63 ± 6 at 1 year; P = .012), SF (58 ± 5 vs 80 ± 4 at 2 months; P = .001), and RE (36 ± 9 vs 68 ± 6 at 1 year; P = .005) domains. The overall results were similar when analyzed excluding those who discontinued PTH (data not shown).
Discussion
We investigated the effect of PTH(1–84) therapy on QOL in hypoparathyroid subjects though 5 years using the RAND 36-Item Health Survey. At baseline, subjects scored significantly lower than the normative reference range in all eight domains of mental and physical health. PTH(1–84) therapy was associated with improvement in the total score, MCS, PCS, and 7 out of the 8 domains, the four mental health domains (VT, SF, RE, and MH) and three of the physical health domains (PF, RF, and GH). In addition, the proportions of subjects with positive T-scores increased with therapy. Beneficial effects were noted early and persisted through 5 years. Serum calcium was maintained in the intended low-normal range (≥8.0 mg/dL) for most subjects. However, the proportion of subjects with serum calcium values within the reference range (8.6–10.2 mg/dL) was lower.
Complaints of cognitive dysfunction in hypoparathyroid patients are common. Our group and others have shown that hypoparathyroidism is associated with reduced QOL using standardized measures (13–15). We previously reported our results showing that PTH(1–84) therapy was associated with an improvement in QOL measures through 1 year of therapy (14). In that smaller group of subjects, improvement in QOL was noted in the total score, MCS, PCS, three of the mental health domains (VT, SF, and MH) and two of the physical health domains (PF and GH). There is some evidence that PTH may have central nervous system (CNS) effects (22–26), which may provide an explanation for reduced QOL in hypoparathyroid subjects despite relative eucalcemia and the capacity of PTH therapy to alleviate some of these complaints.
Sikjaer et al published the results of a randomized clinical trial investigating the effect of PTH(1–84) 100 μg daily vs placebo on QOL over 6 months (15). They found an improvement in QOL measures in both the placebo and PTH arms but no between-group differences. Mean serum calcium levels in the PTH-treated group were significantly increased during their study and there was a relatively high incidence of hypercalcemia. The authors noted that the large fluctuations in serum calcium might have negated any potential advantage of the PTH therapy. Considering that hypoparathyroid subjects may acclimate to relatively lower serum calcium values, these results may indicate that the reference range for serum calcium in normal subjects may not be suitable for all hypoparathyroid subjects with regard to their mental and physical well-being.
The strengths of this study include the remarkably large cohort of subjects with hypoparathyroidism followed longitudinally through 5 years of therapy. These long-term QOL data with PTH(1–84) in the therapy of hypoparathyroidism are unique. Among the limitations is the absence of a placebo control. While the persistence of the findings through 5 years is reassuring against the results being solely due to a placebo effect, this does not remove the possibility of confounding. A majority of the subjects remained in the study protocol, although subjects who discontinued PTH therapy may not have benefited to the same extent and this may have resulted in attrition bias despite our use of an intention-to-treat analysis. It should also be noted that the SF-36 instrument is a generalized one and not tailored to the specific QOL measures that subjects with hypoparathyroidism typically experience. It is impressive, nevertheless, that this general measurement tool would so clearly demonstrate improvements in QOL among subjects with hypoparathyroidism. A QOL instrument more specifically designed to a hypoparathyroid cohort might conceivably demonstrable even more dramatic improvements with PTH (1–84) therapy. Since our study design called for serum measurements after the first 24 h of PTH administration, earlier changes, if they occurred, would not have been captured. We do not believe our subjects would have experienced significant elevations in serum calcium in the time period preceding our measurement at the administered doses titrated to subjects' biochemistries based on the results of pharmacologic studies in other subjects (27, 28). In addition, a number of subjects discontinued the intervention due to adverse events that may or may not have been related to study drug. Our clinical trial subjects may not be representative of the hypoparathyroid population in general and the results of this trial may not reflect a common use experience. PTH therapy may not be appropriate for all patients.
PTH(1–84) therapy has previously been shown to be associated with improvement in biochemical and skeletal indices (7, 8, 10–12). The results of this study now show an association with improvement in mental and physical health over a substantial period of time. Further study in a randomized, placebo-controlled manner using PTH doses titrated by biochemical parameters would help to confirm these observations. The effect of a more physiologic mode of PTH delivery on QOL remains of interest.
Acknowledgments
This work was supported by National Institutes of Health grant DK069350, NPS Pharmaceuticals.
Disclosure Summary: Dr Bilezikian is a consultant for Amgen, Eli Lilly, Radius, NPS Pharmaceuticals, Merck, and GSK, and receives research support from NPS Pharmaceuticals and Amgen. Dr. Rubin receives research support from NPS Pharmaceuticals. No conflicts of interest reported for the remaining authors.
Footnotes
- BP
- bodily pain
- GH
- perception of general health
- MCS
- mental component summary
- MH
- mental health
- PCS
- physical component summary
- PF
- physical functioning
- QOL
- quality of life
- RA
- rheumatoid arthritis
- RE
- role limitations due to emotional health problems
- RF
- role limitations caused by physical health problems
- rh
- recombinant human
- SF
- social functioning
- SF-36
- RAND 36-Item Short Form
- VT
- vitality.
References
- 1. Bilezikian JP, Khan A, Potts JT, Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoback D. Clinical practice. Hypoparathyroidism. New Engl J Med. 2008;359:391–403 [DOI] [PubMed] [Google Scholar]
- 3. Cusano NE, Rubin MR, Sliney J, Jr, Bilezikian JP. Mini-review: new therapeutic options in hypoparathyroidism. Endocrine. 2012;41:410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97:4507–4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winer KK, Ko CW, Reynolds JC, et al. Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab. 2003;88:4214–4220 [DOI] [PubMed] [Google Scholar]
- 6. Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, Cutler GB., Jr Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1–34 versus calcitriol and calcium. J Clin Endocrinol Metab. 2010;95:2680–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin MR, Sliney J, Jr., McMahon DJ, Silverberg SJ, Bilezikian JP. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int. 2010;21:1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubin MR, Dempster DW, Sliney J, Jr., et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res. 2011;26:2727–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winer KK, Zhang B, Shrader JA, et al. Synthetic human parathyroid hormone 1–34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab. 2012;97:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sikjaer T, Rejnmark L, Rolighed L, Heickendorff L, Mosekilde L. The effect of adding PTH(1–84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res. 2011;26:2358–2370 [DOI] [PubMed] [Google Scholar]
- 11. Cusano NE, Rubin MR, McMahon DJ, et al. Therapy of hypoparathyroidism with PTH(1–84): a prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2013;98:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mannstadt M, Clarke BL, Vokes T, et al. Efficacy and safety of recombinant human parathyroid hormone (1–84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1:275–283 [DOI] [PubMed] [Google Scholar]
- 13. Arlt W, Fremerey C, Callies F, et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol. 2002;146:215–222 [DOI] [PubMed] [Google Scholar]
- 14. Cusano NE, Rubin MR, McMahon DJ, et al. The effect of PTH(1–84) on quality of life in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98:2356–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sikjaer T, Rolighed L, Hess A, Fuglsang-Frederiksen A, Mosekilde L, Rejnmark L. Effects of PTH(1–84) therapy on muscle function and quality of life in hypoparathyroidism: results from a randomized controlled trial. Osteoporos Int. 2014;25:1717–1726 [DOI] [PubMed] [Google Scholar]
- 16. Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483 [PubMed] [Google Scholar]
- 17. RAND Health 2010 Medical Outcomes Study: 36-Item Short Form Survey Scoring Instructions. Available at: http://wwwrandorg/health/surveys_tools/mos/mos_core_36item_scoringhtml
- 18. te Vaarwerk MJ, Gaal EA. Psychological distress and quality of life in drug-using and non-drug-using HIV-infected women. Eur J Public Health. 2001;11:109–115 [DOI] [PubMed] [Google Scholar]
- 19. O'Brien PE, Dixon JB, Brown W, et al. The laparoscopic adjustable gastric band (Lap-Band): a prospective study of medium-term effects on weight, health and quality of life. Obes Surg. 2002;12:652–660 [DOI] [PubMed] [Google Scholar]
- 20. van Baalen B, Odding E, van Woensel MP, Roebroeck ME. Reliability and sensitivity to change of measurement instruments used in a traumatic brain injury population. Clin Rehabil. 2006;20:686–700 [DOI] [PubMed] [Google Scholar]
- 21. Seguin R, Lamonte M, Tinker L, et al. Sedentary behavior and physical function decline in older women: findings from the women's health initiative. J Aging Res. 2012;2012:271589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem. 1995;270:15455–15458 [DOI] [PubMed] [Google Scholar]
- 23. Bagó AG, Dimitrov E, Saunders R, et al. Parathyroid hormone 2 receptor and its endogenous ligand tuberoinfundibular peptide of 39 residues are concentrated in endocrine, viscerosensory and auditory brain regions in macaque and human. Neuroscience. 2009;162:128–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balabanov S, Tollner U, Richter HP, Pohlandt F, Gaedicke G, Teller WM. Immunoreactive parathyroid hormone, calcium, and magnesium in human cerebrospinal fluid. Acta Endocrinol (Copenh). 1984;106:227–233 [DOI] [PubMed] [Google Scholar]
- 25. Joborn C, Hetta J, Niklasson F, et al. Cerebrospinal fluid calcium, parathyroid hormone, and monoamine and purine metabolites and the blood-brain barrier function in primary hyperparathyroidism. Psychoneuroendocrinology. 1991;16:311–322 [DOI] [PubMed] [Google Scholar]
- 26. Care AD, Bell NH. Evidence that parathyroid hormone crosses the blood-brain barrier. Proceedings of the IXth International Conference on Calcium Regulating Hormones and Bone Metabolism; Nice, France; 1986 [Google Scholar]
- 27. Fox J, Wells D, Garceau R. Relationships between pharmacokinetic profile of human PTH(1–84) and serum calcium response in postmenopausal women following 4 different methods of administration. J Bone Miner Res. 2011;26 Available at: http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=cb1c7954-4672-41f1-8b55-9a70ec107b6f Accessed April 23, 2014
- 28. Sikjaer T, Amstrup AK, Rolighed L, Kjaer SG, Mosekilde L, Rejnmark L. PTH(1–84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects after 6 months of treatment. J Bone Miner Res. 2013;28:2232–2243 [DOI] [PubMed] [Google Scholar]