Abstract
Context:
Studies of hormone changes in the peripubertal period note increases in adrenal hormones prior to increases in sex steroids. It is unclear how these processes are related to each other, except through this temporal relationship.
Objective:
Examine relationships in adrenal and sex hormones in 252 peripubertal girls.
Setting and Design:
Longitudinal observation study. School districts, at the Cincinnati site of the Breast Cancer and the Environment Research Centers, between 2004–2010. Participants were recruited between ages 6 and 7 years of age and were seen every 6 months. Main outcome measures included height, weight, maturation status, and fasting blood specimen. Serum was analyzed for selected hormones every six months, beginning 30 months prior to, and extending to 6 months after, breast development. Androstenedione, estradiol, estrone, and T were measured by high-performance liquid chromatography (HPLC) with tandem mass spectrometry. Dehydroepiandrosterone-sulfate (DHEA-S) and SHBG also were measured.
Results:
DHEA-S concentrations increased 24 months before breast development; androstenedione and estrone between 12 to 18 months before breast development; whereas estradiol and T increased, and SHBG fell between 6 and 12 months before breast development. Girls with greater body mass index had lower estradiol concentrations at onset of breast development as well as 6 months after pubertal onset.
Conclusions:
Serum estrone and DHEA-S increased prior to estradiol concentrations, and the increase in estradiol occurred prior to breast development. Heavier peripubertal girls have lower estradiol levels at puberty, suggesting peripheral conversion of adrenal androgens to estrone.
Studies of hormone changes in the peripubertal period have noted increases in adrenal hormones prior to increases in the sex steroids (1–5); Adrenarche refers to the reactivation of the adrenal cortex with the production of adrenal androgens, specifically dehydroepiandrosterone and its sulfate (DHEA and DHEAS), and androstenedione. In girls, gonadarche refers to the reactivation of the hypothalamic-pituitary-ovarian axis, with the production of estrogen by the ovaries, and the subsequent appearance of secondary sex characteristics (typically breast tissue), and is usually noted after adrenarche. Many have reflected on the temporal association between adrenarche and gonadarche (eg, Cutler and Loriaux [6]). Of note, estrogens are synthesized not only in the ovary, but also in target tissues (such as breast, bone, and brain) as well as in peripheral tissues (such as adipose tissue), through the conversion of adrenal androgens (particularly androstenedione) by tissue-specific aromatase (as reviewed by Simpson (7) and Stocco [8]). Local production may generate important intracrine effects without affecting circulating levels (9). It is unclear whether or how adrenarche and gonadarche are related to each other, except through this temporal relationship. It is recognized increasingly that there are pubertal changes in girls that predate appearance of secondary sexual characteristics. These changes include an increase in height velocity and the pubertal growth spurt (documented by many studies), as well as changes in uterine dimensions and ovarian volumes noted on pelvic ultrasound (10, 11). In addition, studies have noted an increase in urine and serum concentrations of estradiol in prepubertal girls (12–17).
This article examines the longitudinal relationship between adrenal and sex steroid concentrations in a group of prepubertal girls, measured from 30 months before to 6 months after the appearance of breast development. Estradiol, estrone, androstenedione, and T serum concentrations were analyzed using high-performance liquid chromatography (HPLC) with tandem mass spectrometry, a newer highly sensitive analytic approach (13, 18, 19). Dehydroepiandrosterone sulfate (DHEA-S) and SHBG were measured through established methods.
Materials and Methods
The participants for these analyses represent one site of those enrolled onto a larger, longitudinal epidemiology study of pubertal maturation, through the Cincinnati site of the Breast Cancer and the Environment Research Centers (BCERC). The National Institute of Environmental Health Sciences (NIEHS)/National Cancer Institute (NCI) BCERC were four centers with transdisciplinary research collaborations integrated across biologic, epidemiologic, and community outreach projects. The epidemiology projects of the BCERC have been conducted at three sites: Mount Sinai School of Medicine; Kaiser Permanente Northern California; and Cincinnati Children's Hospital/University of Cincinnati. Study aims and design have been detailed elsewhere (20). Briefly, the participants at the Cincinnati site were recruited through public and parochial schools in the greater Cincinnati metropolitan area (85% of participants) as well as through the Breast Cancer Registry of Greater Cincinnati (15% of participants). Recruitment through the Registry targeted those with a first- or second-degree family member with breast cancer. Participants were recruited between ages 6 and 7 years, and enrollment at the Cincinnati site lasted 29 months. Girls were seen every six months between 2004–2010, with a visit window of ±4 weeks. This study was approved by the Institutional Review Board of Cincinnati Children's Hospital Medical Center. Informed consent was obtained from parents and or guardians.
Trained and certified female staff members performed standardized anthropometric measurements, with two or more height and weight measurements, as detailed previously (20). In addition, a limited number of female staff were trained and certified in assessment of pubertal maturation, using the criteria established by Marshall and Tanner for breast maturation (with palpation) (21) and pubic hair stages, with photographs that demonstrated the maturation stages, published by van Wieringen (22).
Early morning fasting blood specimens were drawn at every visit. Serum was frozen at −80°C and later analyzed for selected hormones at the onset of breast development, and at each of three preceding visits (up to 18 months before breast development) as well as 6 months after breast development, for androstenedione, T, and SHBG, and up to 30 months for estrone, estradiol, and DHEA-S.
DHEA-S was measured by RIA after enzymolysis. Briefly, samples were diluted in buffer-containing sulfatase from Helix pomatia, and after incubation, measured in duplicate by RIA using a rabbit antibody made against a DHEA-7-BSA antigen. Tritiated DHEA was used as tracer for the RIA. Each assay batch contained a complete standard curve and three levels of quality control samples. SHBG was measured by automated immunoradiometric assay. Androstenedione, estradiol, estrone, and T were measured by high-performance liquid chromatography (HPLC) with tandem mass spectrometry at Esoterix Laboratories. Each steroid was first extracted with hexane/ethyl acetate; the extract was dried and reconstituted in solvent compatible with the HPLC. Solvents were HPLC grade or redistilled in glass. Deuterated steroids, otherwise identical with the analytes, were used as internal standards except for estradiol and estrone where 13C steroids were used for internal standards. HPLC and mass spectrometry conditions were similar to those described previously (23, 24). In the previously published method, in contrast with ours, estradiol and estrone were eluted with water-methanol as well as acetonitrile, an Applied BioSystems API5000 triple quadrupole mass spectrometer was used, and derivatization was not used. The first and third quadrupoles were tuned for each analyte using unit resolution, and conditions were optimized for maximum signal intensity foreach steroid and internal standard. T and estradiol standards were from USP; others were from Sigma Chemical. Internal standards were from Cambridge Isotopes or Sigma. Each assay batch for all hormones contained duplicate standard sets of eight or more charcoal-stripped serum-based standards and duplicate sets of three to five serum quality-control samples prepared by selected serum, from individual donations, and with dilution using stripped serum or spiking if required. Assay performance was demonstrated during validation to bioanalytical validation standards. The lower limits of quantitation were defined as the level at which a coefficient of variation was less than 20% (25% for RIA). The quantitation levels were DHEA-S, 10 μg/dl; estrone, 2.5 pg/ml; estradiol, 1 pg/ml; androstenedione, 10 ng/dl; and T 3 ng/dl. Because of the volume of serum necessary to run the hormone assays, and limited availability of specimen, the serum was diluted 1:1 with water, resulting in a limit of detection twice as great as noted above.
Interassay precision was expressed as percent coefficient of variation for low, medium, and high control serum samples was for DHEA-S, 6.5, 8.4, and 7.3%; for estrone, 4.9, 4.6, and 4.7%; for estradiol, 4.4, 3.5, and 3.3%; for androstenedione, 4.5, 3.6, and 3.4; and for T, 9.9, 7.9, and 5.0%. Midrange control coefficients of variation were androstenedione, 4.3%; estradiol, 3.5%; estrone, 4.6%; and T, 4.3%.
These analyses were performed on the initial 252 girls who had entered puberty, as defined by breast stage 2 or greater, within the first 4 years of the study. Specimens were identified for hormone analysis after the participant had attained puberty (breast stage 2 or greater), selecting specimens at 6, 12, and 18 months before breast development, at the time of breast development, and 6 months after breast development. Additional specimens at 24 and 30 months before breast development were selected for analyses of DHEA-S, estrone, and estradiol.
Statistical analyses
The initial analyses examined the distributions of hormone concentrations using histogram plots and Kolmogorov-Smirnov (Goodness-of-Fit) tests. Log transformation was applied to each of the hormones and SHBG, as the Kolmogorov-Smirnov tests demonstrated better fit with log-normal transformation. There were varying numbers of missing values for different hormone assays, typically due to insufficient volume. The missing hormone data were assumed to be randomly distributed; mixed modeling was applied to the log-transformed hormone values. Mixed modeling was chosen to understand better the profile of hormone changes around the time of breast development. That is, the mixed model analyses simultaneously considered hormone concentrations at time at onset of breast development, as well as 6, 12, and 18 months before and 6 months after time of breast development. The hormone concentrations at times relative to breast development were the main factor, and corresponding least square means were estimated and presented in the figures. The least square means were adjusted for laboratory batch effect and body mass index (BMI) z score at onset of breast development (by quartile). BMI z score was determined by using the 2000 growth charts from the Centers for Disease Control and Prevention (www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm). The P values noted in the results and figures are based on the least square means at different relative times, contrasted between adjacent 6-month intervals, to define the earliest significant increase in concentration for a given hormone. Multiple comparisons were adjusted using the Hochberg step-down procedure. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc).
Results
There were 379 girls recruited into the Cincinnati BCERC. This article reports on the initial 252 girls who entered puberty, as defined by breast stage 2 or greater, and had two or more available blood specimens. Among the girls included in this analysis, the average age at the first visit was 7.09 ± 0.62 years, whereas those not included had a mean age of 6.92 ± 0.62 years (P = .046). Race and ethnicity as defined by parents/guardians in this cohort included 32.5% African-American, 4.4% Hispanic, 61.9% white non-Hispanic, and 0.8% Asian participants. There were no differences in race or ethnicity in those who were or were not included in these analyses. The average age of breast development in this subset was 8.78 years (SD, 1.16) (25), not different from Cincinnati participants without hormone analyses (P = .37). “Pathway” (initial manifestation of puberty (26)) could be determined in 70.2%, with 52.8% experiencing breast-first (thelarche) pathway, and 17.4% pubic hair–first (pubarche) pathway.
The 252 participants with hormone analyses generated 871 specimens obtained between 18 months before and 6 months after onset of breast development, as well as an additional 122 specimens from 24 and 30 months prior to breast development. The vast majority of “missing” specimens were from participants recruited less than 18 months before puberty, who could not contribute specimens at −18 or −24 months. Other missing data occurred because the girls had missed an individual study visit, or they refused phlebotomy at an individual visit. In addition, serum volume available from any single visit may have been insufficient for all hormonal analyses; the number of observations included in the analyses varied between 701 (for T) to 772 (for SHBG) for the four-time point observations, and 994 for DHEA-S for the six-time point observations. There were 70 participants who had four specimens, and 63 with specimens from all five time periods. There were no significant difference of estradiol values at onset of breast development (P = .67), contrasting with those who had specimens missing at −12 or −18 months to those not missing specimens at those times, demonstrating there was no bias created by the missing samples from girls who matured earlier.
Serum hormone concentrations increased at different times preceding breast development. As noted in Figures 1, 2, and 3, hormone concentrations of DHEA-S increased 30 to 18 months prior to breast development (P = .0022); androstenedione (P = .0110) and estrone (P = .0027) increased 12 and 18 months before onset of breast development (see also Supplemental Figures 1–5). Estradiol (P = .0040) and T (P = .0004) concentrations increased and SHBG decreased (Figure 4) (P = .0069) 6 to 12 months before breast development. When examining by “pathway” (initial manifestation of puberty) (26), those with the pubarche pathway had higher levels of DHEAS (34.9 vs 25.5, P = .026), T (6.49 vs 5.11, P = .027), and androstenedione (33.2 vs 22.1, P = .0009), and no difference in estradiol (P = .35) or estrone levels (P = .8) at entry into puberty. When comparing participants by BMI z-score, those with BMI levels above the median had significantly lower estradiol levels at the onset of breast development, as well as 6 months later (Figure 5). At the time of breast development, girls with BMI below the median had a greater estradiol-to-androstendione ratio than girls above the median BMI (1.53 vs 1.22, P = .05), whereas there was no difference in estrone-to-androstenedione ratio (2.28 vs 1.96, P = .10).
Figure 1.
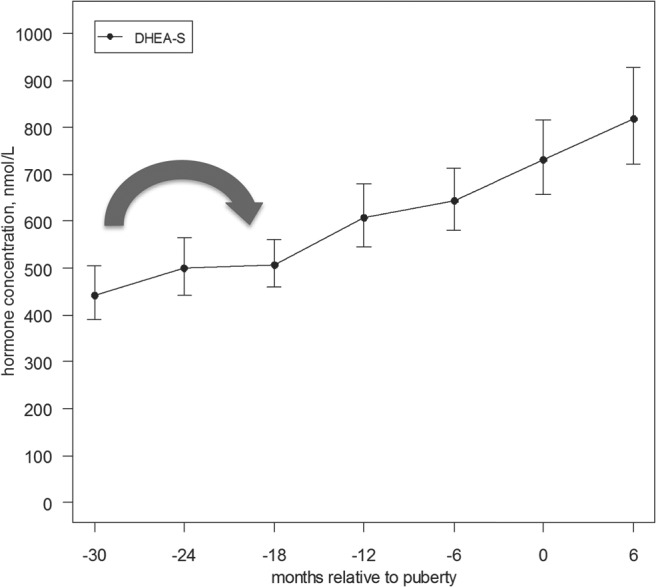
Serum hormone concentration of DHEAS relative to onset of puberty (with confidence interval). This figure demonstrates mean DHEAS concentrations relative to onset of puberty (as defined by onset of breast development), with 95th percentile interval. The initial increase in DHEAS was noted between 30 to 18 months prior to the onset of puberty (P = .0022) (large arrow).
Figure 2.
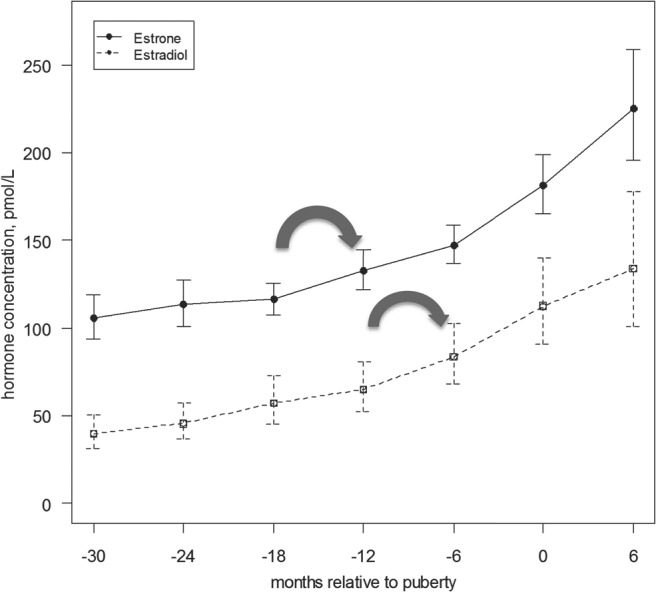
Serum hormone concentration of estradiol and estrone relative to onset of puberty (with confidence interval). This figure demonstrates mean estradiol and estrone concentrations relative to onset of puberty (as defined by onset of breast development), with 95th percentile interval. The initial increase in estradiol was noted between 12 and 6 months (P = .0040) prior to the onset of puberty, and the initial increase in estrone was between 18 to 12 months (P = .0027) (large arrows).
Figure 3.
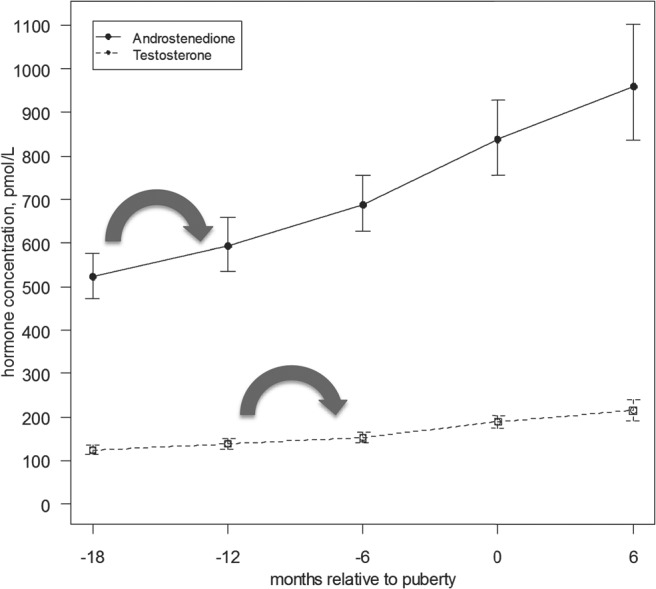
Serum hormone concentration of androstenedione and T relative to onset of puberty (with confidence interval). This figure demonstrates mean androstenedione and T concentrations relative to onset of puberty (as defined by onset of breast development), with 95th percentile interval. The initial increase in androstenedione was noted between 18 to 12 months (P = .0110) prior to the onset of puberty, and the initial increase in T was noted between 12 and 6 months (P = .0004) (large arrows).
Figure 4.
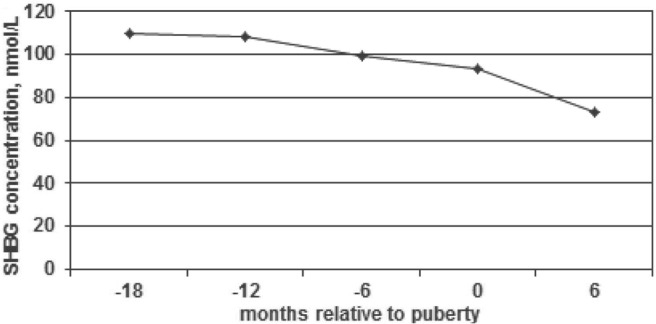
SHBG concentration relative to onset of puberty. This figure demonstrates mean SHBG concentrations drop significantly at 6 to 12 months prior to breast development (P = .0069).
Figure 5.
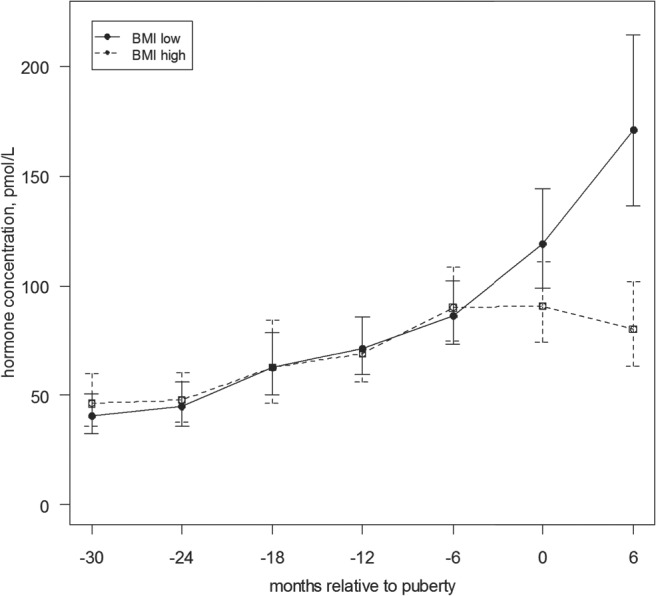
Serum hormone concentration of estradiol by BMI group relative to onset of puberty (with confidence interval). This figure demonstrates mean estradiol concentrations are significantly greater at onset of puberty (as defined by onset of breast development), as well as six months after onset, in girls in those with BMI below the median.
Discussion
This study examined the hormone changes in peripubertal girls at 6-month intervals prior to breast development and identified the time point of the significant increase in sex steroids. These analyses demonstrate as well as amplify results of previous studies, that there are hormonal changes that occur with puberty before the appearance of secondary sexual characteristics in girls (13, 15, 16, 18, 27). Previous publications have reported sex steroid concentrations either by chronologic age, often combining ages because of sample size, and incorporating ages regardless of pubertal status (18); or pubertal status, combining all who do not have secondary sexual characteristics as “prepubertal” (or the equivalent) (13, 15, 16, 27). Although a few studies are longitudinal, many studies are cross-sectional (13, 15, 16, 18, 27). Similar to several previous studies, we noted in this longitudinal study that the increase in serum concentrations of hormones associated with adrenarche occurred before the increase in serum concentrations of those hormones associated with gonadarche (1–5). Thus, it is unclear whether the timing of adrenarche has changed, although age of pubertal onset, based on breast development, has decreased (20, 25, 28). Our hormone profiles by pathway (26) are similar to those recently reported by Mouritsen (29); there were no differences in estradiol by pathway, and those with “pubarche” had greater DHEAS levels in both studies. However, we found greater androstenedione levels in the pubarche pathway, consistent with the DHEAS differences, but different from the results of Mouritsen. The differences in androstenedione levels between the two studies may have resulted from greater sensitivity of the HPLC/tandem mass spectrometry, or from differences in race/ethnicity, BMI, or environmental exposures between the two sites.
This study additionally noted that serum estrone concentrations increased after DHEA-S, but 6 months prior to the increase noted in serum estradiol. This finding provides additional support to Ducharme's suggestion of the association of adrenal steroids with the reinitiation of the hypothalamic pituitary gonadal (HPG) axis (30). Estrone production outside the ovary has been reported in postmenopausal women, with conversion of androstenedione to estrone (rather than T to estradiol) (31), accounting for most estrogen in postmenopausal women. In postmenopausal women, estrone is noted as the most prevalent circulating estrogen (32, 33); as noted, the adipocyte seems to be the major site of extraovarian estrogen synthesis (34). Studies in adults also have noted that the highest activity of aromatase, an enzyme that converts androgens to estrogens, occurred in stromal adipose tissue in the buttocks (35). Previous studies have documented that adiposity in peripubertal girls is associated with suppression of the hypothalamic-pituitary-ovarian axis (36, 37). The current study suggests that prepubertal girls approaching puberty, with limited ovarian activity, may have a similar estrogen synthetic pathway, through peripheral conversion of adrenal hormones such as DHEA and androstenedione in adipose tissue, and without activation of the HPG axis. This might account for three recent findings: 1) the weaker correlation in more recent cohorts between age of breast development with age of menarche (38, 39); 2) the slower tempo of breast development to menarche noted in contemporary girls overall and earlier-maturing girls (38–43); and 3) the finding of Aksglaede and colleagues (28), who reported that girls in Copenhagen matured at earlier ages without an earlier increase in estradiol, through a mechanism independent of gonadotropins and activation of the HPG axis. The findings of Aksglaede and the current study, placed in the context of the obesity epidemic and consequent increase in adiposity (44, 45) and/or exposure to endocrine-disrupting chemicals acting on the adipocyte or other hormone-responsive tissues (46–55), may account for earlier maturation of girls noted two decades earlier (56) as well as noted more recent cohorts (20, 25, 28). In addition, this mechanism might account for isolated breast development without the activation of the hypothalamic-pituitary-gonadal axis, the thelarche variant, (57) (in contrast with pubertal thelarche pathway (26)).
Although this study provides unique contributions to the field of pubertal development, there are several limitations to consider. We reported a difference in estradiol levels between those with high vs low BMI; although fat tissue can be confused as breast tissue, those with high BMI had height velocity values similar to those with low BMI, and both were consistent with the pubertal growth spurt (data not shown). We used HPLC/tandem mass spectroscopy, a newer analytic approach for hormone analyses, which provides greater sensitivity than more traditional methods. This approach has gained increasing popularity recently (13, 18, 19), although a recent study demonstrated poor precision with all assays at low T levels (58). Of note, the laboratory that performed the hormone analyses has been certified by the Centers for Disease Control and Prevention and continues to be engaged in the proficiency phase to maintain certification; the average bias estimations from the proficiency studies are less than 2%. We used DHEA-S and androstenedione as the two hormones representative of adrenarche. Remer noted that adrenarche may start earlier than indicated by the increase in DHEA excretion rate, if one examines direct metabolites of DHEA (3). Hopper noted that serum DHEA-S and DHEA levels do not increase in parallel in peripubertal girls, although DHEA-S does seem to increase earlier than DHEA (59). The most important limitation is that we included only two thirds of the cohort, those who were first to enter puberty, as defined by breast maturation during the course of the longitudinal follow-up of the study. Those not included in this analysis either presented with breast development at enrollment or were recruited later in the study and had not entered puberty at the time of hormone analyses, or they were entering puberty at older ages. In addition, those participants who contributed DHEA-S, estrone, and estradiol specimens at 24 and 30 months prior to breast development are overrepresented by girls who matured later. Therefore, some analyses are underrepresented by early- and others by late-maturing girls. The missing data in the mixed model analyses did not interject a bias; although the mean age of breast development was younger in those with samples at −12 and −18 months prior to breast development, there were no significant differences in estradiol levels at onset of breast development in these groups. Lastly, we reported that the initial significant increase in DHEAS occurred between −30 and −18 months, and androstenedione −18 and −12 months before breast development; those increases occurred with the initial measurement, and possibly could have occurred earlier.
In conclusion, the new HPLC/tandem mass spectrometry analyses have enabled us to evaluate pubertal hormone changes in girls, as well as other physiologic parameters, prior to any evident breast development. In this group of girls, hormonal changes associated with adrenarche occurred prior to hormonal changes associated with gonadarche. Hormones associated with adrenarche, as well as estrone, increased 12–24 months before breast development, and the hormones associated with gonadarche increased 6–12 months before breast development. These findings suggest a mechanism, especially in heavier girls, for pubertal changes without activating the hypothalamic-pituitary-ovarian axis.
Acknowledgments
We gratefully thank the research staff, study helpers, as well as participants and their families, and the clerical assistance of Lynn Hanrahan.
This work was supported by the Breast Cancer and the Environment Research Centers Grant U01 ES/CA ES-12770 from the National Institute of Environmental Health Sciences (NIEHS), the National Cancer Institute (NCI), UL1 RR026314 (USPHS), P30 ES006096 (NIEHS), 8 UL1 TR000077–05 (NIH and CTSA), and ES-019453. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCI, NIH, or the CTSA.
Disclosure Summary: F.M.B., S.M.P., B.H., E.R.B., and L.D.D. have nothing to disclose. D.W.C. is employed by Endocrine Sciences.
Footnotes
- BCERC
- Breast Cancer and the Environment Research Centers
- BMI
- body mass index
- DHEA
- dehydroepiandrosterone
- DHEAS
- dehydroepiandrosterone sulfate
- HPG
- hypothalamic pituitary gonadal
- NCI
- National Cancer Institute
- NIEHS
- National Institute of Environmental Health Sciences.
References
- 1. Meikle AW, Kushnir MM, Rockwood AL, et al. Adrenal steroid concentrations in children seven to seventeen years of age. J Pediatr Endocrinol Metab. 2007;20:1281–1291 [DOI] [PubMed] [Google Scholar]
- 2. Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr. 1977;90:766–770 [DOI] [PubMed] [Google Scholar]
- 3. Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab. 2005;90:2015–2021 [DOI] [PubMed] [Google Scholar]
- 4. Sizonenko PC, Paunier L, Carmignac D. Hormonal changes during puberty. IV. Longitudinal study of acrenal androgen secretions. Horm Res. 1976;7:288–302 [DOI] [PubMed] [Google Scholar]
- 5. Tung YC, Lee JS, Tsai WY, Hsiao PH. Physiological changes of adrenal androgens in childhood. J Formos Med Assoc. 2004;103:921–924 [PubMed] [Google Scholar]
- 6. Cutler GB, Jr., Loriaux DL. Andrenarche and its relationship to the onset of puberty. Fed Proc. 1980;39:2384–2390 [PubMed] [Google Scholar]
- 7. Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230 [DOI] [PubMed] [Google Scholar]
- 8. Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson E, Rubin G, Clyne C, et al. The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab. 2000;11:184–188 [DOI] [PubMed] [Google Scholar]
- 10. Orbak Z, Sasöz N, Alp H, Tan H, Yildirim H, Kaya D. Pelvic ultrasound measurements in normal girls: Relation to puberty and sex hormone concentration. J Pediatr Endocrinol Metab. 1998;11:525–530 [DOI] [PubMed] [Google Scholar]
- 11. Salardi S, Orsini LF, Cacciari E, Bovicelli L, Tassoni P, Reggiani A. Pelvic ultrasonography in premenarcheal girls: Relation to puberty and sex hormone concentrations. Arch Dis Child. 1985;60:120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bay K, Andersson AM, Skakkebaek NE. Estradiol levels in prepubertal boys and girls–Analytical challenges. Int J Androl. 2004;27:266–273 [DOI] [PubMed] [Google Scholar]
- 13. Courant F, Aksglaede L, Antignac JP, et al. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J Clin Endocrinol Metab. 2010;95:82–92 [DOI] [PubMed] [Google Scholar]
- 14. Ikegami S, Moriwake T, Tanaka H, et al. An ultrasensitive assay revealed age related changes in serum oestradiol at low concentrations in both sexes from infancy to puberty. Clin Endocrinol. 2001;55:789–795 [DOI] [PubMed] [Google Scholar]
- 15. Janfaza M, Sherman TI, Larmore KA, Brown-Dawson J, Klein KO. Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: A 10 year experience. J Pediatr Endocrinol Metab. 2006;19:901–909 [DOI] [PubMed] [Google Scholar]
- 16. Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB., Jr Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest. 1994;94:2475–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi L, Remer T, Buyken AE, Hartmann MF, Hoffmann P, Wudy SA. Prepubertal urinary estrogen excretion and its relationship with pubertal timing. Am J Physiol Endocrinol Metab. 2010;299:E990–E997 [DOI] [PubMed] [Google Scholar]
- 18. Kushnir MM, Rockwood AL, Bergquist J, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129:530–539 [DOI] [PubMed] [Google Scholar]
- 19. Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384 [DOI] [PubMed] [Google Scholar]
- 20. Biro FM, Galvez MP, Greenspan LC, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126:e583–e590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Wieringen JC, Roede MJ, Wit JM. Growth diagrams for patient care. Tijdschr Kindergeneeskd. 1985;53:147–152 [PubMed] [Google Scholar]
- 23. Grant R, Wagner A, Morr M, Patel M, Inventors. US Patent US7935921 B2. 2011 Methods and systems for the quantitative analysis of biomarkers.
- 24. Kushnir MM, Rockwood AL, Roberts WL, et al. Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clin Chem. 2006;52:1559–1567 [DOI] [PubMed] [Google Scholar]
- 25. Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biro FM, Lucky AW, Simbartl L, et al. Pubertal maturation in girls and the relationship to anthropometric changes: Pathways through puberty. J Pediatr. 2003;142:643–646 [DOI] [PubMed] [Google Scholar]
- 27. Paris F, Servant N, Térouanne B, Balaguer P, Nicolas JC, Sultan C. A new recombinant cell bioassay for ultrasensitive determination of serum estrogenic bioactivity in children. J Clin Endocrinol Metab. 2002;87:791–797 [DOI] [PubMed] [Google Scholar]
- 28. Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: The Copenhagen Puberty Study. Pediatrics. 2009;123:e932–939 [DOI] [PubMed] [Google Scholar]
- 29. Mouritsen A, Aksglaede L, Soerensen K, et al. The pubertal transition in 179 healthy Danish children: Associations between pubarche, adrenarche, gonadarche, and body composition. Eur J Endocrinol. 2013;168:129–136 [DOI] [PubMed] [Google Scholar]
- 30. Ducharme JR, Forest MG, De Peretti EDE, Sempé M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42:468–476 [DOI] [PubMed] [Google Scholar]
- 31. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Falk RT, Gentzschein E, Stanczyk FZ, et al. Measurement of sex steroid hormones in breast adipocytes: Methods and implications. Cancer Epidemiol Biomarkers Prev. 2008;17:1891–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reed MJ, Purohit A. Aromatase regulation and breast cancer. Clin Endocrinol. 2001;54:563–571 [DOI] [PubMed] [Google Scholar]
- 34. Bellemare V, Laberge P, Noel S, Tchernof A, Luu-The V. Differential estrogenic 17beta-hydroxysteroid dehydrogenase activity and type 12 17beta-hydroxysteroid dehydrogenase expression levels in preadipocytes and differentiated adipocytes. J Steroid Biochem Molecul Biol. 2009;114:129–134 [DOI] [PubMed] [Google Scholar]
- 35. Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78:428–432 [DOI] [PubMed] [Google Scholar]
- 36. Bordini B, Littlejohn E, Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab. 2009;94:1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: Evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biro FM, Huang B, Crawford PB, et al. Pubertal correlates in black and white girls. J Pediatr. 2006;148:234–240 [DOI] [PubMed] [Google Scholar]
- 39. de Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, Erich WB. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. 1992;75:442–446 [DOI] [PubMed] [Google Scholar]
- 40. Jasik CB, Lustig RH. Adolescent obesity and puberty: The “perfect storm” Ann NY Acad Sci. 2008;1135:265–279 [DOI] [PubMed] [Google Scholar]
- 41. Martí-Henneberg C, Vizmanos B. The duration of puberty in girls is related to the timing of its onset. J Pediatr. 1997;131:618–621 [DOI] [PubMed] [Google Scholar]
- 42. Palmert MR, Malin HV, Boepple PA. Unsustained or slowly progressive puberty in young girls: Initial presentation and long-term follow-up of 20 untreated patients. J Clin Endocrinol Metab. 1999;84:415–423 [DOI] [PubMed] [Google Scholar]
- 43. Pantsiotou S, Papadimitriou A, Douros K, Priftis K, Nicolaidou P, Fretzayas A. Maturational tempo differences in relation to the timing of the onset of puberty in girls. Acta Paediatr. 2008;97:217–220 [DOI] [PubMed] [Google Scholar]
- 44. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249 [DOI] [PubMed] [Google Scholar]
- 45. Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405 [DOI] [PubMed] [Google Scholar]
- 46. Biro FM, Wolff MS, Kushi LH. Impact of yesterday's genes and today's diet and chemicals on tomorrow's women. J Pediatr Adolesc Gynecol. 2009;22(1):3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buck Louis GM, Gray LE, Jr., Marcus M, et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics. 2008;121:S192–207 [DOI] [PubMed] [Google Scholar]
- 48. Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics. 2008;121:S167–S171 [DOI] [PubMed] [Google Scholar]
- 49. Rogan WJ, Ragan NB. Some evidence of effects of environmental chemicals on the endocrine system in children. Int J Hygiene Environ Health. 2007;210:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. N Eng J Med. 2003;348:1527–1536 [DOI] [PubMed] [Google Scholar]
- 51. Thomsen AR, Almstrup K, Nielsen JE, et al. Estrogenic effect of soy isoflavones on mammary gland morphogenesis and gene expression profile. Toxicol Sci. 2006;93:357–368 [DOI] [PubMed] [Google Scholar]
- 52. Windham GC, Pinney SM, Sjodin A, et al. Body burdens of brominated flame retardants and other persistent organo-halogenated compounds and their descriptors in US girls. Environ Res. 2010;110:251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wolff MS, Britton JA, Boguski L, et al. Environmental exposures and puberty in inner-city girls. Environ Res. 2008;107:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wolff MS, Teitelbaum SL, Pinney SM, et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect. 2010;118:1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. girls: The Third National Health and Nutrition Examination Survey, 1988–1994. Environ Health Perspect. 2003;111(5):737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99:505–512 [DOI] [PubMed] [Google Scholar]
- 57. Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. 2009;20:237–242 [DOI] [PubMed] [Google Scholar]
- 58. Legro RS, Schlaff WD, Diamond MP, et al. Total testosterone assays in women with polycystic ovary syndrome: Precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95:5305–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hopper BR, Yen SS. Circulating concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate during puberty. J Clin Endocrinol Metab. 1975;40:458–461 [DOI] [PubMed] [Google Scholar]


