Abstract
AIDS-related mortality remains a leading cause of preventable death among African-Americans. We sought to determine if community health workers could improve clinical outcomes among vulnerable African-Americans living with HIV in Miami, Florida. We recruited 91 medically indigent persons with HIV viral loads ≥1,000 and/or a CD4 cell count ≤350. Patients were randomized to a community health worker (CHW) intervention or control group. Viral load and CD4 cell count data were abstracted from electronic medical records. At 12 months, the mean VL in the intervention group was log 0.9 copies/μL lower than the control group. The CD4 counts were not significantly different among the groups. Compared to the control group, patients randomized to CHWs experienced statistically significant improvements in HIV viral load. Larger multi-site studies of longer duration are needed to determine whether CHWs should be incorporated into standard treatment models for vulnerable populations living with HIV.
Keywords: Community health workers, HIV viral load, African-Americans, HIV disparities
Introduction
Dramatic declines in AIDS-related mortality have been observed since the introduction of highly active antiretroviral (HAART) medications. Yet this decline has not been experienced equally by all populations living in the United States [1–3]. Minority groups such as African-Americans and Latinos suffer disproportionately from HIV disease, and AIDS remains a leading cause of preventable death among these groups [1–8]. Miami-Dade County has one of the highest HIV mortality rates in the US and Blacks and Latinos comprise 81 % of the HIV positive population [9, 10].
In less developed countries, community health workers (CHWs) are increasingly being used as a practical and cost-effective approach to improve outcomes among patients living with HIV [11–14]. Our prior review of the effectiveness of CHWs addressing HIV in the US found most of the randomized trials reporting clinical outcomes were limited by relatively short follow-up or focused on a very targeted group (e.g. injection drug users) [11]. Two studies utilized outreach or peer workers to provide directly observed therapy (DOT) in an effort to improve HAART adherence among substance-abusers [15, 16]. One quasi-experimental study with indigent women who were new HAART users allocated patients to one of three arms: one in which patients received care from a management team consisting of a social worker, peer case worker and a pharmacist; another in which patients worked with a case manager who provided education and assessed for social needs and provided social services; and an arm which patients received a peer worker who provided DOT and positive reinforcement [17]. Another study used volunteers to provide DOT to 18 men for 6 weeks [18]. Data from these studies suggested that utilizing CHWs may be a promising approach to improving outcomes among underserved people living with HIV (PLWH) [15–18] and in a number of other chronic conditions [19–21].
In this manuscript, we present findings from the counseling on adherence and community health (COACH) study. Several aspects of our study distinguished it from prior studies, including a longer follow-up period of 12 months, enrollment of a more diverse sample of men and women who may or may not have had a history of substance abuse, and facility based recruitment for those with a history of HAART non-adherence. The objective of COACH was to conduct a randomized study to examine whether a CHW intervention could improve outcomes among indigent PLWH in Miami-Dade County who were not achieving treatment goals. Our hypothesis was that as compared to the control group, persons randomized to a CHW intervention would achieve statistically significant improvements in viral load and CD4 counts after 12 months of study participation.
Methods
Participant Accrual
We recruited patients from the Special Immunology Clinics of Jackson Memorial Hospital (JMH). JMH is Miami-Dade’s only public hospital and de-facto safety net institution for the majority of the County’s medically indigent residents. Between March 2008 and June 2010, we asked health care providers at the practice to refer potentially eligible patients. Inclusion criteria were age over 18 years, a history of HAART non-adherence (as documented by physician notes in medical records), an HIV viral load of>1,000 copies/μL and/or a CD4 count of <350 cells/μL. Patients with severe psychiatric impairments or other major co-morbidities (e.g. end-stage renal disease) and those who were incarcerated or residing in a rehabilitation facility were excluded from enrollment into the study.
If a potential participant was interested, we arranged to meet the participant at the clinic to discuss the program details and conduct a brief screening questionnaire. For persons whom met the study criteria and wished to participate, we obtained written informed consent and a signed release to access medical records. After completion of these documents, a baseline questionnaire lasting about an hour was conducted to obtain demographic, socioeconomic, and information on health-seeking behaviors. Medical records were also reviewed to obtain the most recent CD4 cell count and HIV viral load.
Randomization
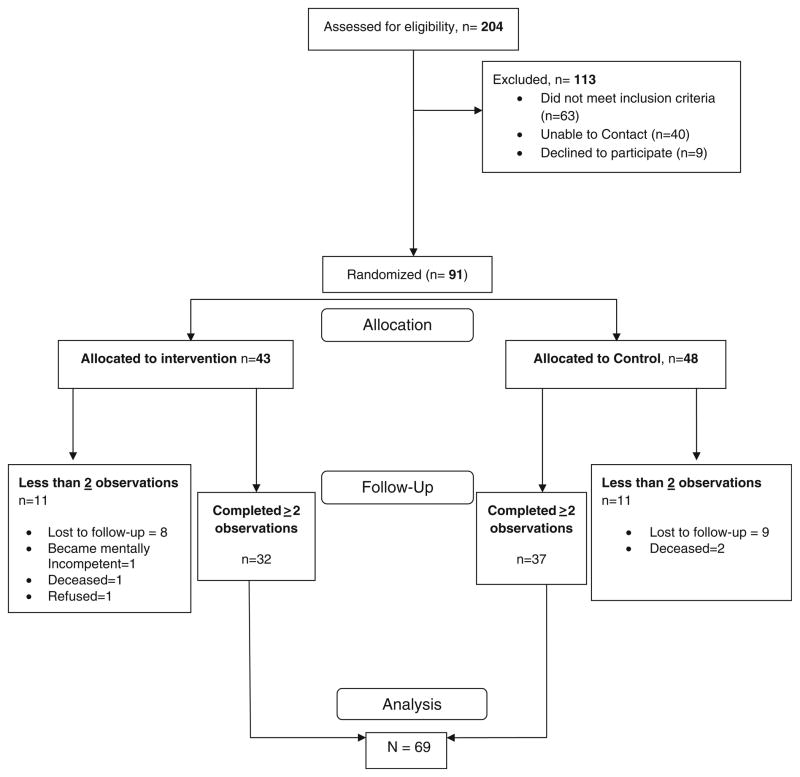
During the 15 month recruitment period (March 2008–June 2009), we successfully enrolled 91 HIV-patients into our study (see Fig. 1) which were randomized to an intervention or control group. To maintain equal participants in each group, we used a block randomization scheme and a random number generator to allocate each block of four patients recruited.
Fig. 1.
COACH recruitment and analysis
Intervention
The design of our intervention was informed by the Partners in Health (PIH) Prevention, Access, Care, and Treatment (PACT) programs in Haiti and in Boston [14, 22]. These programs use community health workers to help address barriers to HIV care and improve HAART adherence among PLWH who are not achieving optimal outcomes. Published reports on the programs suggest CHWs improve self reported medication adherence, reduce hospital admissions and improve overall health status among residents in Haiti’s Central Plateau [12] and in low-income communities in Boston [14]. Staff from the PIH/PACT were involved in the design of COACH from conceptualization, implementation through evaluation. We utilized their prior experience to train our CHWs to deliver individualized treatment strategies. With guidance and input on key programmatic issues from PIH/PACT, we modified their program to reflect the local context of our community.
Each participant randomized to the CHW intervention was assigned to one of four CHWs for 12 months. Prior to patient contact, each CHW underwent required online institutional training on patient protections in social/behavioral research and patient confidentiality. Subsequently, CHWs underwent a specialized, 4 week training on HIV, barriers to medication adherence, and behavioral strategies to improve clinical HIV outcomes. The study PI and a licensed clinical social worker supervised CHW activities and met with each CHW weekly to review cases and make recommendations regarding intervention activities designed to help each patient achieve treatment goals.
During the CHWs’ initial contact with each participant, an assessment of social and system barriers to obtaining health care was conducted. This included insurance status, stability of housing, food availability, medication adherence, social service needs, social support, transportation resources, and mental health. CHWs and the CHW supervisor reviewed each participant’s unique circumstances and designed an individualized action plan with emphasis on the most pressing needs. For example, as all PLWH in the US are eligible for insurance via the Ryan White program, CHWs prioritized obtaining insurance for those who were uninsured at the initial assessment. For those with unstable housing, CHWs sought assistance from Housing Opportunities for Persons with AIDS (HOPWA) and local shelters. Those in need of food were shown important community resources such as food banks in their neighborhood and/or linked to publicly funded food assistance programs. Those with limited or no social support were referred to existing HIV support groups and in some instances family meetings were conducted to discuss the importance of social support.
With each new study subject, CHWs conducted on average one face to face visit per week during the first 3 months to provide education and support focused on HIV management. In-person visits typically lasted 1–2 h depending on the participant’s needs at the time. CHW led peer health education focused on general HIV education, maximizing health-seeking behaviors and the importance of medication adherence using the educational curriculum adopted from the PIH/PACT program to provide peer-education on HIV. The curriculum was designed to teach participants how HIV affected their physical health and immune system functioning, basic information on CD4 cells and HIV viral load, modes of HIV transmission, the impact of HAART on HIV progression, and the effects of non-adherence, including developing resistance to HAART.
After the first few months, CHWs gradually replaced the weekly in-person visits with telephone support. All participants without phones were enrolled into existing programs for low income persons which provide free cell phones, unlimited incoming calls and up to 250 outgoing minutes per month [23]. During this period, the CHWs contacted participants by telephone 6–10 times per month for added support and counseling. In many instances, participants contacted CHWs at the last minute to cancel or reschedule a planned CHW visit or phone call. This was expected among our highly challenged population with many competing demands and CHWs were instructed to be as flexible as possible in ensuring patients received the planned intervention.
Some participants required more intense face-to-face support throughout the entire study. CHWs discussed each of these cases with the study PI and social work supervisor, who ultimately approved any changes, such as modifying service intensity or altering the type of support delivered to participants. Thus, participants who required more intense support received in person visits more frequently than others who were receptive to telephone support. As the 12 month study period was nearing conclusion, an integral aspect of the intervention involved empowering participants with the skills to independently manage their health.
CHWs also accompanied participants to the majority of their medical appointments. Following medical appointments, the CHW reviewed treatment recommendations with the patient to ensure they understood the provider’s advice. In addition, CHWs facilitated access to social service benefits and communicated with treating providers, in an effort to improve adherence and health care utilization patterns of their participants. For example, CHWs often referred participants to The Healing Place, a mental health provider for PLWH.
Sixty to ninety days before participants were scheduled to exit the study, CHWs introduced patients to HIV support groups in their community, reviewed HAART adherence strategies, as well as the process of maintaining health insurance and social service benefits, including food stamps, housing assistance, transportation vouchers, and mental health support. As part of the exit process, participants received an HIV resource booklet created by the CHW supervisor that included detailed information on accessible support services and health care programs for PLWH in Miami, FL.
Control Group
Patients randomized to the control group continued to receive care at the JMH Special Immunology clinic, which also cared for the majority of intervention patients. This includes a hospital based case manager who is charged with assisting patients with medical follow-up, assessing medical, financial, educational, psychosocial, and referral needs for each client, and developing an individualized plan of care to meet those needs. The case manager was also responsible for providing one-on-one education and group education sessions on HIV and AIDS, transportation assistance, and help acquiring food vouchers. The success of these services was largely dependent on the unique relationship between the case manager and each patient and some control group members did not utilize the case managers. CHWs visited control group patients every 3 months to collect study related information. Though CHWs did not provide any direct assistance, patients were encouraged to follow up with their primary care providers. CHWs were able to complete three or more such visits among 67 % of control group participants and 17 % completed all five scheduled visits.
Viral Load and CD4 Counts
In the JMH Special Immunology clinic, patients are expected to have CD4 cell count and HIV viral load checked every 3 months by their physician as part of routine medical care.
Two study physicians blinded to participants’ group assignment abstracted CD4 cell count and viral load data from the electronic medical records (EMR). However, not all patients had their CD4 cell count and viral load assessed at 3 month intervals. Thus, participants who only had a baseline evaluation and no further laboratory determinations were excluded from the final analysis. Also, as very few patients had clinical data for all five time points, our analysis included only those with two or more observations. To examine the robustness of our findings, we performed a series of sensitivity analyses restricted to subjects having a minimum of 3–5 observations and found no substantial differences in the results.
Data Analysis
To evaluate for statistically significant changes in our primary outcomes of CD4 and viral load between our intervention and control groups we applied an alpha of .05 and used a repeated measures analysis of variance. A mixed linear models approach was used with group (intervention/control), time (baseline/3 months/6 months/9 months/12 months), and the group × time interaction as fixed factors. Subjects were nested within groups as the random factor. An unstructured covariance matrix was used to represent the correlated structure of the data. In these analyses, viral load data was log transformed to achieve a more normal distribution.
There were no baseline differences in sociodemographic covariates, CD4 cell counts, VL, or HAART adherence. Thus in our final models we did not adjust for these covariates. SAS 9.2 (SAS Institute, Inc., Cary, NC) statistical package was used to analyze data.
Results
A total of 91 participants were randomized, with 43 in the intervention group and 48 in the control group. The majority of our participants (89 %) were African-American. Most of our subjects (>90 %) had Medicaid and/or Ryan White as their source of insurance coverage, over half had not graduated from high school and 89 % reported incomes under $12,000 per year. Of the 91 patients enrolled, 69 had two or more follow-up observations (32 in the intervention; 37 in the control). Nearly half had been diagnosed with depression, and one quarter had a history of anxiety disorder (see Table 1). Sixty-three percent of participants reported that a loved one, such as a friend or family member, helps them with their most difficult medical problems. There were no significant differences in sociodemographic characteristics (age, race, gender, education, and income), or clinical HIV status (CD4 and VL) between participants lost to follow-up (did not have at least two viral load and CD4 observations) and those included in our final analysis.
Table 1.
Baseline demographic, mental health, social support, and clinical data
| Variablea | Control (n = 37) Frequency (%) |
Intervention (n = 32) Frequency (%) |
|---|---|---|
| Gender | ||
| Male | 59 | 49 |
| Female | 41 | 51 |
| Race/ethnicity | ||
| Non-hispanic Black | 89 | 88 |
| Hispanic | 8 | 9 |
| Non-hispanic White | 3 | 3 |
| Age (years) | ||
| 18–24 | 0 | 3 |
| 25–44 | 57 | 38 |
| 45–60 | 38 | 50 |
| 61+ | 5 | 9 |
| Socio-demographic characteristics | ||
| <High school | 51 | 53 |
| <$1,000/month | 84 | 94 |
| % with Medicaid and/or Ryan White | 92 | 97 |
| Self reported history of mental health disorder | ||
| Depression | 49 | 69 |
| Anxiety disorder | 27 | 31 |
| Bipolar disorder | 11 | 16 |
| Post-traumatic stress disorder | 8 | 19 |
| Social support | ||
| Has spouse/significant other | 62 | 81 |
| Disclosed HIV status to partner | 54 | 72 |
| Family support for health problems | 81 | 84 |
| Primary outcomes | ||
| Mean log viral load | 4.304 | 4.303 |
| Mean CD4 count | 67 | 75 |
No significant differences in baseline characteristics between groups
Primary Outcomes
Viral Loads
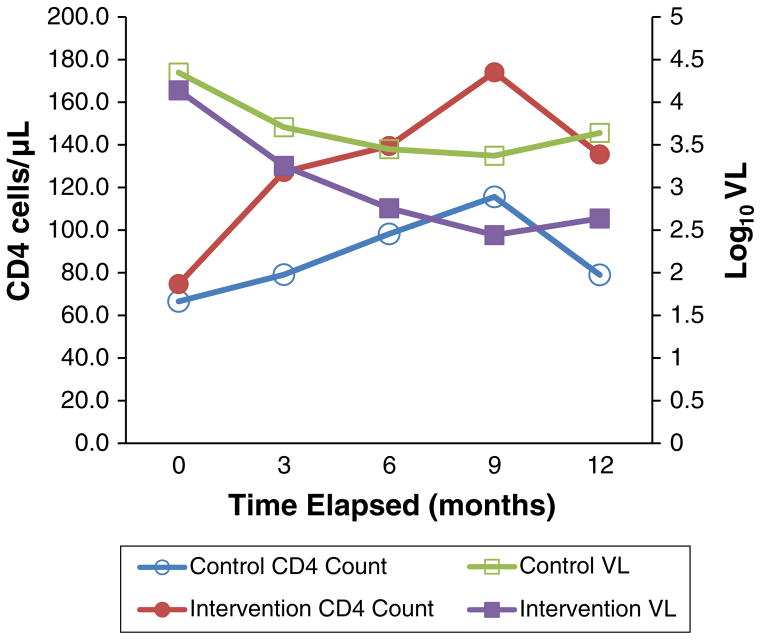
In pre and post analysis, participants in both groups showed marked reductions in VL. At 9 and 12 months, the patients randomized to the CHW intervention arm had significantly lower viral loads than those in the control group. Of some concern, the mean viral load among both groups began to increase after 9 months. However, even with these increases, significant differences between both groups persisted at 12 months, and the mean VL in intervention group was log 0.9 copies/μL lower than the control group at post-study (t = 2.76, df = 67, p <.01, see Fig. 2).
Fig. 2.
COACH clinical outcomes
CD4 Counts
Compared to baseline, both groups of patients showed some improvements in CD4 cell counts. While the mean CD4 cell count of the intervention group at 12 months was 0.23 log units higher than control group, this difference did meet our criteria for statistical significance.
Neither sociodemographic characteristics, history of mental disorders or social support were independent predictors of our outcomes, and in models that adjusted for these covariates, there were no interaction effects on our primary outcomes of CD4 and viral load.
Discussion
We found that in a cohort of predominantly low income African-American patients with poorly controlled HIV, as compared to a control group, our CHW intervention resulted in some improvements in viral load. We also observed large improvements in viral load among the control group. This finding among control group participants is encouraging and shows the impact that a safety net clinic can have on vulnerable populations living with HIV. However, our study also demonstrates the important additional role a CHW has in such settings. Our findings are consistent with non-randomized evaluations of existing PIH programs in both rural Haiti and inner city Boston [12–14]. Randomized trials conducted over shorter time periods also report similar results for CHW studies addressing HIV disparities in the United States [15–18]. Thus, this study builds upon the increasing evidence base demonstrating CHW interventions are effective for addressing a number of chronic health conditions among underserved populations [19–21].
CHWs in our study delivered an intervention to HIV positive clients who were medically indigent and reliant on public health benefits to support their healthcare needs. Among barriers CHWs assisted with were transportation challenges, lack of funds for co-payments or prescriptions, or difficulties establishing a permanent residence within our local communities. For example, fifteen participants in unstable housing situations received CHW assistance to obtain more appropriate housing. Often such housing was located far away from our public hospital and it became more feasible to help participants establish a medical home closer to their residence. In such cases, CHWs helped participants transition from the public hospital into other community-based service providers.
Although we saw some improvements in CD4 cell count, the changes were not statistically significant. This is similar to other studies [24, 25] and has been attributed to the fact that CD4 cell counts are also influenced by co-morbidities and other health modulating factors that are less impacted by HAART [26–28].
We also observed an increase in viral loads and decreasing CD4 cell counts towards the end of the study. The intervention was originally designed to empower participants to independence and CHWs gradually decreased support services throughout the program. However, the increase in viral load after 9 months of participation suggests CHW activities need to be maintained at a high intensity level in order to sustain the improvements achieved during earlier months.
The total annual cost to conduct the intervention activities was $118,121 which averages to a cost of $2,747 per intervention participant. Though a formal economic evaluation was not part of our study design, estimates from the PIH/PACT program, on which we modeled our intervention, showed it decreased total inpatient days by 35 %, median length of inpatient by 50 % and was associated with an average cost savings of $10,000 per participant per year [29].
While consistent with other research, our trial was focused in one community where the vast majority of patients were African-American and reliant on public assistance for housing and health benefits. Thus our findings may not be generalizable to other diverse populations of people living with HIV. Among some participants whom our CHWs assisted in relocating to more distant residences, we were unable to obtain all planned CD4 and viral load data as they now received care at other facilities.
Lastly, with respect to implementation our goal was to have each CHW follow their assigned patient for 12 months. However there was substantial turnover among community health workers. As reported in prior studies, long-term employee retention in CHW programs is a challenge [30]. Two of the original four CHWs left to pursue career advancement and needed to be replaced. CHW is an entry level position and those that do well in this role typically evolve into more advanced positions. As the study was internally funded, budgetary restrictions towards the end of the study resulted in CHW staff reductions and increased case-load per CHW. More stable funding and ability to match higher wages from competing positions may help retain CHWs in such programs.
Despite these challenges, our results suggest that CHWs can help improve viral loads among some of the most vulnerable HIV positive patients. Informal qualitative feedback on the program demonstrated that some of the most promising outcomes stemmed from an improved sense of self-worth that resulted from the relationship established between CHWs and participants. Based on our findings, we believe the next step would be a large multi site clinical trial testing the CHW intervention in diverse patient populations over a longer period of time. Mortality, an outcome in which a small study such as ours is not powered to detect, would also need to be included as a primary outcome in such a study that follows participants over a longer term. In addition, quantifying the cost effectiveness of CHW intervention would help inform governments, payers, managed care HIV programs, and other key stakeholders who direct health benefits for people living with HIV.

“When I met my chw, I didn’t care about life –but now I’m living. I was unhealthy and nowI’m healthy. And I want to share my story to letother people like me know that they can behealthy too.”
—COACH participant, September 24, 2008
Contributor Information
Sonjia Kenya, Email: skenya@med.miami.edu, Division of General Medicine, Jay Weiss Center Social Medicine and Health Equity, University of Miami Miller School of Medicine, 1400 NW 10th Avenue, Suite 801, Miami, FL 33136, USA.
Jamal Jones, Email: jjones3@med.miami.edu, Jay Weiss Center for Social Medicine and Health Equity, University of Miami Miller School of Medicine, Miami, FL, USA.
Kristopher Arheart, Department of Epidemiology and Public Health, University of Miami Miller School of Medicine, Miami, FL, USA.
Erin Kobetz, Department of Epidemiology and Public Health, Jay Weiss Center for Social Medicine and Health Equity, University of Miami Miller School of Medicine, Miami, FL, USA.
Natasha Chida, Jay Weiss Center for Social Medicine and Health Equity, University of Miami Miller School of Medicine, Miami, FL, USA.
Shelly Baer, Department of Pediatrics, Mailman Center for Child Development, University of Miami Miller School of Medicine, Miami, FL, USA.
Alexis Powell, Division of Infectious Diseases, University of Miami Miller School of Medicine, Miami, FL, USA.
Stephen Symes, Division of Infectious Diseases, Jay Weiss Center Social Medicine and Health Equity, University of Miami Miller School of Medicine, Miami, FL, USA.
Tai Hunte, Jay Weiss Center for Social Medicine and Health Equity, University of Miami Miller School of Medicine, Miami, FL, USA.
Anne Monroe, Jay Weiss Center for Social Medicine and Health Equity, University of Miami Miller School of Medicine, Miami, FL, USA.
Olveen Carrasquillo, Division of General Medicine, Jay Weiss Center Social Medicine and Health Equity, University of Miami Miller School of Medicine, 1400 NW 10th Avenue, Suite 801, Miami, FL 33136, USA.
References
- 1.Centers for Disease Control and Prevention (CDC) [Accessed 24 Jul 2009];HIV/AIDS in the United States. 2008 http://www.cdc.gov/hiv/resources/factsheets/us.htm.
- 2.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV infected patients. Analysis of the pre-, early, and late HAART eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 3.CDC. [Accessed 10 Jul 2009];Cases of HIV Infection and AIDS in the United States and dependent areas. 2008 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2007report/table8.htm.
- 4.CDC. [Accessed 5 Mar 2009];HIV/AIDS among African Americans, Fact-Sheet. 2008 http://www.cdc.gov/HIV/topics/aa/resources/factsheets/pdf/aa.pdf.
- 5.CDC. [Accessed 7 Feb 2012];HIV among Latinos, Fact Sheet. 2011 http://www.cdc.gov/hiv/resources/factsheets/pdf/latino.pdf.
- 6. [Accessed 10 Jul 2009];UNAIDS update: AIDS epidemic update regional summary. 2008 http://data.unaids.org/pub/Report/2008/jc1532_epibriefs_namerica_europe_en.pdf.
- 7.Florida Department of Health, Miami-Dade County. [Accessed 10 Feb 2009];AIDS/HIV among Blacks in Miami/Dade. 2007 http://www.dadehealth.org/downloads/FS_2007_BLACKS.pdf.
- 8.World Health Organization (WHO) Adherence to long-term therapies: evidence for action. Geneva: WHO; 2003. [Google Scholar]
- 9.Centers for Disease Control. [Accessed 7 Feb 2012];Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas, 2009. 2011 http://www.cdc.gov/hiv/surveillance/resources/reports/2009report/index.htm.
- 10.Health Council of South Florida. [Accessed 2 Mar 2012];2011 District 11 Health Profile: Miami-Dade County, Monroe County. 2011 http://www.healthcouncil.org/publications/District_Health_Profile_2011_02_09_2012.pdf.
- 11.Kenya S, Chida N, Symes S, Shor-Posner G. Can community health workers improve adherence to highly active antiretroviral therapy in the USA. HIV Med. 2011;12(9):525–34. doi: 10.1111/j.1468-1293.2011.00921.x. [DOI] [PubMed] [Google Scholar]
- 12.Farmer P, Léandre F, Mukherjee JS, et al. Community-based approaches to HIV treatment in resource-poor settings. Lancet. 2001;358:404–9. doi: 10.1016/s0140-6736(01)05550-7. [DOI] [PubMed] [Google Scholar]
- 13.Farmer P, Léandre F, Mukherjee J, Gupta R, Tarter L, Kim JY. Community-based treatment of advanced HIV disease: introducing DOTHAART (directly observed therapy with highly active antiretroviral therapy) Bull World Health Organ. 2001;79:1145–51. [PMC free article] [PubMed] [Google Scholar]
- 14.Behforouz H, Farmer P, Mukherjee J. From directly observed therapy to accompagnateurs: enhancing AIDS treatment outcomes in Haiti and in Boston enhancing adherence to AIDS treatment. CID. 2004;38(Suppl 5):S429–36. doi: 10.1086/421408. [DOI] [PubMed] [Google Scholar]
- 15.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–7. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-injected drug users. J Acquir Immune Defic Syndr. 2006;43:S48–53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- 17.Visnegarwala F, Rodriguez-Barradass MC, Graviss EA, Caprio M, Nykyforchyn M, Laufman L. Community outreach with weekly delivery of anti-retroviral drugs compared to cognitive-behavioral health care team-based approach to improve adherence among indigent women newly starting HAART. AIDS Care. 2006;8:332–8. doi: 10.1080/09540120500162155. [DOI] [PubMed] [Google Scholar]
- 18.Khanlou H, Vijayabhaskar RK, Yeh V, et al. Pilot study of directly observed therapy in highly nonadherent HIV-infected patients in an urban community-based institution. J Acquir Immune Defic Syndr. 2003;33:651–3. doi: 10.1097/00126334-200308150-00017. [DOI] [PubMed] [Google Scholar]
- 19.Babamoto KS, Sey KA, Camilleri AJ, Karlan VJ, Catalasan J, Morisky DE. Improving diabetes care and health measures among hispanics using community health workers results from a randomized controlled trial. Health Educ Behav. 2009;36(1):113–26. doi: 10.1177/1090198108325911. [DOI] [PubMed] [Google Scholar]
- 20.Parker EA, Israel BA, Robins TG, et al. Evaluation of community action against asthma: a community health worker intervention to improve children’s asthma-related health by reducing household environmental triggers for asthma. Health Educ Behav. 2008;35(3):376–95. doi: 10.1177/1090198106290622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32(5):435–47. doi: 10.1016/j.amepre.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Smith Fawzi MC, Jagannathan P, Cabral J, et al. Limitations in knowledge of HIV transmission among HIV-positive patients accessing case management services in a resource-poor setting. AIDS Care. 2006;18(7):764–71. doi: 10.1080/09540120500373844. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed 8 Jan 2013];Lifeline Program for Low-Income Consumers. http://www.fcc.gov/lifeline.
- 24.Behforouz HM, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an Urban US setting. J Acquir Immune Defic Syndr. 2004;36:642–5. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 25.Stenzel MS, McKenzie M, Mitty JA, Flanigan TP. Enhancing adherence to HAART: a pilot program of modified directly observed therapy. AIDS Read. 2001;11(317–9):324–8. [PubMed] [Google Scholar]
- 26.Sledjeski EM, Delahanty DL, Bogart LM. Incidence and impact of posttraumatic stress disorder and comorbid depression on adherence to HAART and CD4+ counts in People Living with HIV. AIDS Patient Care STDs. 2005;19(11):728–36. doi: 10.1089/apc.2005.19.728. [DOI] [PubMed] [Google Scholar]
- 27.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV epidemiology research study. JAMA. 2001;285(11):1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 28.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:288–92. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed 6 Dec 2012];Massachusetts General Hospital Disparities Solutions Center, using multi-disciplinary teams to address disparities: The use of culturally competent navigators, health coaches, and community health workers. 2006 http://www2.massgeneral.org/disparitiessolutions/z_files/dsc%20navigator_chw_5.20.08.pdf.
- 30.Rahman SM, Ali NA, Jennings L, et al. Factors affecting recruitment and retention of community health workers in a newborn care intervention in Bangladesh. Hum Resour Health. 2010;8:12. doi: 10.1186/1478-4491-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]