Abstract
The magnitude of the suspected increase in risk of acute interstitial nephritis among proton pump inhibitor users is uncertain. Here, we conducted a nested case-control study using routinely collected national health and drug dispensing data in New Zealand to estimate the relative and absolute risks of acute interstitial nephritis resulting in hospitalization or death in users of proton pump inhibitors. The cohort included 572,661 patients without a history of interstitial nephritis or other renal diseases who started a new episode of proton pump inhibitor use between 2005 and 2009. Cases had a first diagnosis after cohort entry of acute interstitial nephritis confirmed by hospital discharge letter or death record, and renal histology (definite, 46 patients), or discharge letter or death record only (probable, 26 patients). Ten controls, matched by birth year and sex, were randomly selected for each case. In the case-control analysis based on definite cases and their controls, the unadjusted matched odds ratio (95% confidence interval) for current versus past use of proton pump inhibitors was 5.16 (2.21–12.05). The estimate was similar when all cases (definite and probable) and their corresponding controls were analyzed, and when potential confounders were added to the models. The crude incidence rates and confidence intervals per 100,000 person-years were 11.98 (9.11–15.47) and 1.68 (0.91–2.86) for current and past use, respectively. Thus, current use of a proton pump inhibitor was associated with a significantly increased risk of acute interstitial nephritis, relative to past use.
Keywords: drug nephrotoxicity, epidemiology and outcomes, nephritis
Concern about a possible increased risk of acute interstitial nephritis among omeprazole users was first raised in 1992.1 Subsequently, several published case reports and case series suggested a class effect of proton pump inhibitors (PPIs) (including omeprazole, pantoprazole, lansoprazole, rabeprazole, and esomeprazole) on the occurrence of acute interstitial nephritis.2, 3, 4, 5, 6 Anecdotal case reports received by national drug safety authorities prompted regulators in several countries to urge caution when prescribing PPIs7, 8, 9, 10, 11, 12; however, despite these warnings, several studies have shown concerning and continuing levels of inappropriate prescribing of PPIs in both hospital and primary care settings.13, 14, 15, 16
Surprisingly, however, only one study has formally explored the risk of acute interstitial nephritis in PPI users. This study comparing the use of omeprazole on the index date with nonuse reported an odds ratio of 3.20; however, the diagnoses were not histologically validated and chance could not be ruled out as a possible explanation (95% confidence interval (95% CI) 0.80–12.79).17 Other research has suggested that the absolute risk is very low, but has relied on unsystematic case identification methods and imprecise estimates of PPI exposure.9, 18 We did a population-based case-control study nested in a cohort of New Zealand users of omeprazole, pantoprazole, or lansoprazole (the PPIs available in New Zealand) to estimate the relative and absolute risks of acute interstitial nephritis resulting in hospitalization or death in current and recent users of these drugs compared with past users.
RESULTS
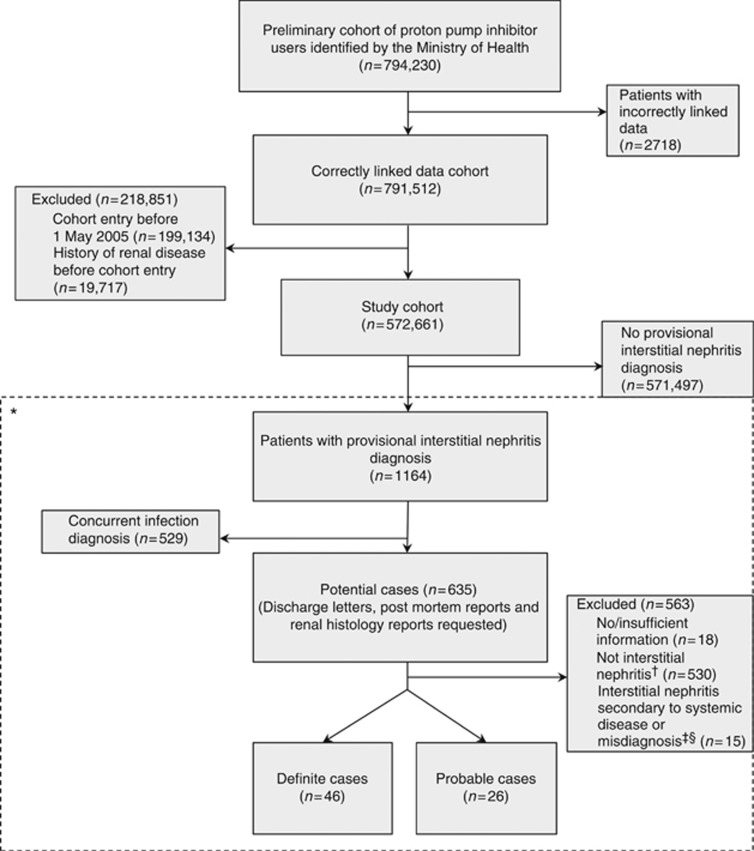
From 1 January 2005 to 31 August 2009, the Ministry of Health identified 794,230 patients from the Pharmaceutical Collection who had been dispensed at least one course of PPI treatment (Figure 1). The study cohort comprised 572,661 patients who had correctly linked health and dispensing data, begun an episode of PPI use between 1 May 2005 and 31 August 2009, and did not have a history of renal disease (including interstitial nephritis) before cohort entry. From the study cohort, we identified 1164 patients as potential cases. We excluded 529 patients whose additional diagnoses indicated an infection of the kidney or urinary tract, and requested hospital discharge letters, postmortem reports, and renal histology reports for the remaining 635 potentially eligible cases, receiving information for 617 patients (97.2%). On the basis of this information, an additional 545 patients were excluded (most had pyelonephritis). The case-control study therefore included 72 validated cases who presented acutely with interstitial nephritis (46 definite, histologically confirmed; 26 probable, discharge letter confirmed) and 719 matched controls (460 definite and 259 probable). There were no fatal cases. Owing to a data management oversight, only nine controls were selected for one case.
Figure 1.
Study flow diagram. *The dashed line indicates that this part of the figure only describes the case identification process. †Determined after reviewing patients' hospital discharge information. ‡Chronic interstitial nephritis with early amyloid disease (n=1); focal segmental glomerulosclerosis (n=1); glomerulonephritis (n=1); light chain cast nephropathy (n=3); minimal change disease with nephrotic syndrome (n=1); multiple myeloma (n=2); systemic lupus erythematosus (n=1); vasculitis (n=1); acute tubular necrosis (n=2); nonsteroidal anti-inflammatory drug nephropathy in context of dehydration (n=1); ‘interstitial nephritis on USS (ultrasound)' (n=1). §Determined after consultation with a renal physician.
Table 1 shows the characteristics of the cases and controls. Cases (definite and all) were more likely than controls to be of European ethnicity, to be current users of drugs other than PPIs associated with increased risk of acute interstitial nephritis, to have been hospitalized in the previous year for any reason, and to live in the most deprived socioeconomic areas (Supplementary Table S1 online). Omeprazole was the most commonly dispensed PPI at the last dispensing before the index date, and almost two-thirds of cases and controls who were current users were dispensed a ‘standard' daily dose at the last dispensing before the index date (Supplementary Table S2 online).
Table 1. Characteristics of cases and matched controls at index date.
|
Definite |
All |
|||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| (n=46) | (n=460) | (n=72) | (n=719) | |
| Age (years) (mean (s.d.)) | 65.4 (11.3) | 65.4 (11.2) | 64.7 (13.9) | 64.7 (13.8) |
| Female (n (%)) | 26 (56.5) | 260 (56.5) | 44 (61.1) | 440 (61.2) |
| Ethnicitya (n (%)) | ||||
| European | 43 (93.5) | 370 (80.4) | 63 (87.5) | 582 (81.0) |
| Māori | 2 (4.4) | 19 (4.1) | 4 (5.6) | 35 (4.9) |
| Pacific Island | 1 (2.2) | 9 (2.0) | 2 (2.8) | 18 (2.5) |
| Asian | — | 25 (5.4) | 1 (1.4) | 36 (5.0) |
| Other | — | 4 (0.9) | — | 4 (0.6) |
| Missing | — | 33 (7.2) | 2 (2.8) | 44 (6.1) |
| PPI type at last dispensing before the index date (n (%)) | ||||
| Omeprazoleb | 41 (89.1) | 419 (91.1) | 65 (90.3) | 652 (90.7) |
| Pantoprazole | 4 (8.7) | 39 (8.5) | 6 (8.3) | 62 (8.6) |
| Lansoprazole | 1 (2.2) | 2 (0.4) | 1 (1.4) | 5 (0.7) |
| Use of other drugs associated with increased interstitial nephritis risk in the 30 days before index datec,d (n (%)) | ||||
| Yes | 26 (56.5) | 190 (41.3) | 42 (58.3) | 289 (40.2) |
| Hospitalization in the year before the index date for any diagnosis (n (%)) | ||||
| Yes | 23 (50.0) | 136 (29.6) | 36 (50.0) | 214 (29.8) |
Abbreviation: PPI, proton pump inhibitors.
Multiple recorded ethnicities were categorized according to a prioritization algorithm developed by Statistics New Zealand: Māori, Pacific Island, Asian, Other, European.
Includes patients dispensed Helicobacter pylori triple therapy, which consists of omeprazole and two antibiotics.
10 cases (13.9%) and 40 controls (5.6%) had incomplete dispensing information because their index dates occurred <30 days after cohort entry.
Nonsteroidal anti-inflammatory drugs, other analgesics, aspirin and other anticoagulants, antibiotics and other antimicrobials, anxiolytics, anti-epileptics, diuretics, ACE inhibitors, angiotensin II antagonists, beta-blockers, calcium channel blockers, H2 receptor antagonists, immune modulators and miscellaneous other drugs (see Supplementary Table S9 online for a complete listing).
The results of the main analysis are shown in Table 2. In the matched analysis confined to definite cases and controls, the unadjusted odds ratio was 5.16 (95% CI 2.21–12.05; P<0.001) for current use of any study PPI compared with past use (all cases and controls odds ratio 4.82; 95% CI 2.43–9.58; P<0.001). Recent use was associated with elevated risk; however, the findings were not significant. In secondary analyses, we found no conclusive evidence of a dose-response relationship or duration effect (Supplementary Tables S3 and S4 online). Among patients who were only ever dispensed omeprazole during the study period, the unadjusted matched odds ratio for definite cases and controls was 4.00 (95% CI 1.70–9.42; P=0.002) for current use compared with past use (all cases and controls odds ratio 4.78; 95% CI 2.34–9.78; P<0.001). Recent use was associated with a nonsignificant increase in risk.
Table 2. Risk of acute interstitial nephritis in users of the proton pump inhibitors omeprazole, pantoprazole, or lansoprazole.
| Cases | Controls | Matched odds ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Definite casesa | n=46 | n=460 | ||
| Current use | 35 | 207 | 5.16 (2.21–12.05) | <0.001 |
| Recent use | 4 | 56 | 2.38 (0.65–8.67) | 0.188 |
| Past use | 7 | 197 | 1.0 | — |
| All casesb | n=72 | n=719 | ||
| Current use | 55 | 332 | 4.82 (2.43–9.58) | <0.001 |
| Recent use | 5 | 89 | 1.72 (0.57–5.22) | 0.337 |
| Past use | 12 | 298 | 1.0 | — |
Abbreviation: CI, confidence interval.
Verified by discharge letter and renal biopsy.
Definite and probable (verified by discharge letter only) cases.
Adjusting for ethnicity, socioeconomic status, use of other drugs associated with increased risk of interstitial nephritis in the 30 days before the index date (including nonsteroidal anti-inflammatory drugs and antibiotics), and hospital admissions in the year before the index date for any reason, including for specific conditions associated with renal disease in general, had a negligible effect on the results of the primary and secondary analyses.
Table 3 shows that the crude absolute risk of acute interstitial nephritis per 100,000 person-years was substantially higher for current users compared with past users (11.98 vs. 1.68), and was intermediary in recent users (4.28). The absolute risk for current users who were 60 years and above was considerably higher than for younger current users; for instance, for every 100,000 PPI users in the 60 plus age group, about 20 per year developed acute interstitial nephritis as compared with 2 per year in those aged 15–49 years.
Table 3. Incidence rates for acute interstitial nephritis in users of the proton pump inhibitors omeprazole, pantoprazole, or lansoprazole.
| All cases | Person-years | Incidence rate (95% CI) per 100,000 person-years | |
|---|---|---|---|
| Entire study cohort | n=72 | ||
| Current users | 55 | 459 241 | 11.98 (9.11–15.47) |
| Recent users | 5 | 116 735 | 4.28 (1.57–9.49) |
| Past users | 12 | 714 116 | 1.68 (0.91–2.86) |
| Current users by age (years) at cohort entry | n=55 | ||
| 15–49a | 3 | 135 421 | 2.22 (0.56–6.03) |
| 50–59 | 9 | 101 278 | 8.89 (4.33–16.31) |
| 60–69 | 19 | 99 575 | 19.08 (11.83–29.25) |
| 70–79 | 15 | 73 476 | 20.41 (11.86–32.92) |
| 80+ | 9 | 40 488 | 22.23 (10.84–40.79) |
Abbreviation: CI, confidence interval.
The youngest case was 17 years old.
DISCUSSION
We found that the current use of the PPIs omeprazole, pantoprazole, or lansoprazole was associated with a significantly increased risk of acute interstitial nephritis resulting in hospitalization compared with past use. The absolute risks were very low, but appeared substantially higher in older users. Although these rates should be interpreted with some caution owing to small numbers and wide CIs, the apparent age-related effect has clinically important implications given the aging population and the higher proportionate use of PPIs in older patients (using population data from the 2006 Census19 we estimated that about 31% of the New Zealand population aged 60 years or more was dispensed a PPI at least once between 1 January 2005 and 31 August 2009, as compared with 12% in those aged 15–49 years). PPIs were also used by children, including ∼4% of New Zealand infants per year.
We searched MEDLINE up to October 2013 to find analytical epidemiological studies that investigated PPIs and the risk of interstitial nephritis. A case-control study using data from the United Kingdom General Practice Research Database (based on only five exposed, nonvalidated, cases) found an odds ratio of 3.20 (95% CI 0.80–12.79) when comparing the use of omeprazole on the index date with its nonuse.17 Moreover, the strength of the association may have been underestimated because the cohort included a mix of new and long-term users (although we found no evidence of a duration effect), and the reference category included both past and never users. A recent United States study based on information from a private insurance database reported a significant relationship between PPI use and acute kidney injury (odds ratio 1.72; 95% CI 1.27–2.32; P<0.001); however, there was considerable potential for misclassification of exposure, the cases were not validated, and only 3 of the 854 cases had an interstitial nephritis diagnostic code.20 We found only one published account of absolute risk, which used cases reported to the authors and estimated dispensing information from PPI distributors in the Auckland area of New Zealand. The authors found an incidence similar to ours of 8.0 per 100,000 person-years (95% CI 2.6–18.7) based on 15 cases and an estimated 750,000 1-month treatments dispensed annually.18 In addition, two cases of interstitial nephritis from a cohort of 22,050 first-time omeprazole users were reported in the 1990s as part of a prescription event monitoring program in New Zealand.9 Although both studies actively searched for cases, the systematic identification of cases used in the present research likely resulted in more complete case ascertainment, whereas the use of detailed dispensing information allowed for more accurate estimates of person-years at risk for each exposure category. Our study also provides more clinically useful information by estimating the incidence of acute interstitial nephritis in past users to compare with the incidence in current and recent users.
Our study has a number of additional strengths compared with previous research. We minimized selection bias by using routinely collected health and dispensing data for an entire country where there is universal access to the health-care system. This information is likely to be accurate, complete, and consistent owing to national clinical coding standards for recording hospital discharge and mortality information, and a pharmaceutical claims system that reimburses pharmacists only if key dispensing details are provided. Our case definition only included patients whose interstitial nephritis was severe enough to cause hospitalization or death (although no fatal cases were found), minimizing the possibility that current users of PPIs were more likely to be referred and diagnosed because doctors were aware of a possible link between PPI use and acute interstitial nephritis (referral and diagnostic bias). In addition to using a broad search strategy in the preliminary identification of potential cases, we verified diagnoses with clinical information. Although renal histology is the only way to definitively diagnose acute interstitial nephritis, we also collected information on patients without histological confirmation, as biopsy referral patterns may differ by treating clinician or patient age. The findings were similar when considering histologically confirmed (definite) cases and all cases. We also likely identified virtually all users of the study PPIs. These drugs were available only by prescription during the study period, and a very high proportion of PPI dispensing records included National Health Index numbers, unique patient identifiers, which allowed us to link dispensing and health data (90% in 2005, increasing to 98% in 2009). In addition, we compared the risk of acute interstitial nephritis in similar groups of users by restricting cohort eligibility to patients who initiated a new episode of PPI use during the study period. Because of the incomplete National Health Index coverage in the earlier period of the study, it is possible that some long-term users may have been included in the study cohort, although they would comprise a very small proportion. We also determined exposure on the basis of dispensing data, and not on prescription data. Although it is impossible to know whether patients actually took their medicines as prescribed, any non-adherence would likely be similar between cases and controls and would result in an underestimation of effects.
Confounding by age, sex, concomitant drug use (including nonsteroidal anti-inflammatory drugs and antibiotics), or other medical conditions is unlikely to explain the key findings. Cases and controls were matched by birth year and sex, and we excluded patients with a recorded history of kidney disease before cohort entry. Confounding by indication should have been avoided as all study members were prescribed a PPI at some time during the study period. Potential confounding by other subsidized prescription medicines possibly associated with interstitial nephritis, or non-renal medical conditions associated with increased risk of renal disease in general, was explored in the analysis with little effect on the results. However, we did not have information about unsubsidized or over-the-counter drugs (which are not recorded in the Pharmaceutical Collection), diagnoses made in primary care, or lifestyle factors such as smoking and obesity. Although aspirin and several nonsteroidal anti-inflammatory drugs were available over-the-counter throughout the study period, we expected that most chronic users of these drugs would be obtaining them by subsidized prescription owing to financial considerations. We were able to partially account for diagnoses made in primary care through analyzing the dispensing data, and found that adjustment for a range of medicines usually prescribed for conditions such as hypertension, diabetes, or congestive heart failure did not affect the estimates. Although we lacked important lifestyle information, the lower bound of the CI for current users was so large that a single unmeasured confounder would have to exert a large effect to nullify our results.
Our study had several limitations, including a relatively small number of identified cases, which affected the precision of the findings as shown by the wide CIs. However, our cases represent the largest series to be published to date, and the detailed dispensing data available for the entire study cohort enabled more precise estimates of absolute risk than previous studies. We were limited by the unavailability of electronic records before 1988, which meant that we did not have full lifetime hospitalization data for most people in the study cohort. Although considerable care was taken to exclude patients with previous interstitial nephritis or other renal disease from the study cohort, the inadvertent inclusion of any non-incident cases may have led to a slight overestimation of the association, assuming the recurrent episode of interstitial nephritis occurred while the patient was currently taking the PPI.
In conclusion, this study contributes to a growing body of evidence suggesting that current users of PPIs are at an increased risk of developing acute interstitial nephritis, and is the first study to take a systematic approach to estimating absolute risks. Although PPIs have revolutionized the care of patients diagnosed with gastric acid–related disorders, these drugs are not without risks. Greater caution is warranted considering the extremely large number of patients using these drugs, evidence of widespread inappropriate use, and over-the-counter availability of these medicines in many countries, including the United States and the United Kingdom.
MATERIALS AND METHODS
Data sources
We used routinely collected information from New Zealand's National Minimum Dataset (hospital discharges from 1988)21 and Mortality Collection (all hospital and community deaths).22 Diagnoses in these data sets were recorded using the International Classification of Diseases 9th and 10th revision, Australian Modification codes (ICD-9-AM, ICD-10-AM). We also used information on dispensed drugs held in the Pharmaceutical Collection,23 which records pharmacist dispensing claims for all publicly funded prescription medicines in New Zealand. Patient information in these data sets was linked using National Health Index numbers, unique identifiers assigned to ∼98% of the New Zealand population.24 The study was approved by the New Zealand Multi-region Ethics Committee (MEC/11/EXP/098).
Identification of study cohort
All patients who were dispensed a study PPI (including users of Helicobacter pylori triple therapy) at least once between 1 January 2005 and 31 August 2009 were identified from the Pharmaceutical Collection by the Ministry of Health. The Ministry used the National Health Index numbers of these patients to link their dispensing and health information, providing us with the patients' demographic data, details of all dispensings of the study PPIs and all other medicines from 2005 to 2009, hospital admission details from 1988, and, where applicable, death details. Unique patient identifiers were provided in lieu of National Health Index numbers for all patients except those identified by the Ministry as potential cases (see below). Cohort entry was the date of the first dispensing of a study PPI between 1 January 2005 and 31 August 2009.
We excluded linked records in which the dispensing and health information obviously could not have referred to the same person (e.g., patients who supposedly received medicines before their recorded birth date). To ensure that the study cohort included only those patients who initiated a new episode of PPI use during the study period (first-time users and those restarting after a break), we excluded all patients who were dispensed a study PPI between 1 January 2005 and 30 April 2005 (New Zealand allows a maximum 90-day dispensed supply of a PPI at one time). We also excluded patients with a recorded history of interstitial nephritis or other renal diseases before their cohort entry date (Supplementary Tables S5 and S6 online).
Identification of cases and controls
We asked the Ministry to identify all patients who were potentially diagnosed with acute interstitial nephritis after cohort entry by searching the hospital discharge and mortality data using the ICD-10-AM rubrics (determined in consultation with a professional clinical coder) under which interstitial nephritis may be coded (N10, N118, N119, N12, N141, N142, and N144). As mortality information for patients who died in 2009 had not yet been coded, we searched the free text causes of death for these patients for ‘interstitial nephritis'. Next, we devised an algorithm to exclude patients whose additional diagnoses indicated an infection of the kidney or urinary tract (Supplementary Tables S7 and S8 online). Finally, to verify the diagnoses of the remaining potential cases, hospital discharge letters, postmortem reports, and any renal histology reports were requested and independently reviewed by M-LB and LP who were blinded to the patients' PPI exposure status. In cases where there was some uncertainty about a patient's diagnosis (17 cases) a renal physician was consulted, and patients in whom interstitial nephritis was secondary to a systemic disease, or who were misdiagnosed, were excluded. Definite cases were patients who presented acutely with interstitial nephritis that was verified by discharge letter or death record, and renal histology. Probable cases were patients with only discharge letter or death record confirmation. The diagnosis date was taken as the index date for each case and their matched controls. We used risk set sampling25 from the study cohort to randomly select 10 controls (blinded to PPI exposure status) for each case, matched by birth year and sex; hence, controls were members of the study cohort who were at risk of developing acute interstitial nephritis on the index date.
PPI use
Cases and controls
Cases and controls were defined as current users of PPIs if their dispensed supply extended into the 30-day period before the index date, recent users if their supply extended to within 31–90 days before the index date, and past users if their supply terminated >90 days before the index date. Because they shared the same index date, the PPI exposure status of each case and their matched controls was therefore determined at the same calendar time.
Person-years of exposure in the study cohort
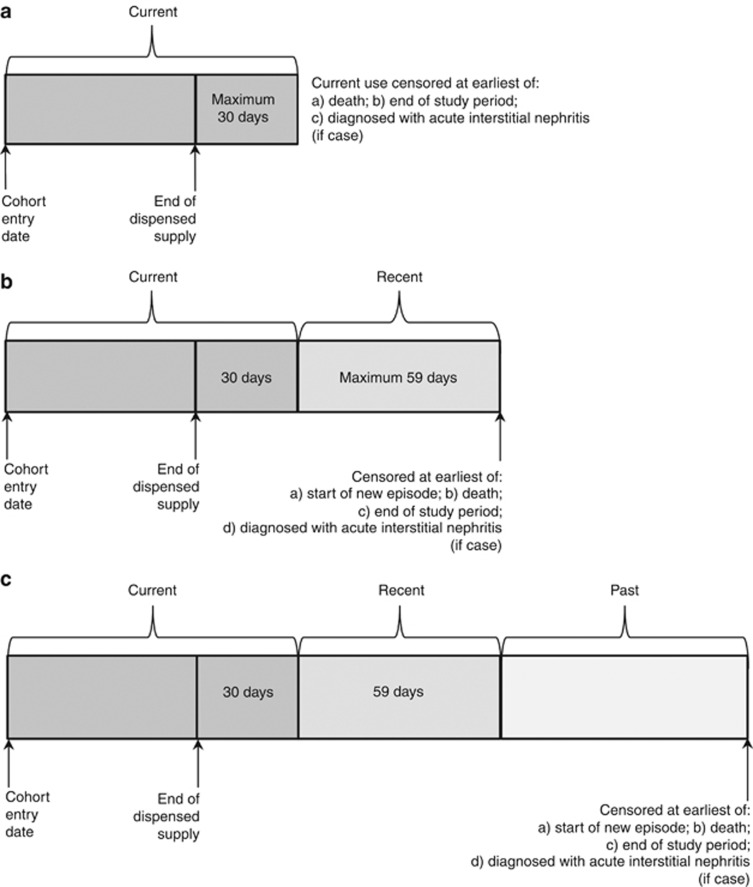
Person-years at risk for current users, regardless of PPI type or total daily dose, were determined by summarizing the dispensing data provided by the Ministry into continuous episodes of use (defined as a series of dispensings in which there was no more than a 30-day gap between the end of one dispensed supply and the start of the next). Episodes of current use were censored if the patient died, reached the end of the study period, or if a case was diagnosed with acute interstitial nephritis. The durations of all episodes were summed to determine the total person-years of current use for the study cohort. For patients who stopped taking a PPI, we estimated person-years of follow-up in recent users by adding the amount of time occurring 31–90 days after the end of each continuous episode (59 days maximum). Similarly, person-years of follow-up in past users were determined by adding the amount of time occurring 91 or more days after the end of each continuous episode. Follow-up in recent and past users was censored if a new episode was started, the patient died or reached the end of the study period, or if a case was diagnosed with acute interstitial nephritis. The durations of follow-up in recent and past users were, respectively, added together to give the total recent and past person-years for the entire study cohort. According to our definitions, some patients only contributed current person-years, some current and recent person-years, and some current, recent, and past person-years (Figure 2).
Figure 2.
Determination of current, recent, and past person-years. (a) Patients who only contributed current person-years. (b) Patients who contributed current and recent person-years. (c) Patients who contributed current, recent, and past person-years.
Potential confounding factors
In addition to birth year and sex, we assessed information on the following potential confounders: ethnicity; socioeconomic status; use of other drugs in the 30 days before the index date, which are associated with increased risk of interstitial nephritis (including nonsteroidal anti-inflammatory drugs and antibiotics Supplementary Table S9 online); and hospital admissions in the year before the index date for any reason, and for specific conditions associated with increased risk of renal disease in general (atherosclerosis, hyperlipidemia, hypertension, congestive heart failure, Type I and Type II diabetes, hyperglycemia, cancer, hepatitis C, and HIV/AIDS).
Statistical analysis
Conditional logistic regression was used to estimate odds ratios and 95% CIs. We estimated the risk of acute interstitial nephritis resulting in hospitalization or death in definite cases and their controls, and then in all (definite and probable) cases and controls. The primary analysis explored the risk of acute interstitial nephritis in current and recent users, respectively, of the study PPIs, with past users as the reference group. In secondary analyses, we explored the risk of acute interstitial nephritis according to duration of use (⩽180 days, >180 days) in the subset of users who had taken PPIs continuously between cohort entry and the index date (patients who discontinued PPI use during the study period were excluded from this analysis), investigated the impact of total daily dose (low, standard, and high) taken by current users of any PPI, and examined the risk in the subset of cases and controls who were only ever dispensed omeprazole. Confounding was tested by entering the potential confounders one at a time into the conditional logistic regression model and the 10% change in estimate convention26 was used to identify confounders.
Crude incidence rates in current, recent, and past users and age-stratified rates in current users were calculated using the cohort data by dividing the total number of cases in a particular exposure category by that category's person-years of follow-up. The Poisson distribution was used to determine 95% CIs. Stata version 12 was used for all analyses.
Acknowledgments
We thank Dr Rob Pollock and Mr Dave Barson for database programming, Professor Robert Walker for reviewing selected patient records, Mr Peter MacCallum for advising us on the appropriate diagnostic codes, and Professor Sir David Skegg for commenting on an earlier draft of this manuscript. Funding support: Primary financial support was provided by the Health Research Council of New Zealand and Medsafe (grant number 10/031). Additional support was provided to M-LB through a University of Otago Masters' scholarship. The funders had no role in the design of the study, or in the collection, analysis, or interpretation of the data.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Table S1. Socioeconomic status of cases and matched controls.
Table S2. Total daily dose in current users of the proton pump inhibitors omeprazole, pantoprazole, and lansoprazole at the last dispensing before the index date.
Table S3. Risk of acute interstitial nephritis in current users of the proton pump inhibitors omeprazole, pantoprazole, or lansoprazole (by total daily dose) compared to past users.
Table S4. Risk of acute interstitial nephritis in current users of the proton pump inhibitors omeprazole, pantoprazole, or lansoprazole, by duration of current use.
Table S5. International Classification of Diseases 9th revision Australian Modification codes (ICD-9-AM) used to exclude study cohort members with kidney disease diagnoses before cohort entry.
Table S6. International Classification of Diseases 10th revision Australian Modification codes (ICD-10-AM) used to exclude study cohort members with kidney disease diagnoses before cohort entry.
Table S7. International Classification of Diseases 10th revision Australian Modification codes (ICD-10-AM) that were used to identify, and then exclude, potential cases who had a kidney or urinary tract infection (patients concurrently diagnosed with N10 or N12 and any of the ICD-10-AM codes listed in the table were excluded, regardless of whether other ICD-10-AM codes were listed).
Table S8. International Classification of Diseases 10th revision Australian Modification codes (ICD-10-AM) that were used to identify, and then exclude, potential cases who had a kidney or urinary tract infection and had not already been excluded according to Table S7 (patients concurrently diagnosed with N10 or N12 and any of the ICD-10-AM codes listed in the table were excluded, provided there were no other ICD-10-AM codes listed).
Table S9. Government-subsidized prescription drugs listed in the New Zealand formulary known or suspected of increasing the risk of interstitial nephritis, by therapeutic class.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Ruffenach SJ, Siskind MS, Lien YHH. Acute interstitial nephritis due to omeprazole. Am J Med. 1992;93:472–473. doi: 10.1016/0002-9343(92)90181-a. [DOI] [PubMed] [Google Scholar]
- Baudeau C, Dard S, Zylberberg H, et al. Rabeprazole induced acute interstitial nephritis. Rev Med Interne. 2008;29:834–836. doi: 10.1016/j.revmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Chan MR, Yevzlin AS, Zhong W, et al. A 78-year-old woman with proton pump inhibitor-induced acute interstitial nephritis. Hosp Phys. 2009;2:43–47. [Google Scholar]
- Eken J, Phadke G, Ahmed S, et al. Lansoprazole-induced acute interstitial nephritis. South Med J. 2009;102:335–336. doi: 10.1097/SMJ.0b013e318191e77c. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, Coleman PL, Webster AC, et al. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol. 2006;4:597–604. doi: 10.1016/j.cgh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Klassen S, Krepinsky JC, Prebtani APH. Pantoprazole-induced acute interstitial nephritis. CMAJ. 2013;185:56–59. doi: 10.1503/cmaj.120954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Labour and Welfare . Pharmaceuticals and Medical Devices Safety Information No.215. Ministry of Health, Labour and Welfare: Tokyo; 2005. [Google Scholar]
- Ministry of Health Labour and Welfare . Pharmaceuticals and Medical Devices Safety Information No. 234. Tokyo: Ministry of Health, Labour and Welfare; 2007. [Google Scholar]
- Medsafe Omeprazole-induced interstitial nephritis. Prescriber Update. 2000;20:11–13. [Google Scholar]
- Medsafe Watching Briefs-Proton pump inhibitors and interstitial nephritis. Prescriber Update. 2006;27:2–3. [Google Scholar]
- Medsafe Proton pump inhibitors and interstitial nephritis. Prescriber Update. 2011;32:25. [Google Scholar]
- Therapeutic Goods Administration. Proton pump inhibitors and acute interstitial nephritis Australian Prescriber. 2011. p. 190.
- Batuwitage BT, Kingham JGC, Morgan NE, et al. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007;83:66–68. doi: 10.1136/pgmj.2006.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SM, Boueiz A, Paranji S, et al. Patterns and predictors of proton pump inhibitor overuse among academic and non-academic hospitalists. Intern Med. 2010;49:2561–2568. doi: 10.2169/internalmedicine.49.4064. [DOI] [PubMed] [Google Scholar]
- Grant K, Al-Adhami N, Tordoff J, et al. Continuation of proton pump inhibitors from hospital to community. Pharm World Sci. 2006;28:189–193. doi: 10.1007/s11096-006-9028-4. [DOI] [PubMed] [Google Scholar]
- Reid M, Keniston A, Heller JC, et al. Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7:421–425. doi: 10.1002/jhm.1901. [DOI] [PubMed] [Google Scholar]
- Leonard CE, Freeman CP, Newcomb CW, et al. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf. 2012;21:1155–1172. doi: 10.1002/pds.3329. [DOI] [PubMed] [Google Scholar]
- Simpson IJ, Marshall MR, Pilmore H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology. 2006;11:381–385. doi: 10.1111/j.1440-1797.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- Statistics New Zealand . 2006 Census Data. Statistics New Zealand: Wellington; p. 2007. [Google Scholar]
- Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150. doi: 10.1186/1471-2369-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health . Ministry of Health: Wellington; 2012. National Minimum Dataset (hospital events) Data Dictionary Version 7.4. [Google Scholar]
- Ministry of Health . Mortality Collection Data Dictionary Version 1.3. Ministry of Health: Wellington; 2009. [Google Scholar]
- Ministry of Health . Pharmaceutical Claims Data Mart (PHARMS) Data Mart-Data Dictionary Version 4.1. Ministry of Health: Wellington; 2012. [Google Scholar]
- Ministry of Health . National Health Index Data Dictionary Version 5.3. Ministry of Health: Wellington; 2009. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL.Case-control studiesIn Rothman KJ, Greenland S, Lash TL edsModern Epidemiology3rd edn, Lippincott Williams & Wilkins: Philadelphia; 2008111–127. [Google Scholar]
- Rothman KJ, Greenland S.Cohort StudiesIn Rothman KJ, Greenland S, Lash TL (eds). Modern Epidemiology3rd edn, Lippincott Williams & Wilkins: Philadelphia; 2008100–110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.