Abstract
Objective
To determine the prevalence and persistence of new onset clinical remission in rheumatoid arthritis (RA) patients.
Methods
The Consortium of Rheumatology Researchers of North America (CORRONA) cohort was used to examine the prevalence of remission and associated comorbidities and RA therapies according to the 2011 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) remission criteria. Factors influencing the likelihood of remaining in remission were identified by logistic regression with generalized estimating equations. Analysis of variance and Tukey’s test were used to determine differences in disability according to whether RA patients had been in remission or only low disease activity (LDA).
Results
A total of 2,105 individuals met ACR/EULAR remission criteria at the most recent visit within CORRONA, yielding an 8% point prevalence of remission. Patients with certain comorbidities (e.g. heart failure) were significantly less likely to achieve or remain in remission compared to those without these conditions (p<0.001 for each). Among prednisone users, the prevalence of remission was 1%–6% (depending on dose), compared to those not on prednisone (10%). More than 50% of patients who had consistently been in remission for ≥ 1 year were able to remain in remission over the next year. Patients consistently in remission had less disability than patients who achieved LDA or who fluctuated between remission and LDA.
Conclusion
Patients consistently in remission for at least one year had a high likelihood to remain in remission. These individuals might be considered the most likely candidates for de-escalation or withdrawal of RA treatments.
Keywords: rheumatoid arthritis, remission, CRP
Background
Clinical remission in rheumatoid arthritis (RA) is an achievable goal for some patients, especially in light of the availability of many new therapeutic options like anti-tumor necrosis factor (anti-TNF), anti CTLA-4 and anti-interleukin (IL)-6 receptor blockers. Remission in RA has been previously defined in several ways using different measurement instruments (1–3); however these instruments’ definition of remission varied in terms of their stringency (4). In 2011, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) (5) proposed a new definition of RA remission, predominantly for use in clinical trials, with the goal of establishing a standard definition to improve RA outcomes and decrease disability. Given the stringent requirements of these new criteria compared to some past remission definitions, the applicability of this definition in the real world is unclear. Among reasons why this new definition may be problematic in real-world settings is that age-associated comorbidities may preclude patients being able to meet the definition of remission for reasons unrelated to RA disease activity (6–7). Comorbidities play an important role in disability and can influence the patients’ perception of their global health. These comorbidities may consequently influence patients’ ability to achieve and maintain RA remission as measured by the new 2011 remission criterion that incorporates patient global scores as a required element.
Recent studies that applied the ACR/EULAR remission definition to data from clinical trials (8) or observational cohorts (9–12) have shown that this remission criterion had a point prevalence that ranged from 6 to 16 percent (8–9, 11). However, longitudinal data examining the durability of remission remains scarce. One study observed that remission was sporadic and that the durability of remission was short (11). A key limitation of many studies that examine the durability of remission is that remission was often not considered from the time of first onset, and the duration of time that patients had already been in remission was not well characterized. Because the duration of time that patients have been in remission is likely to be a key factor in the likelihood that they will stay in remission, it is essential to identify patients at the time they first achieve remission, and then observe them over time. Given increasing interest in the possibility of withdrawing biologic therapy for some patients, there might be a population of RA patients that have been in remission long enough to make this a reasonable consideration. However, it is not well understood how the duration of time patients have been in remission, along with other factors such as comorbidities, might influence the likelihood of remaining in remission. The clinical usefulness of being able to identify such patients could translate to helping identify those individuals for whom RA medications might successfully have their doses decreased, or even completely withdrawn, without experiencing a flare of their RA.
The objectives of this study were: 1) to evaluate the point prevalence of RA remission using the new ACR/EULAR RA remission criteria in a large United States (US) registry of RA patients according to various comorbidities and RA treatments; 2) to evaluate the durability of remission based upon the length of time patients had been in remission; 3) to evaluate the impact on disability associated with having consistently versus inconsistently been in remission compared to having achieved only low disease activity.
Methods
Population and Definition of Remission
This study used data from the Consortium of Rheumatology Researchers of North America (CORRONA) from 2001–2011, which has been previously described (13–14). RA patients satisfying the 1987 American College of Rheumatology diagnosis criteria (15) were enrolled from academic and community rheumatology practices. Data from both physicians and patients were prospectively collected at enrollment and follow up clinical encounters occurring at approximately 3–4-month intervals; at each visit, common measures of RA disease activity, functional status, and use of RA medications were collected. All of the subjects gave written informed consent and the study protocol was approved by both central and local institutional review boards.
Definition and Prevalence of Remission and Associated Characteristics
The remission definition used for these analyses was the Boolean ACR/EULAR definition that consists of swollen joint count (SJC) and tender joint count (TJC) ≤ 1, CRP ≤ 1 mg/dL and patient global ≤ 1 (on a 0–10 scale). In cross-sectional analyses, the most recent CORRONA visit with complete data to evaluate remission was used to establish the point prevalence of remission according to use of various RA medications, smoking status and presence of comorbidities including chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM) and congestive heart failure (CHF). These data were captured at each CORRONA visit.
Durability and Factors Associated with Remaining in Remission over Time
Longitudinal analyses examined the durability of remission according to various factors which included the length of time that patients had been in remission, as well as concomitant medical comorbidities, RA treatments, and various demographic characteristics. Because the length of time patients had been in remission was expected to be a strong predictor of remaining in remission, patients were identified at the time they newly met the remission definition, described as ‘new onset’ remission. New onset remission was defined as the first CORRONA visit in remission where the prior CORRONA visit was not in remission. Usable visits consisted of those with complete data available to assess remission status. If a visit occurred at which an individual was known to have failed to meet remission criteria because of non-CRP related factors (e.g. SJC), CRP was therefore not required and the visit was included in the analyses. CORRONA visits had to occur within 210 days (7 months) of one another. This restriction was required in order to avoid having too much time elapse during which medical comorbidities and RA treatments could have changed, resulting in misclassification of the main independent variables of interest. Fewer than thirteen percent of remission episodes were censored because they lacked a visit with the necessary CRP data within the required 210 day interval.
Impact of Different Disease Activity Levels on Patient’s Functionality
In order to assess the influence of remission on changes in functional status and disability, three mutually exclusive subpopulations were defined to compare subsequent changes in functionality assessed by the modified health assessment questionnaire (mHAQ). These populations were characterized based upon disease activity status over three clinical visits, anchored in time by the occurrence of new onset remission or low disease activity. These three cohorts were categorized as 1) ‘new-onset consistent remission’, defined as the first time that the patient achieved new onset remission and remained in remission at the next two subsequent visits; 2) ‘new-onset inconsistent remission’ defined as the first time that the patient achieved new onset remission but failed to remain in remission at either or both of the next two subsequent visits (e.g. fluctuated between remission and low disease activity); and 3) ‘new-onset low disease activity’, defined as the first time that a patient who had been in moderate or high disease activity achieved new onset low disease activity (LDA, DAS28(ESR) > 2.6 and ≤ 3.2) and remained in LDA at the next two visits. Follow-up time for this disability analysis began after the three qualifying visits and was examined at the following three visits.
Covariates
Comorbidities considered were selected based upon clinical interest and included congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), diabetes (DM) and current tobacco use. Cormorbidities were assessed at each visit and were considered as present if were present at the time of remission or within the subsequent one year. This approach minimized missing data and was made under the assumption that these chronic illnesses could have been present for some time before a definitive diagnosis was made, recognized, and recorded by the CORRONA rheumatologist.
RA medications were grouped in the following categories: non-biologic disease modifying anti-rheumatic drugs (DMARDs) [sulfasalazine (SSA), hydroxychloroquine (HCQ), leflunomide, methotrexate (MTX)]; anti-tumor necrosis factor (TNF) biologics [infliximab, adalimumab, etanercept, golimumab, certolizumab] with or without non-biologic DMARDs; and non-anti-TNF biologics [rituximab, abatacept, tocilizumab] with or without non-biologic DMARDs. Prednisone use was categorized as no use, < 5 mg, 5–9 mg or ≥ 10 mg of prednisone. Rheumatoid factor, socio-economic status and being disabled from work according to patient self-report, were considered as factors potentially associated with achieving or maintaining remission.
Statistical Analysis
The point prevalence of remission by various comorbidities and RA medications, and the distribution of pain, fatigue, ESR and mHAQ for patients in remission were descriptively analyzed cross-sectionally at the same time of the most recent CORRONA visit. For longitudinal analysis, logistic regression was used to identify factors associated with remaining in remission, with the start of follow-up for this analysis beginning at the visit at which patients achieved new onset remission. Clustering of visits within patients was accounted for using generalized estimating equations (GEE) with an auto-regressive working correlation matrix that used a fixed correlation coefficient ranging between 0.20 and 0.25. Kaplan-Meier life tables were used to illustrate the probability of remaining in remission based on the number of previous consecutive visits in remission. For this analysis, patients were censored if they reached the endpoint of interest (failure to remain in remission), or if more than 210 days elapsed until the next visits. These censored patients could be included again in the analysis if they qualified at a later time as achieving new onset remission.
The impact of consistent remission, inconsistent remission, and consistent low disease activity on functionality (measured by mHAQ) was evaluated descriptively. Mean differences in mHAQ and days lost from work because of RA (collected at each CORRONA visit) were compared between these 3 populations using analysis of variance (ANOVA). Tukey’s Studentized Range test was done to determine differences between these 3 populations at each visit. The proportion of patients that had a change of +0.3 (the minimal clinically important difference for worsening) (16) in mHAQ also was compared between the 3 groups.
Results
There were 2,351 patients in RA remission according to the ACR/EULAR Boolean criteria at the most recently recorded CORRONA visit and 25,879 were not in remission. Table 1 shows the characteristics of patients in CORRONA according to remission status. The overall prevalence for new-onset RA remission was low (8%). Patients in remission uncommonly had comorbidities such as COPD, diabetes, or heart failure. The majority of patients in remission had low pain scores (86% with pain ≤ 1 on 0–10 visual analog scale), low fatigue scores (72% with score ≤ 1 on 0–10 visual analog scale) and low disability (79%, mHAQ = 0). Among patients in remission, 45% had ESR < 10 mm/hr, and an additional 27% had an ESR between 10 and 20 mm/hr. The most common treatments used among RA patients in remission were MTX monotherapy (28% of remission patients) and MTX + anti-TNF therapy (31% of remission patients).
Table 1.
Characteristics of RA Patients within CORRONA by ACR/EULAR Remission Status using Boolean Criteria*
| Variable | Remission N = 2,351 (8%) |
Not Remission N = 25,879 (92%) |
|---|---|---|
|
| ||
| Age, years (Median, IQR) | 60 ± 8 | 61 ± 17 |
| Female, (%) | (74) | (76) |
| Race, (%) | ||
| Caucasian, (%) | (85) | (82) |
| Other, (%) | (15) | (17) |
| Rheumatoid Factor (RF) positive, (%) | (46) | (41) |
| Disease duration, years (%) | ||
| <2 | (7) | (11) |
| 2–5 | (27) | (24) |
| 6–10 | (27) | (22) |
| >10 | (39) | (42) |
| COPD, (%) | (0.6) | (2) |
| Diabetes, (%) | (5) | (9) |
| Heart Failure, (%) | (0.4) | (2) |
| Current smoking, (%) | (10) | (16) |
| Pain (0–10 visual analog scale), (%) | ||
| 0 | (28) | (4) |
| 1 | (58) | (16) |
| 2 | (9) | (14) |
| ≥ 3 | (5) | (63) |
| Fatigue (0–10 visual analog scale), (%) | ||
| 0, | (29) | (10) |
| 1 | (43) | (14) |
| 2 | (12) | (11) |
| ≥ 3 | (15) | (65) |
| mHAQ | ||
| 0 | (79) | (32) |
| >0, ≤ 0.5 | (18) | (35) |
| > 0.5 | (3) | (33) |
| Physician global (0–10), Median, IQR | 0.3 ± 0.5 | 1.5 ± 2.5 |
| ESR (mm/hr), (%) | ||
| < 10 | (45) | (29) |
| 10 – < 20 | (27) | (24) |
| ≥ 20 | (28) | (47) |
Boolean criteria is defined by tender joint count, swollen joint count, and patient global health assessment ≤ 1 and CRP ≤ 1 mg/dL
As shown in Table 2, the likelihood of being in remission according to use of various RA treatments was lowest for non-anti-TNF biologic + MTX + (HCQ) ± SSA (2%) and highest for anti-TNF biologics + MTX or MTX + HCQ (both with a point prevalence of 11%). Remission was less common among RA patients treated with prednisone (1%– 6%, depending on dose) compared to those not on prednisone (10%). There were significant differences in the prevalence of remission depending on whether patients had comorbidities including CHF, DM and COPD. Similarly, current smokers had a lower prevalence of remission compared to non-smokers.
Table 2.
Point prevalence of RA patients in Remission according to Subgroups Defined by RA Treatments and Various Comorbidities
| Total, n | Prevalence of Remission, % | p-value* | |
|---|---|---|---|
|
| |||
| DMARD/Biologic treatment | <0.001 | ||
| MTX | 7301 | 9 | |
| MTX + HCQ | 1,435 | 11 | |
| MTX + HCQ + SSA ± Lef | 174 | 5 | |
| MTX + SSA | 239 | 7 | |
| Lef ± (HCQ + SSA) | 777 | 6 | |
| Non-TNF Biologic | 732 | 4 | |
| Non-TNF Biologic + Lef ± (HCQ + SSA) | 206 | 3 | |
| Non-TNF Biologic + MTX | 974 | 6 | |
| Non-TNF Biologic + MTX + HCQ ± SSA | 124 | 2 | |
| Anti-TNF monotherapy | 2,889 | 10 | |
| Anti-TNF + Lef ± (HCQ + SSA) | 559 | 8 | |
| Anti-TNF + MTX | 5,469 | 11 | |
| Anti-TNF + MTX + HCQ ± (SSA + Lef) | 586 | 6 | |
|
| |||
| Prednisone (mg/day) | <0.001 | ||
| None | 22,285 | 10 | |
| < 5 | 1,777 | 6 | |
| 5–9 | 2,803 | 4 | |
| ≥ 10 | 1,365 | 1 | |
|
| |||
| COPD | <0.001 | ||
| Yes | 432 | 3 | |
| No | 20,576 | 10 | |
|
| |||
| Diabetes | <0.001 | ||
| Yes | 2,357 | 5 | |
| No | 25,696 | 9 | |
|
| |||
| Heart Failure | <0.001 | ||
| Yes | 407 | 2 | |
| No | 27,646 | 8 | |
|
| |||
| Smoking, | <0.001 | ||
| Current | 4,294 | 5 | |
| Not current | 15,388 | 8 | |
MTX = methotrexate; HCQ = hydroxychloroquine; SSA = sulfasalazine; Lef = leflunomide; anti-TNF = anti-tumor necrosis alpha; non-anti-TNF biologic = abatacept, rituximab and tocilizumab combined COPD = Chronic Obstructive Pulmonary Disease
p-value calculated using Chi-squared
Figure 1 describes the probability of remaining in remission conditional on whether patients had 1, 2, 3, 4 or 5 consecutive previous visits where they were in remission. Patients who were in remission for 4 (n = 231) or 5 (n = 140) consecutive visits (≥ 1 year for the majority of patients) had a > 75% probability of remaining in remission at the next visit and had approximately a 50% probability of remaining in remission at the next year. In contrast, patients with fewer consecutive visits in remission were less likely (< 50%) to remain in remission over time.
Figure 1.
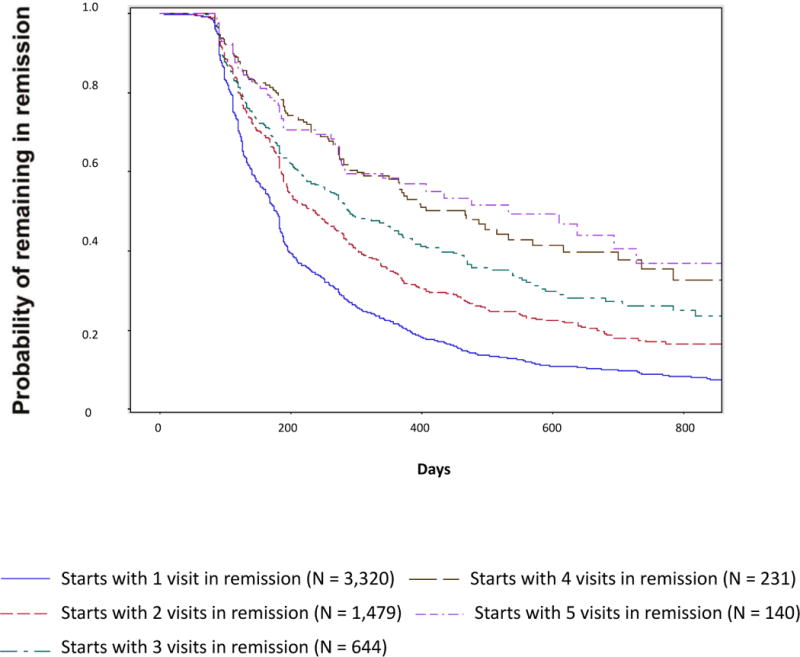
Probability of Remaining in Remission Based on Number of Previous Consecutive Visits in Remission
Multivariable analysis in Table 3 describes various factors associated with the likelihood of remaining in remission. The number of previous visits in remission was a significant factor associated with remaining in remission. After multivariable adjustment, having been in remission for at least 4 consecutive visits was a strong predictor of future remission. Smoking, being disabled from work, higher pain scores and higher doses of prednisone were associated with a decreased likelihood of remaining in remission. The associations between remission and various comorbidities did not persist after multivariate adjustment except for heart failure. Several RA medication groups were associated with a lower likelihood of remaining in remission; patients on biologics with non-anti-TNF mechanisms of action were associated with a significantly lower likelihood of remaining in remission.
Table 3.
Probability of Remaining in RA Remission at the Next Visit by RA Treatment and Comorbidities
| OR (95% CI) for Remaining in Remission at Next Visit | ||
|---|---|---|
|
| ||
| Bivariate | Multivariable-adjusted* | |
|
| ||
| Consecutive previous visits in remission | ||
| 1 previous visit | Referent | Referent |
| 2 previous visits | 1.05 (0.92, 1.19) | 0.96 (0.84. 1.10) |
| 3 previous visits | 1.19 (0.99, 1.44) | 1.04 (0.85, 1.27) |
| 4 previous visits | 2.50 (1.93, 3.23) | 2.07 (1.56, 2.73) |
| 5 previous visits | 3.39 (2.53, 4.54) | 2.56 (1.88, 3.50) |
|
| ||
| Current Smoking (Referent to not current) | 0.78 (0.61, 0.98) | 0.76 (0.61, 0.93) |
|
| ||
| Pain, | ||
| 0 | Referent | Referent |
| 1 | 0.66 (0.57, 0.77) | 0.66 (0.57, 0.76) |
| 2 | 0.37 (0.30, 0.45) | 0.40 (0.33, 0.50) |
| ≥3 | 0.27 (0.21, 0.36) | 0.31 (0.23, 0.41) |
|
| ||
| Disabled | 0.45 (0.30, 0.68) | 0.56 (0.37, 0.85) |
|
| ||
| Heart Failure | 0.16 (0.06, 0.43) | 0.23 (0.08, 0.64) |
|
| ||
| DMARD/Biologic treatment** | ||
| Anti-TNF + DMARD | Referent | Referent |
| MTX monotherapy | 0.87 (0.74, 1.03) | 0.87 (0.74, 1.02) |
| MTX-containing DMARD combinations | 0.98 (0.76, 1.26) | 0.98 (0.77, 1.24) |
| Non-MTX DMARD(s) | 0.77 (0.58, 1.03) | 0.79 (0.59, 1.07) |
| Anti-TNF monotherapy | 0.97 (0.79, 1.21) | 0.91 (0.74, 1.13) |
| Non-anti-TNF Bio monotherapy | 0.26 (0.13, 0.54) | 0.29 (0.14, 0.57) |
| Non-anti-TNF Biologic + DMARD | 0.51 (0.35, 0.76) | 0.48 (0.33, 0.71) |
|
| ||
| Prednisone (mg/day) None |
Referent | Referent |
| < 5 | 0.57 (0.44, 0.73) | 0.62 (0.48, 0.81) |
| 5–9 | 0.49 (0.37, 0.65) | 0.56 (0.41, 0.76) |
| ≥ 10 | 0.58 (0.31, 0.61) | 0.69 (0.36, 1.33) |
Also controlling for age, gender, rheumatoid factor, RA disease duration, education level, chronic obstructive pulmonary disease, diabetes and narcotic use, none of which were significantly associated with the outcome.
MTX = Methotrexate; DMARDs = Disease Modifying anti-rheumatic drug including MTX unless specified as not included; anti-TNF = anti-tumor necrosis alpha; non-anti-TNF biologic = abatacept, rituximab and tocilizumab combined.
Mean mHAQ scores varied according to whether patients achieved new onset consistent remission, new onset inconsistent remission, or new onset LDA (Figure 2A). While the longitudinal changes in mHAQ were parallel to each other in these 3 groups, the absolute value of the mHAQ was lowest for those with consistent remission. In terms of days lost from work due to RA, there were no significant differences between the remission, LDA and inconsistent remission populations (Figure 2B) except at visit 3 where the LDA population was significantly different from the consistent remission and inconsistent remission populations. The mean days lost from work due to RA were not different between the remission and inconsistent remission populations.
Figure 2.
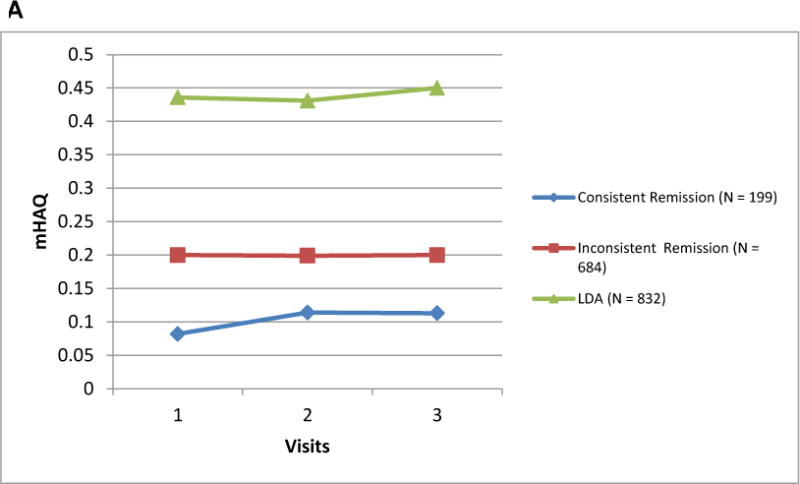
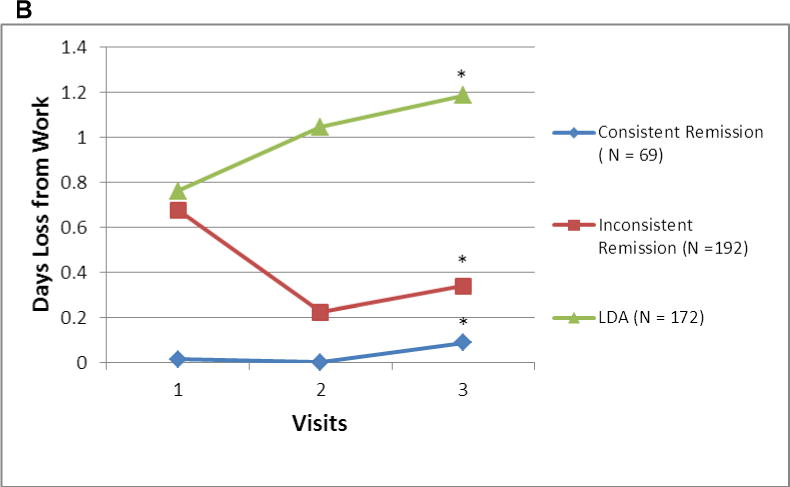
A and B Mean mHAQ† and Days Lost from Work due to RA‡ among RA Patients Achieving New Onset Consistent Remission, New Onset Inconsistent Remission, and New Onset Low Disease Activity
† The consistent remission, inconsistent remission, and LDA subpopulations were defined based upon the disease activity status of 3 consecutive CORRONA visits, the first of which was when patients experienced new onset remission or new onset low disease activity. The results presented here are the mHAQ scores for the next 3 visits. Differences in the mean mHAQ were significantly different between the groups at each of the three time points shown (p <0.001 for each).
‡ The consistent remission, inconsistent remission, and LDA subpopulations were defined based upon the disease activity status of 3 consecutive CORRONA visits, the first of which was when patients experienced new onset remission or new onset low disease activity.
*The only significant differences observed were at visit 3 comparing the LDA and the other two populations (p < 0.001 for both comparisons). No other differences at any other time point were significantly different.
mHAQ = Modified Health Assessment Questionnaire; LDA = Low disease activity; N = number of patients.
After averaging the mHAQ over the 3 ‘baseline’ visits used to define each population, the proportion of patients that subsequently had a change of +0.3 in mHAQ (the minimal clinically important difference for worsening) (16) at each of the following 3 visits compared to the mean mHAQ from the 3 ‘baseline’ visits was low for the consistent remission population (1.5%, 4.5%, and 4.0%). The corresponding proportions were higher for the inconsistent remission population (6.6%, 6.3%, and 6.7%), and were highest for the LDA population (9.1%, 8.7%, and 11.4%).
Discussion
After applying the ACR/EULAR Boolean remission criteria to a national US cohort, the overall point prevalence of clinical remission was low (8% overall). Based upon the presence or absence of varying comorbidities and use of different RA treatments, the prevalence of remission in various subgroups of RA patients was at most 10%. The likelihood of future remission varied modestly by RA therapies, especially for prednisone where higher doses were associated with a lower likelihood of remission. Of high importance, the length of time that a patient had been in remission was a strong predictor of remaining in remission. Patients had a high likelihood of remaining in remission if they had been in remission for at least 4 visits, which in this cohort translated to at least one year for the majority of patients. Factors associated with a decreased likelihood of remaining in remission were current smoking, higher patient-reported pain levels, and comorbidities such as heart failure and tobacco use.
The prevalence of remission in this study was relatively similar to two other published studies that applied the Boolean ACR/EULAR criteria (8, 11) to U.S. cohorts, although we intentionally favored use of the very conservative Boolean remission definition, which has been found to be more stringent than the simplified disease activity index (SDAI)-based remission definition or DAS28-based remission thresholds. Compared to the prevalence of remission we found in the CORRONA cohort, an analysis of tocilizumab (TCZ) clinical trial data showed a somewhat higher remission incidence (16% at 12 weeks and 20% at 24 weeks) (8). These differences in the incidence of remission may be accounted for by differences in the RA populations; for example, the TCZ trial excluded patients on prednisone doses > 10 mg, those with high comorbidity burdens and severe RA functional class (Steinbrocker IV) (8). In another study, where two U.S. cohorts were compared, the point prevalence was 8–9% for both analyzed cohorts which was very similar to our results (8% overall) (11). There were 2 prior analyses conducted within CORRONA that examined the point prevalence of remission and the effect of sex and RA disease duration (17–18). The unique feature of this analysis focused on the durability of remission for patients who newly achieved remission and examined varying lengths of time that patients had been in remission as a predictor for the subsequent durability of remission. In addition, we evaluated the impact of patients being in consistent versus inconsistent remission compared to low disease activity on functional status and work productivity.
Several published studies have shown that the durability of remission was low (11–12). However, unless one identifies patients at the time they first achieved remission (i.e. ‘new onset remission’) and follows them forward, it is difficult to interpret the results of such studies given that the duration of time patients have been in remission is an important predictor of the likelihood of remaining in remission over time. There was one study that was able to start follow-up time at new onset remission (12) to avoid this ‘left censoring’ problem, but did not determine how time in consistent remission influences future time in remission.
The effect of comorbidities on remission could be related to the independent effect of these conditions, their interaction with RA (e.g. if patients had multiple reasons for functional limitations), or their impact on how remission is measured. Patient global and CRP are two of the four components of remission, and other studies have shown that comorbidities can affect inflammatory markers (19–20) and patient’s measurement of their global health (7).
The association of prednisone dose and the likelihood of remission using the new ACR/EULAR remission definition found that patients treated with prednisone were less likely to achieve or to remain in remission. These patients presumably had more refractory disease. Similarly, although we found that non-TNF biologic therapies were associated with a significantly lower likelihood of remaining in remission compared to MTX + anti-TNF agents, it is likely that patients treated with non-TNF biologics had more refractory disease despite controlling for RA disease duration and other factors.
This study provides not only information regarding the applicability of the new ACR/EULAR remission criteria outside clinical trials but also identifies factors associated with the durability of remission. Among the strengths of our study are 1) large numbers of patients with long term follow up, allowing for use of the most stringent of the various remission definitions, the ACR/EULAR Boolean criteria; 2) examination of many risk factors like RA therapies, prednisone dose and smoking that were hypothesized to influence remission; and 3) inclusion only of patients with “new onset” remission, which avoided the inclusion of prevalent cases of remission (i.e. left censored data) and allowed the characterization of the time in remission to be examined as predictor of remaining in remission in the future.
There are several potential limitations with our study. We allowed no more than a maximum interval of 210 days between visits (CORRONA visits are generally spaced 3–4 months apart), in order to avoid long periods of time where treatment changes or events that could occur and/or misclassify the main independent variables of interest. Moreover, CRP is optionally collected within CORRONA and was therefore missing at some visits. However, less than 13% of episodes of new onset remission had to be censored because of missing CRP or an extended gap between visits. As an alternative to having used the Boolean remission definition, we could have used the Clinical Disease Activity Index (CDAI) to define remission (CDAI ≤ 2.8). However, our interest was to examine the new ACR/EULAR remission definition that requires CRP. We also elected not to use the SDAI-based remission definition (SDAI ≤ 3.3) given that this is a less stringent definition for remission; it is likely that the overall prevalence and durability of remission would have been somewhat higher using the SDAI remission definition. Finally, some comorbidities of interest like fibromyalgia were only recently begun to be collected within CORRONA and could not be examined in this analysis. Fibromyalgia might be of particular interest since it often affects fatigue and pain which could influence remission status. Patients with fibromyalgia and RA should be examined with respect to their ability to achieve and maintain remission in future studies. We also recognize the possibility that for patients with other medical comorbidities, physicians might be less willing to use DMARDs and biologics, making patients less able to achieve remission; this should be examined in future studies.
In conclusion, this study demonstrated that remission is achievable and can be maintained even using a stringent definition like the ACR/EULAR Boolean criterion. For patients who have been in remission for at least one year, the likelihood of remaining in remission is at least 50%. The ability to maintain remission is greater for patients who have lower levels of pain and who are not treated with glucocorticoid therapy, likely a marker for less refractory disease. Patients treated with combination treatments (e.g. MTX + anti-TNF therapy) appear to have the greatest likelihood of maintaining sustained remission, suggesting the possibility of tapering the dose or withdrawing one of these RA therapies. An interventional trial testing this hypothesis may be worth pursuing.
Acknowledgments
Funding:
This work was supported by the Agency for Healthcare Research and Quality (R01HS018517) and the National Institutes of Health (AR053351). CORRONA has received general support in the last two years from Abbott, Amgen, Astra Zeneca, Genentech, Janssen (Centocor), Lilly and Pfizer. Dr. Greenberg is a shareholder in CORRONA and has received consulting fees from AstraZeneca, CORRONA, Novartis, Pfizer. Dr. Curtis receives support from the NIH (AR053351) and the Agency for Healthcare Research and Quality (R01HS018517)
References
- 1.Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003 Feb;42(2):244–57. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D. Activity assessments in rheumatoid arthritis. Curr Opin Rheumatol. 2008 May;20(3):306–13. doi: 10.1097/BOR.0b013e3282fbd382. [DOI] [PubMed] [Google Scholar]
- 3.Makinen H, Kautiainen H, Hannonen P, Sokka T. Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis? Ann Rheum Dis. 2005 Oct;64(10):1410–3. doi: 10.1136/ard.2005.037333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mierau M, Schoels M, Gonda G, Fuchs J, Aletaha D, Smolen JS. Assessing remission in clinical practice. Rheumatology (Oxford) 2007 Jun;46(6):975–9. doi: 10.1093/rheumatology/kem007. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011 Mar;70(3):404–13. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 6.Radner H, Smolen JS, Aletaha D. Impact of comorbidity on physical function in patients with rheumatoid arthritis. Ann Rheum Dis. 2010 Mar;69(3):536–41. doi: 10.1136/ard.2009.118430. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan E, Hakkinen A, Sokka T, Hannonen P. Impact of age and comorbidities on the criteria for remission and response in rheumatoid arthritis. Ann Rheum Dis. 2005 Sep;64(9):1350–2. doi: 10.1136/ard.2005.037903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iking-Konert C, Aringer M, Wollenhaupt J, Mosch T, Tuerk S, Feist E, et al. Performance of the new 2011 ACR/EULAR remission criteria with tocilizumab using the phase IIIb study TAMARA as an example and their comparison with traditional remission criteria. Ann Rheum Dis. 2011 Aug 29; doi: 10.1136/ard.2011.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther. 2011 Jun 8;13(3):R83. doi: 10.1186/ar3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulfe A, Aletaha D, Saxne T, Geborek P. Disease activity level, remission and response in established rheumatoid arthritis: performance of various criteria sets in an observational cohort, treated with anti-TNF agents. BMC Musculoskelet Disord. 2009;10:41. doi: 10.1186/1471-2474-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahouri SH, Michaud K, Mikuls TR, Caplan L, Shaver TS, Anderson JD, et al. Remission of rheumatoid arthritis in clinical practice: Application of the ACR/EULAR 2011 remission criteria. Arthritis Rheum. 2011 Jul 7; doi: 10.1002/art.30524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prince FH, Bykerk VP, Shadick NA, Lu B, Cui J, Frits M, et al. Sustained rheumatoid arthritis remission is uncommon in clinical practice. Arthritis Res Ther. 2012 Mar 19;14(2):R68. doi: 10.1186/ar3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer J. The CORRONA database. Ann Rheum Dis. 2005 Nov;64(Suppl 4):iv37–41. doi: 10.1136/ard.2005.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer JM. The CORRONA database. Autoimmunity reviews. [Review] 2006 Jan;5(1):46–54. doi: 10.1016/j.autrev.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. [Comparative Study, Research Support, Non-U.S. Gov’t, Research Support, U.S. Gov’t, P.H.S.] 1988 Mar;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Pope JE, Khanna D, Norrie D, Ouimet JM. The minimally important difference for the health assessment questionnaire in rheumatoid arthritis clinical practice is smaller than in randomized controlled trials. J Rheumatol. [Comparative Study Research Support, Non-U.S. Gov’t] 2009 Feb;36(2):254–9. doi: 10.3899/jrheum.080479. [DOI] [PubMed] [Google Scholar]
- 17.Furst DE, Pangan AL, Harrold LR, Chang H, Reed G, Kremer JM, et al. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: results from the Consortium of Rheumatology Researchers of North America registry. Arthritis Care Res (Hoboken). [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] 2011 Jun;63(6):856–64. doi: 10.1002/acr.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jawaheer D, Messing S, Reed G, Ranganath VK, Kremer JM, Louie JS, et al. Men are significantly more likely than women to achieve sustained remission in the CORRONA Cohort of rheumatoid arthritis patients. Arthritis Care Res(Hoboken) 2012 Jun 21; doi: 10.1002/acr.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004 Jul;59(7):574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004 Mar;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]


