Abstract
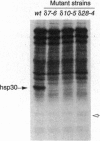
The alpha-crystallin-related heat shock proteins are produced by all eukaryotes, but the role of these proteins in thermoprotection remains unclear. To investigate the function of one of these proteins, we disrupted expression of the single-copy hsp30 gene of Neurospora crassa, using repeat-induced point mutagenesis, and we generated and characterized mutant strains that were deficient in hsp30 synthesis. These strains could grow at high temperature and they acquired thermotolerance from a heat shock. However, the hsp30-defective strains proved to be extremely sensitive to the combined stresses of high temperature and carbohydrate limitation, enforced by the addition of a nonmetabolizable glucose analogue. Under these conditions, their survival was reduced by 90% compared with wild-type cells. This sensitive phenotype was reversed by reintroduction of a functional hsp30 gene into the mutant strains. The mutant cells contained mitochondria from which a 22-kDa protein was readily extracted with detergents, in contrast to its retention by the mitochondria of wild-type cells. Antibodies against hsp30 coimmunoprecipitated a protein also of approximately 22 kDa from wild-type cells. Results of this study suggest that hsp30 may be important for efficient carbohydrate utilization during high temperature stress and that it may interact with other mitochondrial membrane proteins and function as a protein chaperone.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abath F. G., Simpson A. J. A simple method for the recovery of purified recombinant peptides cleaved from glutathione-S-transferase-fusion proteins. Pept Res. 1990 Jul-Aug;3(4):167–168. [PubMed] [Google Scholar]
- Amin J., Ananthan J., Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988 Sep;8(9):3761–3769. doi: 10.1128/mcb.8.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Suhan J. P., Welch W. J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988 Dec;8(12):5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambareri E. B., Jensen B. C., Schabtach E., Selker E. U. Repeat-induced G-C to A-T mutations in Neurospora. Science. 1989 Jun 30;244(4912):1571–1575. doi: 10.1126/science.2544994. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Habel D., Plesofsky-Vig N., Brambl R. The respiratory response to heat shock in Neurospora crassa. FEMS Microbiol Lett. 1991 Jul 1;65(3):317–322. doi: 10.1016/0378-1097(91)90234-2. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensenius J. C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46(1):63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Lavoie J. N., Gingras-Breton G., Tanguay R. M., Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993 Feb 15;268(5):3420–3429. [PubMed] [Google Scholar]
- Leicht B. G., Biessmann H., Palter K. B., Bonner J. J. Small heat shock proteins of Drosophila associate with the cytoskeleton. Proc Natl Acad Sci U S A. 1986 Jan;83(1):90–94. doi: 10.1073/pnas.83.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mehlen P., Briolay J., Smith L., Diaz-latoud C., Fabre N., Pauli D., Arrigo A. P. Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type or deletion mutants of the Drosophila 27-kDa heat-shock protein. Eur J Biochem. 1993 Jul 15;215(2):277–284. doi: 10.1111/j.1432-1033.1993.tb18032.x. [DOI] [PubMed] [Google Scholar]
- Merck K. B., Groenen P. J., Voorter C. E., de Haard-Hoekman W. A., Horwitz J., Bloemendal H., de Jong W. W. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993 Jan 15;268(2):1046–1052. [PubMed] [Google Scholar]
- Miron T., Wilchek M., Geiger B. Characterization of an inhibitor of actin polymerization in vinculin-rich fraction of turkey gizzard smooth muscle. Eur J Biochem. 1988 Dec 15;178(2):543–553. doi: 10.1111/j.1432-1033.1988.tb14481.x. [DOI] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Pinelli E., Shapira M. Temperature-induced expression of proteins in Leishmania mexicana amazonensis. A 22-kDa protein is possibly localized in the mitochondrion. Eur J Biochem. 1990 Dec 12;194(2):685–691. doi: 10.1111/j.1432-1033.1990.tb15669.x. [DOI] [PubMed] [Google Scholar]
- Plesofsky-Vig N., Brambl R. Gene sequence and analysis of hsp30, a small heat shock protein of Neurospora crassa which associates with mitochondria. J Biol Chem. 1990 Sep 15;265(26):15432–15440. [PubMed] [Google Scholar]
- Plesofsky-Vig N., Brambl R. Heat shock response of Neurospora crassa: protein synthesis and induced thermotolerance. J Bacteriol. 1985 Jun;162(3):1083–1091. doi: 10.1128/jb.162.3.1083-1091.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesofsky-Vig N., Vig J., Brambl R. Phylogeny of the alpha-crystallin-related heat-shock proteins. J Mol Evol. 1992 Dec;35(6):537–545. doi: 10.1007/BF00160214. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Lindquist S. L. HSP104 required for induced thermotolerance. Science. 1990 Jun 1;248(4959):1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Bentley N. J., Bossier P., Fitch I. T. The structure and function of small heat shock proteins: analysis of the Saccharomyces cerevisiae Hsp26 protein. Antonie Van Leeuwenhoek. 1990 Oct;58(3):147–154. doi: 10.1007/BF00548925. [DOI] [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]