Abstract
Chondrichthyan fishes are a diverse class of gnathostomes that provide a valuable perspective on fundamental characteristics shared by all jawed and limbed vertebrates. Studies of phylogeny, species diversity, population structure, conservation, and physiology are accelerated by genomic, transcriptomic and protein sequence data. These data are widely available for many sarcopterygii (coelacanth, lungfish and tetrapods) and actinoptergii (ray-finned fish including teleosts) taxa, but limited for chondrichthyan fishes. In this study, we summarize available data for chondrichthyes and describe resources for one of the largest projects to characterize one of these fish, Leucoraja erinacea, the little skate. SkateBase ( http://skatebase.org) serves as the skate genome project portal linking data, research tools, and teaching resources.
Introduction
Chondrichthyan fishes are composed of two subclasses, Holocephali and Elasmobranchii. Holocephalans are the more basal of the pair having first appeared more than 400 million years ago and include a single surviving order, Chimaeriformes, the chimaeras, with 39 extant species 1. Elasmobranchs appeared approximately 350 million years ago and include more than 1000 species of sharks, skates, and rays 2. Chondrichthyan fishes occupy a pivotal position at the base of the vertebrate phylogenetic tree. For research that includes an evolutionary component, representation of this diverse class affords a valuable perspective to evaluate all vertebrates.
Chondrichthyan fishes are circumglobal in distribution and occupy a wide range of ecological habitats. Their life history parameters are equally disparate but in general chondrichthyans are slow growing and late maturing fishes with an increased risk of extinction 3– 5. Fecundity is as few as 1 or 2 for viviparous species such as the sand tiger shark, Carcharias taurus 6 and as high as 300 for the whale shark, Rhincodon typus 7. They are of economic importance for fisheries as well as ecotourism. Management and assessment of stock is essential to ensure both ecotourism interests and food resources remain sustainable 8. Management of fish populations has increasingly relied on molecular tools to investigate population structure, properly identify species, and compliance with fishing quotas 9– 13.
Elasmobranchs have been used as a model for biomedical research for more than 100 years. Elasmobranchs, like other cartilaginous fishes, exhibit many fundamental vertebrate characteristics, including a neural crest, jaws and teeth, an adaptive immune system, and a pressurized circulatory system. The skate is a powerful comparative model to study biological processes shared among jawed and limbed vertebrates such as development 14– 16, renal physiology 17– 20, immunology 21– 26, toxicology 27, neurobiology 28, and wound healing and regeneration 29. They are the most ancient vertebrates to posses an adaptive immune system that generates antibodies using a V(D)J combinatorial mechanism 30. Phylogenetically, cartilaginous fishes are the first vertebrates to possess a thymus, a central lymphoid organ that provides a microenvironment for the development of T cells 31. The thymus shares a common organization with more derived vertebrates containing cortical and medullary regions 32, 33.
In addition to shared physiological characteristics, the diversity of specializations between species allows investigations of evolution within a single clade. For example, elasmobranchs use a plethora of reproductive strategies that span the full range of maternal investment from placental viviparity to strict lecitrophic oviparity. Besides sexual reproduction, captive elasmobranchs are capable of asexual parthenogenesis 34– 36. Of these reproductive mechanisms, the most tractable for research purposes is oviparity. Approximately 43% of chondrichthyans utilize oviparity including all Chimaeriformes, Heterodontiformes (bullhead sharks), Rajoidae (skates) and Scyliorhinidae (catsharks) 37. Many species can be maintained in captivity and will breed and lay eggs throughout an annual season 38. Artificial insemination has been reported for two oviparous species, the clearnose skate, Raja eglanteria 39, and the cloudy catshark, Scyliorhinus torazame 40. Additionally, sperm storage allows wild caught females to lay eggs for several years without requiring males or captive mating events 41.
Leucoraja erinacea, the little skate, was chosen for a genome sequencing project to represent this clade of fishes because of their use as a biomedical model, experimental tractability, genome size, existing sequence data, and northeast regional distribution. The sequencing project is an ongoing effort of the North East Bioinformatics Collaborative (NEBC) of the North East Cyberinfrastructure Consortium (NECC), composed of the bioinformatics core facilities from Delaware, Maine, New Hampshire, Rhode Island, and Vermont funded by National Institutes of Health (NIH) Institutional Development Awards (IDeA) and/or National Science Foundation (NSF) Experimental Program to Stimulate Competitive Research (EPSCoR) programs.
Existing resources
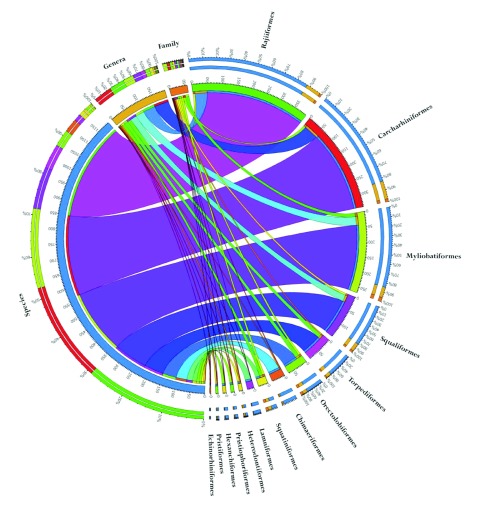
There is a single order of holocephalans and 13 orders of elasmobranchs. The distribution of species in orders, families and genera is shown in Figure 1. The batoids are composed of 4 orders, Rajiiformes, Myliobatiformes, Torpidiformes, and Rhinopristiformes, and contain 54% of extant chondrichthyan species. Sharks are broadly divided into two super orders, Galeomorphii and Squalomorphii that together account for 43% of extant chondrichthyan species. The galean sharks include 4 orders: Heterdontiformes, Orectolobiformes, Lamniormes and Carcharhiniformes, and represent 30% of extant chondrichthyan species. Squalean sharks are composed of 4 orders: Squaliformes, Squatiniformes, Pristophoriformes, and Hexanchiformes, comprising 13% of extant chondrichthyan species. Among individual orders, Rajiiformes, the skates, have the most species (345) followed by Carcharhiniformes, the ground sharks (283) and Myliobatiformes (226) 2. These ‘big three’ orders contain 854 species, 72% of extant chondrichthyans.
Figure 1. Species distribution within chondrichthyan orders.
There is a single order of Holocephalans, Chimaeriformes, and 13 orders of elasmobranchs. The distribution of chondrichthyan species in each of the 14 orders is shown relative to the total number of species, genera and families for the clade. The batoids are composed of 4 orders, Rajiiformes, Myliobatiformes, Torpidiformes, and Rhinopristiformes, and contain 54% of extant chondrichthyan species. Sharks are broadly divided into two super orders, Galeomorphii and Squalomorphii that together include the remaining 9 orders and 43% of extant chondrichthyan species.
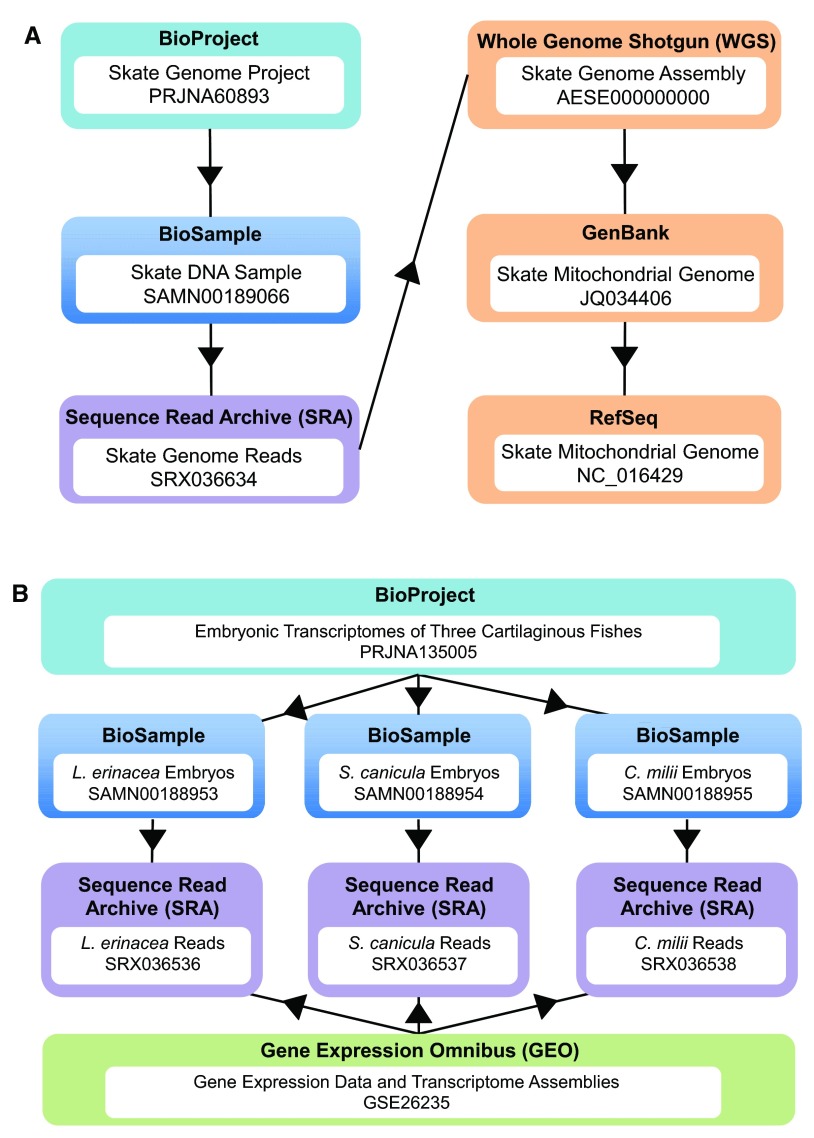
Chondrichthyan conservation, management, and research all benefit from easily accessible and well-documented molecular resources. The organization of data and metadata in archival databases is critically important for efficient use of large and complex datasets. The International Nucleotide Sequence Database Collaboration (INSDC) is composed of three large public nucleotide repositories, DNA Data Bank of Japan (DDBJ), European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI), and GenBank at the National Center for Biotechnology Information (NCBI). Recently, two new NCBI database projects were initiated to collect details of samples, BioSample, and project data, BioProject, and propagate the metadata to all associated database entries in an effort to expand the use of already existing and rapidly expanding molecular resources 42. Figure 2 illustrates the relationship between BioProject, BioSample and the sequence data for SkateBase. Because the BioProject and BioSample databases were established in 2012, not all existing datasets have metadata or details of the biological source to populate a BioSample and BioProject entry. When available, BioProject and BioSample hyperlinks are included for Sequence Read Archive (SRA), Expressed Sequence Tag (EST) and Genome Survey Sequence (GSS) datasets in the tables below.
Figure 2. Representation of SkateBase data within the The National Center for Biotechnology Information (NCBI) databases.
A. The little skate genome project is represented as a BioProject entry that connects all samples and data thematically. A BioSample record describes the DNA sample that was used for genome sequencing that was generated from a single stage 32 skate embryo. The SRA catalogs the unassembled Illumina genome sequence data. The Whole Genome Shotgun (WGS) database contains the contiguous sequences from shotgun sequencing projects. The assembled and annotated mitochondrial genome was deposited in GenBank and subsequently included in the NCBI Reference Sequence Database (RefSeq). B. The project to characterize the embryonic transcriptomes of L. erinacea, C. milii and S. canicula is represented in a BioProject entry. Three BioSample entries, one for each species, lead to three SRA datasets. The transcriptome data is represented also in the Gene Expression Omnibus (GEO), a database of high-throughput functional genomic data derived from microarrays and next-generation sequencing technologies.
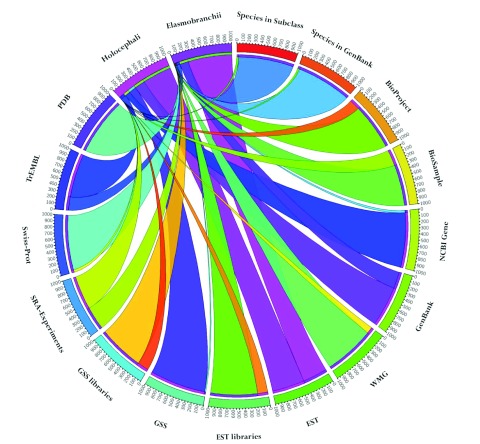
Table 1 is a summary of chondrichthyan sequence data in NCBI databases, UniProtKB, and the Protein Data Bank (PDB) with L. erinacea, Callorhinchus milii and Scyliorhinus canicula, the three species featured at SkateBase listed individually. The distribution of holocephalans and elasmobranchs in public databases is illustrated in Figure 3. Despite the majority of species belonging to Elasmobranchii, the GenBank, UniProtKB/TrEMBL, and Gene databases are dominated by chimaera data derived from the genome sequence of the elephant shark, C. milii 43. Elasmobranch data predominates in UniProtKB/Swiss-Prot, PDB, BioProject and BioSample databases as well as the number of whole mitochondrial genomes (WMG) in GenBank. The EST and SRA databases are nearly equally split between the two subclasses.
Figure 3. Holocephalan and elasmobranch resources in public nucleotide and protein databases.
The distribution of data for Holocephalii (chimaeras) and elasmobranchii (sharks and rays) subclasses of chondrichthyan fishes does not always reflect their species distribution. The number of species represented in GenBank is representative of the actual species distribution but the amount of data in GenBank is not. Holocephalan data forms the majority of the NCBI Gene, GenBank, Genome Survey Sequence (GSS) and UniProt TrEMBL databases. The number of Short Reach Archive (SRA) experiments and EST sequences in nearly equal for each subclass and the remaining databases are primarily populated by elasmobranch data.
Table 1. Chondrichthyan molecular sequence data in public databases.
| National Center for Biotechnology Information (NCBI) databases 1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GenBank | UniProtKB 2 | |||||||||||||||
| Taxonomy | BioProject | BioSample | Gene | GenBank | WMG | EST | EST lib | GSS | GSS
lib |
WGS
(Mbp) |
GEO 3 | SRA | Swiss-
Prot |
TrEMBL | PDB | |
| Chondrichthyes | 7777 | 16 | 75 | 21069 | 55810 | 72 | 192948 | 33 | 28497 | 5 | 2492.3 | 3 | 22 | 276 | 26485 * | 178 |
| Holocephali | 7863 | 3 | 21 | 20201 | 39512 | 8 | 109965 | 6 | 27944 | 1 | 936.9 | 1 | 13 | 12 | 20170 | 0 |
| C. milii | 7868 | 3 | 21 | 20110 | 39232 | 1 | 109965 | 6 | 27944 | 1 | 936.9 | 1 | 13 | 3 | 19989 | 0 |
| Elasmobranchii | 7778 | 13 | 54 | 868 | 16273 | 64 | 82983 | 27 | 553 | 4 | 1555.4 | 2 | 9 | 264 | 6299 | 178 |
| L. erinacea | 7782 | 3 | 7 | 13 | 284 | 1 | 31167 | 5 | 0 | 0 | 1555.4 | 1 | 2 | 6 | 123 | 0 |
| S. canicula | 7830 | 2 | 8 | 13 | 645 | 1 | 1600 | 7 | 0 | 0 | 0 | 1 | 1 | 38 | 283 | 1 |
(WMG) whole mitochondrial genome, (EST) Expressed Sequence Tags, (lib) libraries (GSS) Genome Survey Sequences, (GEO) Gene Expression Omnibus, (WGS) Whole Genome Shotgun, (SRA) Sequence Read Archive, (WMG) whole mitochondrial genomes, (PDB) Protein Data Bank, * includes 16 unidentified fin entries
1 NCBI databases accessed July 25, 2014, 2 Release 2014_07 of 09-Jul-2014, 3 GEO sample accessions
Chondrichthyan genomes
Currently there are multiple efforts to sequence an elasmobranch genome in various stages of completion ( Table 2); however, only the skate genome project currently has data publically available. Efforts to sequence the whale shark are underway at the Georgia Aquarium and Emory University (personal communication, Alistair Dove, Georgia Aquarium). Genoscope leads a project to sequence the genome of another oviparous elasmobranch, the catshark, S. canicula. The current assembly is described in Table 2. A second version of the catshark genome with 200x coverage, including mate pair sequencing, is in progress (personal communication, Sylvie Mazan, French National Centre for Scientific Research). Among holocephalans, the genome of the elephant shark, C. milii, was first described in a 1.4x coverage assembly in 2006 44. With continued sequencing the assembly coverage is currently 19.25x and data has been made available through the project website ( http://esharkgenome.imcb.a-star.edu.sg/) and Genbank 43.
Table 2. Chondrichthyan genome sequencing projects.
| Website | Genome
size (Gb) |
Coverage | Contigs | N50
(bp) |
Platform | Facility | Genbank | Data 1 | BioProject | BioSample | Date | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Holocephali | ||||||||||||
| Callorhinchus milii | esharkgenome | 0.910 | 19.25x | 21,203 | 1466 | Sanger
& 454 |
IMCB | AAVX02000000 | 244 M | PRJNA236996 | SAMN00000800 | 20-Dec-13 * |
| Elasmobranchii | ||||||||||||
| Leucoraja erinacea | skatebase.org | 3.42 | 26x | 2,62,365 | 665 | Illumina
PE |
NECC | AESE010000000 | 105 G | PRJNA60893 | SAMN00189066 | 22-Dec-11 |
| Scyliorhinus canicula | - | 3.5 | 32x | 3,449,662 | 1,292 | Illumina
PE |
Genoscope-CEA | - | - | - | - | - |
| Rhincodon typus | - | 3.44(est.) | 35x | Illumina
& 454 |
Emory University
& Georgia Aquarium |
- | - | PRJNA255419 |
SAMN02918461
SAMN02918462 |
16-Jul-14 | ||
1 (M) Mega or (G) Giga base pairs; (PE) paired end; (est) estimated; (ICMB) Institute of Molecular and Cell Biology, A*STAR, (NECC) North East Cyberinfrastructure Consortium
* replaced original sequence data GenBank AAVX00000000.1 (1.4x coverage) released 20-DEC-2006
A powerful resource for characterizing genomes is large-insert clone libraries where each clone contains a large (~100kb) genomic region. Bacterial artificial chromosome (BAC) and P1-derived artificial chromosome (PAC) libraries are DNA constructs within a plasmid used to transform bacteria. As the bacteria grow the inserted DNA is amplified and subsequently isolated and sequenced. BACs are beneficial for genome sequencing projects because the insert size can be very large, nearly 350 kb, facilitating assembly post-sequencing. BAC/PAC libraries were built for several chondrichthyan species including the nurse shark, Ginglymostoma cirratum 45; elephant shark, C. milli 46; little skate, L. erinacea 47; horn shark, Heterdontus francisci 48; dogfish shark, Squalus acanthias 49, 50; and catshark, S. canicula 51. These libraries were used to successfully characterize a handful of genomic regions such as little skate HoxA cluster 47, 52, horn shark Hox A and D clusters 53, catshark HoxA, B and D clusters 51, 52, C. milii HoxA-D clusters 54, immunoglobulin receptor IgW C regions 30 and neurohypophysial gene loci 46.
RNA databases
Transcriptome sequencing seeks to characterize all genes expressed in a tissue or set of tissues in a sample. Technologies to identify the complete RNA transcript sequence have developed from studies of a small number of transcripts to comprehensive characterizations. The application of large-scale cDNA cloning of Expressed Sequence Tags (ESTs) gave initial characterizations of 5-prime and/or 3-prime ends of transcripts in several elasmobranchs including L. erinacea and S. acanthias ( Table 3). EST sequence data are available in the EST divisions of the GenBank, EMBL and DDBJ databases that make up the INSDC. cDNA clones and their sequences from these EST projects have enabled the complete characterization of the full-length cDNA sequence of several genes. In the last five years, high-throughput RNA sequencing (RNA-Seq) has been applied to comprehensively examine the complete sequence of transcripts in tissues of cartilaginous fishes. Among the most valuable RNA-Seq datasets are those from whole late-stage embryos following organogenesis. Our project has generated these datasets for L. erinacea, S. canicula and C. milii 52. Public RNA-Seq data sets can be found in the NCBI Gene Expression Omnibus and Short Read Archive (SRA) databases or the EBI ArrayExpress and European Nucleic Acid (ENA) archives ( Table 3 and Table 4).
Table 3. National Center for Biotechnology Information (NCBI) Expressed Sequence Tags (EST) and Genome Survey Sequences (GSS) databases (release 130101): Chondrichthyan sequence data.
| BioSample | BioSample Description | Library ID | Organism | Sample
age/sex |
Sample type | ESTs | Facility 1 | Date |
|---|---|---|---|---|---|---|---|---|
| Holocephali | ||||||||
| Chimaeriformes | ||||||||
| 182978 | Whole-genome shotgun library of the elephant shark (aka
elephant fish) |
GSS: LIBGSS_009694 | Callorhinchus milii | - | testis | 27944 | IMCB | 2004 |
| 1000678 | Elephant shark full- length cDNA library from testis | EST: LIBEST_027873 | Callorhinchus milii | - | testis | 29234 | IMCB | 2012 |
| 1000677 | Elephant shark full- length cDNA library from spleen | EST: LIBEST_027872 | Callorhinchus milii | - | spleen | 16664 | IMCB | 2012 |
| 1000676 | Elephant shark full- length cDNA library from liver | EST: LIBEST_027871 | Callorhinchus milii | - | liver | 16573 | IMCB | 2012 |
| 1000675 | Elephant shark full- length cDNA library from kidney | EST: LIBEST_027870 | Callorhinchus milii | - | kidney | 19246 | IMCB | 2012 |
| 1000674 | Elephant shark full- length cDNA library from intestine | EST: LIBEST_027869 | Callorhinchus milii | - | intestine | 12146 | IMCB | 2012 |
| 1000673 | Elephant shark full- length cDNA library from gills | EST: LIBEST_027868 | Callorhinchus milii | - | gills | 16012 | IMCB | 2012 |
| Elasmobranchii: Batoids (rays and skates) | ||||||||
| Torpediformes | ||||||||
| 158311 | Torpedo marmorata electric organ | EST: LIBEST_003755 | Torpedo marmorata | - | electric organ | 8 | CNRS | 2000 |
| 158310 | Torpedo marmorata electric lobe | EST: LIBEST_003754 | Torpedo marmorata | - | electric lobe | 26 | CNRS | 2000 |
| 157461 | pFL61-TEL | EST: LIBEST_002905 | Torpedo marmorata | - | electric lobe | 2 | CNRS | 2000 |
| 157406 | pFL61-EL | EST: LIBEST_002849 | Torpedo marmorata | - | electric lobe | 5 | CNRS | 2000 |
| 154382 | Torpedo californica electric organ | EST: LIBEST_020696 | Torpedo californica | - | electric organ | 10185 | Children’s National
Medical Center, USA |
2006 |
| Rajiformes | ||||||||
| 175126 | Little Skate Multiple Tissues, Normalized | EST: LIBEST_015890 | Leucoraja erinacea | adult | mixed a | 5698 | MDIBL | 2004 |
| 176484 | Little Skate Liver, Normalized | EST: LIBEST_017626 | Leucoraja erinacea | adult | liver | 6016 | MDIBL | 2005 |
| 165533 | Little Skate embryo cell line 1 (LEE-1): 5' sequences | EST: LIBEST_022984 | Leucoraja erinacea | embryonic
cell line |
stage 28 | 4825 | MDIBL | 2006 |
| 154366 | Little skate embryo tissues; 5' sequences | EST: LIBEST_020422 | Leucoraja erinacea | embryo | stage 19, 20, 25 | 5600 | MDIBL | 2006 |
| 166469 | Skate Multiple Tissues, Normalized | EST: LIBEST_023576 | Leucoraja erinacea | adult | mixed a | 9028 | MDIBL | 2008 |
| Elasmonbranchii: Selachii (sharks) | ||||||||
| Carcharhiniformes | ||||||||
| 168576 | Dogfish testis - round spermatids zone (SSH) | EST: LIBEST_025578 |
Scyliorhinus
canicula |
adult | testis | 20 | Caen University | 2009 |
| 168575 | Dogfish testis - spermatogonia zone (SSH) | EST: LIBEST_025577 |
Scyliorhinus
canicula |
adult | testis | 12 | Caen University | 2010 |
| 222714 | Scyliorhinus canicula juvenile library | EST: LIBEST_026904 |
Scyliorhinus
canicula |
juvenile | 5 days post-hatch | 56 | enoscope-CEA | 2011 |
| 222713 | Scyliorhinus canicula embryonic, stages 9–15 library | EST: LIBEST_026903 |
Scyliorhinus
canicula |
embryo | stages 9–15 | 628 | Genoscope-CEA | 2011 |
| 222712 | Scyliorhinus canicula embryonic, stages 19–25 library | EST: LIBEST_026902 |
Scyliorhinus
canicula |
embryo | stages 19–25 | 772 | Genoscope-CEA | 2011 |
| 222711 | Scyliorhinus canicula embryonic, stages 19–24 library | EST: LIBEST_026901 |
Scyliorhinus
canicula |
embryo | stages 19–24 | 33 | Genoscope-CEA | 2011 |
| 222710 | Scyliorhinus canicula adult brain library | EST: LIBEST_026900 |
Scyliorhinus
canicula |
adult | brain | 79 | Genoscope-CEA | 2011 |
| 699400 | cloudy catshark embryo cDNA library | EST: LIBEST_027410 |
Scyliorhinus
torazame |
embryo | stage 31 | 2942 | RIKEN | 2011 |
| Orectolobiformes | ||||||||
| 183175 | GC__Ba | GSS: LIBGSS_009945 |
Ginglymostoma
cirratum |
adult | red blood cells | 178 | University of
Arizona |
2005 |
| 184343 | shark whole genome shotgun library 2 | GSS: LIBGSS_011249 |
Chiloscyllium
plagiosum |
female | ventral fin | 177 | Tgen | 2008 |
| 184342 | shark whole genome shotgun library 1 | GSS: LIBGSS_011248 |
Chiloscyllium
plagiosum |
female | ventral fin | 194 | Tgen | 2008 |
| 166749 | Shark liver regeneration | EST: LIBEST_023789 |
Chiloscyllium
plagiosum |
adult | liver | 2103 | BGI | 2008 |
| 176026 | cDNA library of Shark hepatic regeneration tissues | EST: LIBEST_017019 |
Chiloscyllium
plagiosum |
none | Hour 24 after 2/3
partial hepatectomy |
17 | CPU | 2005 |
| 254067 | Toll like receptor ligand induced Spleen | EST: LIBEST_027180 |
Chiloscyllium
griseum |
male | spleen | 1051 | MVC | 2011 |
| 254066 | Spleen of Chiloscyllium griseum | EST: LIBEST_027179 |
Chiloscyllium
griseum |
male | spleen | 1000 | MVC | 2011 |
| 1797282 | Suppressive subtractive hybridization library from
peptidoglycan induced spleen of the shark |
EST: LIBEST_028031 |
Chiloscyllium
griseum |
male | spleen | 315 | MVC | 2012 |
| Squaliformes | ||||||||
| 175664 | Dogfish Shark Multiple Tissues, Normalized | EST: LIBEST_016552 | Squalus acanthias | adult | mixed b | 15078 | MDIBL | 2004 |
| 176998 | Dogfish Shark Embryo-derived Cell Line SAE, Normalized | EST: LIBEST_018195 | Squalus acanthias | embryonic
cell line |
embryo with
external yolk sac |
5824 | MDIBL | 2005 |
| 154362 | Spiny dogfish shark rectal gland EST library | EST: LIBEST_020417 | Squalus acanthias | - | rectal gland | 5085 | MDIBL | 2006 |
| 150616 | Dogfish Shark Rectal Gland, Normalized | EST: LIBEST_020023 | Squalus acanthias | adult | rectal gland | 6575 | MDIBL | 2006 |
| Hexanchiformes | ||||||||
| 178140 | Hexanchus griseus DNA (Hunter C) | GSS: LIBGSS_003277 |
Hexanchus
griseus |
- | - | 4 | HGMP-RC | 2001 |
1 (ICMB) Institute of Molecular and Cell Biology, A*STAR, (HGMP-RC) Human Genome Mapping Project Resource Centre, Hinxton, (Tgen) Translational Genomics Research Institute AZ, USA, (CNRS) National Center for Scientific Research, France, (MDIBL) Mount Desert Island Biological Laboratory, (CPU) China Pharmaceutical University, (MVC) Madras Veterinary College, TANUVAS, (BGI) Beijing Genomics Institute (SSH) Suppressive subtractive hybridization; (mixed a) liver, kidney, brain, testis, ovary, gill, heart, spleen, rectal gland; (mixed b) rectal gland, kidney, brain, testis, ovary, gill, intestine, heart, spleen
Table 4. National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database: Chondrichthyan sequence data.
| BioProject | BioSample | SRA description | SRA | Organism | Age | Sample
type |
Platform 1 | Data 2 | Facility 3 | Date | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Holocephali | |||||||||||
| PRJNA18361 | SAMN00000800 | 454 sequencing of Callorhinchus milii
genomic fragment library |
SRX001870 * | Callorhinchus milii | adult | testis | LS454 | 244.9 M | IMCB | 2008 | |
| PRJNA135005 | SAMN00188955 |
GSM643959: Callorhinchus milii pooled
Stage 32 embryos |
SRX036538 | Callorhinchus milii | embryos | stage 32 | Illumina SE | 3.3 G | MDIBL | 2011 | |
| PRJNA168475 | SAMN02699939 | Illumina sequencing of elephant shark
thymus RNA |
SRX220387 | Callorhinchus milii | - | thymus | Illumina PE | 9.7 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699938 | Illumina sequencing of elephant shark
testis RNA |
SRX154861 | Callorhinchus milii | - | testis | Illumina PE | 7.3 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699937 | Illumina sequencing of elephant shark
spleen RNA |
SRX154860 | Callorhinchus milii | - | spleen | Illumina PE | 6.3 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699936 | Illumina sequencing of elephant shark
ovary RNA |
SRX154859 | Callorhinchus milii | - | ovary | Illumina PE | 7.9 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699935 | Illumina sequencing of elephant shark
liver RNA |
SRX154858 | Callorhinchus milii | - | liver | Illumina PE | 16.7 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699934 | Illumina sequencing of elephant shark
muscle RNA |
SRX154857 | Callorhinchus milii | - | muscle | Illumina PE | 11.1 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699933 | Illumina sequencing of elephant shark
kidney RNA |
SRX154856 | Callorhinchus milii | - | kidney | Illumina PE | 9 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699932 | Illumina sequencing of elephant shark
intestine RNA |
SRX154855 | Callorhinchus milii | - | intestine | Illumina PE | 11.2 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699931 | Illumina sequencing of elephant shark
heart RNA |
SRX154854 | Callorhinchus milii | - | heart | Illumina PE | 6.9 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699930 | Illumina sequencing of elephant shark
gills RNA |
SRX154852 | Callorhinchus milii | - | gills | Illumina PE | 5.4 G | IMCB | 2013 | |
| PRJNA168475 | SAMN02699929 | Illumina sequencing of elephant shark
brain RNA |
SRX154851 | Callorhinchus milii | - | brain | Illumina PE | 10.5 G | IMCB | 2013 | |
| Elasmobranchii | |||||||||||
| PRJNA60893 | SAMN00189066 | Initial Characterization of Leucoraja
erinacea Genome Using 500bp Paired- End Sequencing |
SRX036634 * | Leucoraja erinacea | embryo | stage 32 | Illumina PE | 105 G | NECC | 2011 | |
| PRJNA135005 | SAMN00188953 |
GSM643957: Leucoraja erinacea pooled
Stage 20–29 embryos |
SRX036536 | Leucoraja erinacea | embryos | stage
20–29 |
Illumina SE | 3.8 G | MDIBL | 2011 | |
| PRJNA135005 | SAMN00188954 |
GSM643958: Scyliorhinus canicula
pooled Stage 24–30 embryos |
SRX036537 | Scyliorhinus canicula | embryos | stage
24–30 |
Illumina SE | 3.9 G | MDIBL | 2011 | |
| PRJDA61447 | SAMD00003843 | Torazame EST | DRX000491 | Scyliorhinus torazame | embryos | stage
23–31 |
LS454 | 43.6 M | RIKEN | 2011 | |
| PRJNA177971 | SAMN01915239 | Carcharodon carcharias cDNA Illumina
sequence reads |
SRX228421 | Carcharodon carcharias | juvenile | heart | Illumina SE | 7.9 G | Cornell | 2013 | |
| PRJNA177971 | SAMN01915239 | Carcharodon carcharias heart
transcriptome |
SRX228332 | Carcharodon carcharias | juvenile | heart | LS454 | 408.4 M | Cornell | 2013 | |
| PRJNA183979 | SAMN01831510 | Illumina sequencing of Nurse Shark
thymus transcripts |
SRX219866 | Ginglymostoma cirratum | - | thymus | Illumina PE | 12 G | IMCB | 2013 | |
| PRJNA183979 | SAMN01831509 | Illumina sequencing of Nurse Shark
spleen transcripts |
SRX219865 | Ginglymostoma cirratum | - | spleen | Illumina PE | 11.2 G | IMCB | 2013 | |
| PRJNA240112 | SAMN02673223 | Neotrygon kuhlii barb venom gland
transcriptome |
SRX481088 | Neotrygon kuhlii | - | barb
venom gland |
Illumina PE | 84.3 M | LSTM | 2014 | |
* genomic data; 1 (SE) single end or (PE) paired end; 2 (M) Mega or (G) Giga base pairs
3 (MDIBL) Mount Desert Island Biological Laboratory, (ICMB) Institute of Molecular and Cell Biology, A*STAR, (LSTM) Liverpool School of Tropical Medicine, (NECC) North East Cyberinfrastructure Consortium
Mitochondrial genomes
Individual mitochondrial genes such as cytochrome c oxidase subunit I (CO1 or COX1) and NADH-ubiquinone oxidoreductase chain 2 (NADH2 or MT-ND2) have been used extensively to construct molecular phylogenies 55– 57. The Fish barcode of life (FISH-BOL) a working group of the International Barcode of Life Project (iBOL), has CO1 barcodes for 54% of elasmobranchs and 62% of holocephalans ( http://www.fishbol.org, accessed July 24, 2014). Recently, whole mitochondrial sequences are increasingly popular for their increased granularity when resolving branches of phylogenetic trees 1. Whole mitochondrial genome sequences currently are available for 72 species of sharks, skates, rays and chimaeras. These sequences are accessible in the GenBank, EMBL and DDBJ databases summarized in Table 5 58.
Table 5. Whole mitochondrial sequences for chondrichthyan fishes.
| Accessions | ||||||
|---|---|---|---|---|---|---|
| BioProject | NCBI Ref_seq | GenBank | Organism | bp | *G+C | Date |
| Holocephali | ||||||
| Chimaeriformes | ||||||
| PRJNA50265 | NC_014281.1 | HM147135.1 | Callorhinchus callorynchus | 16758 | 34 | 21-Oct-10 |
| PRJNA50271 | NC_014284.1 | HM147136.1 | Callorhinchus capensis | 16760 | 34.1 | 21-Oct-10 |
| PRJNA50273 | NC_014285.1 | HM147137.1 | Callorhinchus milii | 16769 | 33.7 | 21-Oct-10 |
| PRJNA11978 | NC_003136.1 | AJ310140.1 | Chimaera monstrosa | 18580 | 38.6 | 14-Nov-06 |
| PRJNA50279 | NC_014288.1 | HM147138 | Chimaera fulva | 21336 | 38.2 | 19-Oct-10 |
| PRJNA50287 | NC_014292.1 | HM147140.1 | Harriotta raleighana | 18024 | 42.5 | 19-Oct-10 |
| PRJNA50283 | NC_014290.1 | HM147139.1 | Hydrolagus lemures | 21233 | 39.4 | 19-Oct-10 |
| PRJNA50289 | NC_014293.1 | HM147141.1 | Rhinochimaera pacifica | 24889 | 41.6 | 19-Oct-10 |
| Elasmobranchii: Batoids (rays and skates) | ||||||
| Myliobatiformes | ||||||
| PRJNA247653 | NC_024102.1 | KJ617038.1 | Gymnura poecilura | 17874 | 45.1 | 7-May-14 |
| PRJNA239601 | NC_023525.1 | KF751650.1 | Himantura granulata | 17657 | 39.1 | 25-Feb-14 |
| PRJNA229016 | NC_022837.1 | KF482070.1 | Aetobatus flagellum | 20201 | 40.9 | 3-Nov-13 |
| PRJNA198706 | NC_021132.1 | KC526959.1 | Dasyatis akajei | 17658 | 40.4 | 10-Mar-14 |
| PRJNA190131 | NC_020352.2 |
KC196067.2
KC633222.1 |
Dasyatis bennetti
Dasyatis bennetti |
17668
17717 |
40.2
40.1 |
22-Jul-13
20-Feb-14 |
| PRJNA182669 | NC_019643.1 | JX524174.1 | Dasyatis zugei | 18264 | 36.6 | 24-May-13 |
| PRJNA15549 | NC_007230.1 | AY597334.1 | Plesiobatis daviesi | 17514 | 41.9 | 20-Mar-07 |
| PRJNA232219 | NC_023116 | KF709642.1 | Potamotrygon motoro | 17448 | 43.3 | 14-Jan-14 |
| PRJNA177278 | NC_018784.1 | JX392983.1 | Mobula japanica | 18880 | 37.4 | 18-Jan-13 |
| PRJNA212605 | NC_021767.1 | KC992792.1 | Neotrygon kuhlii | 18039 | 39.5 | 17-Jul-13 |
| PRJNA182647 | NC_019641.1 | JX827260.1 | Taeniura meyeni | 17638 | 41.6 | 8-Nov-13 |
| Rajiformes | ||||||
| PRJNA239623 | NC_023505.2 | KF318309.2 | Dipturus kwangtungensis | 16912 | 41.6 | 13-Mar-14 |
| PRJNA81399 | NC_016429.1 | JQ034406.1 | Leucoraja erinacea | 16724 | 40.3 | 28-Nov-11 |
| PRJNA13984 | NC_007173.1 | AY525783.1 | Okamejei kenojei | 16972 | 42.4 | 15-Jun-05 |
| PRJNA11877 | NC_000893.1 | AF106038.1 | Amblyraja radiata | 16783 | 40.3 | 22-Apr-09 |
| PRJNA214406 | NC_021964 | KC914434.1 | Raja rhina | 16910 | 41.4 | 11-Sep-13 |
| PRJNA214407 | NC_021963.1 | KC914433.1 | Hongeo koreana | 16905 | 42.2 | 11-Sep-13 |
| PRJNA244226 | NC_023944.1 | KF648508.1 | Zearaja chilensis | 16909 | 41.1 | 1-May-14 |
| Rhinopristiformes | ||||||
| PRJNA228994 | NC_022821.1 | KF381507.1 | Pristis clavata | 16804 | 39.8 | 13-Nov-13 |
| PRJNA229000 | NC_022841.1 | KF534708.1 | Rhinobatos hynnicephalus | 16776 | 40.3 | 13-Nov-13 |
| PRJNA244205 | NC_023951.1 | KJ140136.1 | Rhinobatos schlegelii | 16780 | 39.6 | 6-Apr-14 |
| Elasmonbranchii: Selachii (sharks) | ||||||
| Carcharhiniformes | ||||||
| PRJNA246074 | NC_024055.1 | KF728380.1 | Carcharhinus acronotus | 16719 | 38.4 | 29-Apr-14 |
| PRJNA244183 | NC_023948.1 | KF956523.1 | Carcharhinus amblyrhynchoides | 16705 | 38.2 | 6-Apr-14 |
| PRJNA239607 | NC_023522.1 | KF646785.1 | Carcharhinus leucas | 16704 | 37.4 | 25-Feb-14 |
| PRJNA252486 | NC_024284.1 | KJ720818.1 | Carcharhinus melanopterus | 16706 | 38.6 | 7-Jun-14 |
| PRJNA193929 | NC_020611.1 | KC470543.1 | Carcharhinus obscurus | 16706 | 38.6 | 8-Nov-13 |
| PRJNA239626 | NC_023521.1 | KF612341.1 | Carcharhinus sorrah | 16707 | 38.9 | 25-Feb-14 |
| PRJNA217222 | NC_022193.1 | KF111728.1 | Galeocerdo cuvier | 16703 | 36.9 | 31-Oct-13 |
| PRJNA236275 | NC_023361.1 | KF646786.1 | Glyphis garricki | 16702 | 39.2 | 13-Jan-14 |
| PRJNA212606 | NC_021768.2 | KF006312.2 | Glyphis glyphis | 16701 | 39 | 25-Jul-14 |
| PRJNA239588 | NC_023527.1 | KF889325.1 | Mustelus griseus | 16754 | 39 | 25-Feb-14 |
| PRJNA11875 | NC_000890.1 | AB015962.1 | Mustelus manazo | 16707 | 38.3 | 8-Apr-00 |
| PRJNA228986 | NC_022819.1 | KF356249.1 | Prionace glauca | 16705 | 37.5 | 13-Nov-13 |
| PRJNA226181 | NC_022735.1 | AB560493.1 | Pseudotriakis microdon | 16700 | 36.4 | 29-Oct-13 |
| PRJNA168394 | NC_018052.1 | JQ693102.1 | Scoliodon macrorhynchos | 16693 | 37 | 31-Mar-14 |
| PRJNA11849 | NC_001950.1 | Y16067.1 | Scyliorhinus canicula | 16697 | 38 | 18-Apr-05 |
| PRJNA226138 | NC_022679.1 | JX827259.1 | Sphyrna lewini | 16726 | 39.5 | 8-Nov-13 |
| Orectolobiformes | ||||||
| PRJNA163947 | NC_017882.1 | JQ434458.1 | Chiloscyllium griseum | 16755 | 36.1 | 6-Mar-12 |
| PRJNA37667 | NC_012570.1 | JX162601.1 | Chiloscyllium plagiosum | 16725 | 37.4 | 25-Jul-12 |
| PRJNA81281 | NC_016686.1 | JQ082337.1 | Chiloscyllium punctatum | 16703 | 36.8 | 31-Mar-14 |
| PRJNA217221 | NC_022148.1 | KF111729.1 | Orectolobus japonicus | 16706 | 37.3 | 19-Sep-13 |
| PRJNA238093 | NC_023455.1 |
KF679782.1
KC633221 |
Rhincodon typus
Rhincodon typus |
16875
16928 |
37.1
37.1 |
19-Mar-14
31-Mar-14 |
| Lamniformes | ||||||
| PRJNA239610 | NC_023520.1 | KF569943.1 | Carcharias taurus | 16773 | 39.5 | 5-Feb-14 |
| PRJNA221185 | NC_022415.1 | KC914387.1 | Carcharodon carcharias | 16744 | 40.8 | 31-Oct-13 |
| PRJNA232870 | NC_023266.1 | KF597303.1 | Cetorhinus maximus | 16670 | 40.6 | 14-Jan-14 |
| PRJNA226140 | NC_022691.1 | KF361861.1 | Isurus oxyrinchus | 16701 | 43.2 | 28-Sep-13 |
| PRJNA247657 | NC_024101.1 | KJ616742.1 | Isurus paucus | 16704 | 43.8 | 7-May-14 |
| PRJNA252473 | NC_024269.1 | KF962053.1 | Lamna ditropis | 16699 | 41.8 | 30-May-14 |
| PRJNA207613 | NC_021442.1 | KC702506.1 | Megachasma pelagios | 16694 | 36.7 | 13-May-13 |
| PRJNA33525 | NC_011825.1 | EU528659.1 | Mitsukurina owstoni | 17743 | 38.8 | 29-Dec-08 |
| PRJNA228992 | NC_022822.1 | KF412639.1 | Alopias pelagicus | 16692 | 38.6 | 18-Dec-13 |
| PRJNA207614 | NC_021443.1 | KC757415.1 | Alopias superciliosus | 16719 | 39.3 | 26-Jun-13 |
| Heterodontiformes | ||||||
| PRJNA11979 | NC_003137.1 | AJ310141.1 | Heterodontus francisci | 16708 | 39.9 | 14-Nov-06 |
| PRJNA209901 | NC_021615.1 | KC845548.1 | Heterodontus zebra | 16720 | 40 | 18-Jun-13 |
| Squaliformes | ||||||
| PRJNA246067 | NC_024059.1 | KJ128289.1 | Cirrhigaleus australis | 16543 | 38.8 | 29-Apr-14 |
| PRJNA226141 | NC_022734.1 | AB560492.1 | Somniosus pacificus | 16730 | 39.3 | 29-Oct-13 |
| PRJNA11856 | NC_002012.1 | Y18134.1 | Squalus acanthias | 16738 | 38.8 | 18-Apr-05 |
| Squatiniformes | ||||||
| PRJNA252467 | NC_024276.1 | KJ619663.1 | Squatina japonica | 16689 | 37.9 | 4-Jun-14 |
| Pristiophoriformes | ||||||
| PRJNA247682 | NC_024110.1 | AB721306.1 | Pristiophorus japonicus | 18430 | 44.5 | 10-May-14 |
| Hexanchiformes | ||||||
| PRJNA226134 | NC_022732.1 | AB560490.1 | Hexanchus griseus | 17223 | 36.3 | 29-Oct-13 |
| PRJNA226149 | NC_022733.1 | AB560491.1 | Hexanchus nakamurai | 18605 | 36.3 | 29-Oct-13 |
| PRJNA226155 | NC_022730.1 | AB560488.1 | Heptranchias perlo | 18909 | 35.9 | 29-Oct-13 |
| PRJNA226147 | NC_022729.1 | AB560487.1 | Chlamydoselachus anguineus | 17314 | 35 | 29-Oct-13 |
| PRJNA226123 | NC_022731.1 | AB560489.1 | Notorynchus cepedianus | 16990 | 38.2 | 29-Oct-13 |
*Metazoan Mitochondrial Genomes Accessible dataset Metamiga ( http://amiga.cbmeg.unicamp.br/)
Chondrichthyan Tree of Life
Currently, molecular data for cartilaginous fishes is being collected as part of the Chondrichthyan Tree of Life project ( http://sharksrays.org). The project website currently includes 5 elements: 1) an interactive phylogenetic tree 55; 2) scientific illustrations of specimens; 3) range information for all extant species; 4) interactive comparative anatomy through segmented CT scan data; and 5) DNA sequence for 1265 single copy orthologous genes 59. Project data will be available in public databases as well as through the project website once collection and analysis is complete (personal communication, Gavin Naylor, Medical University of South Carolina).
Protein databases
Given the improved technologies to characterize full-length transcripts using RNA-Seq, there are increasingly more protein sequence data for chondrichthyans. The UniProt Consortium, consists of groups from the European Bioinformatics Institute (EBI), the Swiss Institute of Bioinformatics (SIB) and the Protein Information Resource (PIR). The consortium maintains the UniProt Knowledgebase (UniProtKB), a comprehensive and standardized catalogue of protein sequences and functional annotation knowledgebase 60. Proteins with UniProtKB accessions are first automatically annotated, unreviewed UniProtKB/TrEMBL entries that progress to UniProtKB/Swiss-Prot entries following curator review. Among Chondrichthyes, there are 12 UniProtKB/Swiss-Prot and 20,170 UniProtKB/TrEMBL entries for holocephalans and 264 UniProtKB/Swiss-Prot and 6,299 UniProtKB/TrEMBL entries for elasmobranchs in Release 2014_07 of 09-Jul-2014 of the knowledgebase ( Table 1). An unidentified fin sample accounts for 16 UniProtKB/TrEMBL entries that are not included in either Holocephali or Elasmobranchii. PDB, an archive of protein macromolecular structural data, has 178 entries for Chondrichthyes, all elasmobranchs 61. Of these, 76% are derived from 2 species from a single family, Torpediniformes, the electric rays, and in total only 10 species are represented in PDB.
The distribution of data in NCBI databases, PDB, and UniProtKB for chondrichthyan orders is shown in Figure 4. When order Chimaeriformes is included ( Figure 4A) the distributions are disproportionate due to the large volume of annotated sequence data from the elephant shark genome. The distributions are repeated exclusively for elasmobranchs. To understand if the data distribution is representative of the number of species in each order, a species distribution is included in each chart. A cladogram ( Figure 4B) is linked to the chart legend and illustrates the phylogeny between chondrichthyan orders.
Figure 4. A survey of public data and phylogeny for chondrichthyan orders.
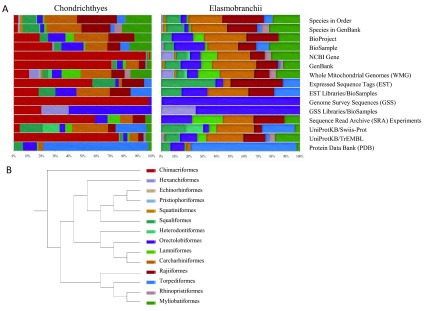
A. The 14 orders of chondrichthyan fish and their relative distribution in public nucleotide and protein databases for Chondrichthyes and Elasmobranchii are shown individually. The species distribution for each Order and GenBank are similar indicating sequence data has been collected for a broad range of chondrichthyans. For Chondrichthyes, the elephant shark genome project data contributes the majority of the data in NCBI Gene, GenBank, Genome Survey Sequence (GSS), and the Short Reach Archive (SRA) databases. The NCBI GSS, GSS libraries, and Protein Data Bank (PDB) are the least diverse with representation of 1–6 of the 14 Orders. The color of each Order as represented in the bar chart is included in the cladogram key with left to right in the bar chart corresponding with top to bottom in the cladogram. B. A cladogram of Chondrichthyes illustrates the phylogeny relationship between the 14 Orders. The color code associated with each Order appears consecutively in the bar chart.
SkateBase
SkateBase ( http://skatebase.org) is the public portal for the little skate genome project and is a valuable collection of data and learning resources. The NEBC little skate genome project team hosted three week-long workshops and a mitochondrial genome annotation jamboree with the goal of using the project data to develop a bioinformatics aware workforce and foster collaborative and distributed big data research. The lecture materials and worked annotation examples are included at SkateBase for educational use ( http://skatebase.org/workshops). The project vitae contains an overview and timeline of the genome project effort along with key personnel, project related publications and presentations, the curation team, and citation information for researchers utilizing the resource in their publication. A Gene Table currently represents manually curated genes derived from workshops and curriculum with extensive annotation evidence. The number of gene entries will continue to grow through usage and expansion of the SkateBase educational modules. Plans to update the annotation interface to enable community annotation by domain experts is planned for the future.
SkateBase provides links to web resources with chondrichthyan data including the Chondrichthyan Tree of Life, Elephant Shark Genome Project ( http://esharkgenome.imcb.a-star.edu.sg), the first described genome for a chimaera 43, and Vertebrate TimeCapsule, ( http://transcriptome.cdb.riken.go.jp/vtcap), a project that aims to develop a gene database to represent evolution and development for vertebrates and currently includes transcriptome data for a hagfish ( Eptatretus burger), shark ( S. torazame) and birchir ( Polypterus senegalus) 62. SkateBase data is linked locally as well as from NCBI in the Gene Expression Omnibus (GSE26235), GenBank (AESE010000000) and Sequence Read Archive (SRA026856) to ensure convenient and easy access. A link to the American Elasmobranch Society ( http://www.elasmo.org), a non-profit organization with the mission of advancing the scientific study of living and fossil sharks, skates, rays, and chimaeras and promoting education, conservation, and wise utilization of natural resources, connects domain scientists to the little skate genome project.
SkateBase data includes embryonic transcriptomes for three chondrichthyan species, a chimaera, C. milii, a shark, S. canicula and the little skate, L. erinacea as well as the first draft of the little skate genome. The assembled skate genome sequence gave a single high-coverage contiguous sequence that represented the entire length of the mitochondrial genome. The mitochondrial genome was subsequently annotated as part of a Jamboree in 2011 63. The annotated sequence is represented by the NCBI Reference Sequence (RefSeq) project, accession NC_016429, and provides extensive information for each gene.
Whole embryos were used to build the transcriptome libraries available at SkateBase 35. Two C. milii embryos, stage 32, were combined and used to build a chimaera library. The transcriptome library for S. canicula was assembled from six pooled embryos, stages 24–30. The embryonic skate transcriptome library was assembled using six pooled embryos ranging in stage from 20–29. This combination of stages encompasses a large portion of the developmental period for these fishes and represents a catalog of genes important for organogenesis of all or part of every physiological system. Early developmental events are similar for nearly all elasmobranchs regardless of reproductive mode or adult body form enabling the data to be useful for more than just the specific species from where it was derived 64. Since all three embryonic transcriptomes contain a similar stage embryo direct comparison for temporal expression patterns is possible. Skatebase includes tools for data investigation, SkateBLAST, a sequence retrieval tool, Skate Contig Lookup, and genome browsers for three skate whole mitochondrial sequences, L. erinacea, the thorny skate, Amblyraja radiata, and, the ocellate spot skate, Okamejei kenojei. Skatebase contains resources that can be used for teaching and research purposes. As an example, two use cases follow, one for sequence or homology based research and the other for education.
SkateBLAST
A common task for researchers is searching for genes of interest in a genome or transcriptome. Knowledge of the gene sequence at the DNA or RNA level is needed for many different studies, including phylogenetic analysis or designing primers for quantitative PCR gene expression studies. Here we describe the major steps necessary to identify relevant sequences for a gene of interest using the BLAST sequence similarity tool at SkateBase. SkateBase features a web interface to BLAST, named SkateBLAST, that builds upon the ViroBLAST package version 2.2 65, with custom modifications allowing parallel cluster-based execution of queries and enhanced display of results. The overall workflow consists of a) entering a query sequence and selecting the database to search; b) evaluating the alignments returned; c) retrieving the sequence from one of the SkateBLAST databases; and d) checking to make sure that the retrieved sequence aligns best to the query sequence. The following description provides a brief tutorial on the overall workflow while describing tools at SkateBase.
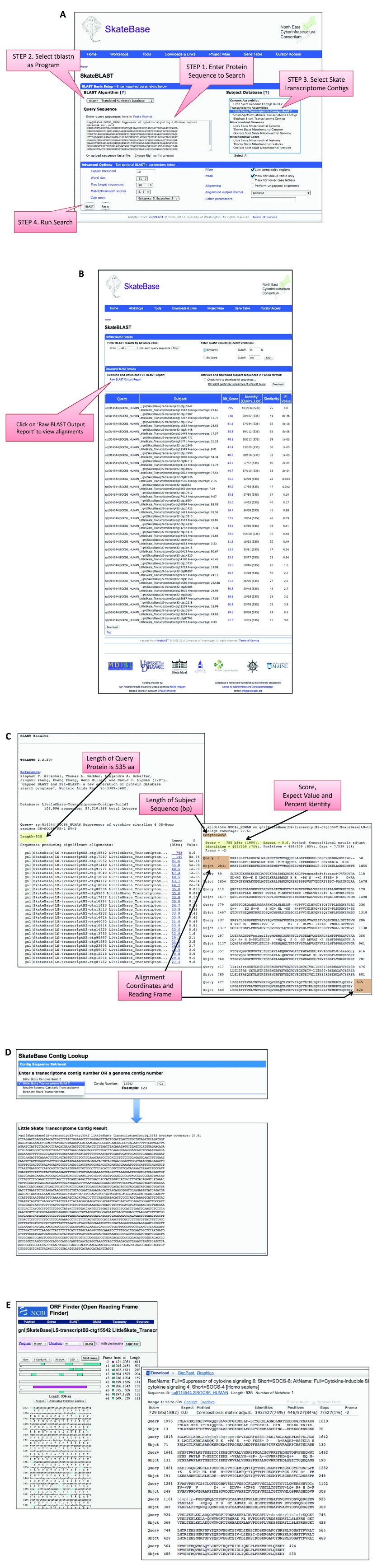
Figure 5 demonstrates the use of SkateBLAST to find expressed sequences for the gene, suppressor of cytokine signaling 6 ( SOCS6). SOCS6 is a E3 ubiquitin ligase that interacts with c-KIT to suppress cellular proliferation through its SH2 domain 66. The first step to identify SOCS6 in the skate transcriptome begins with entering the protein sequence for human SOCS6 that was obtained from UniProt and searching this sequence against the skate transcriptome using the tblastn program. The next step is to evaluate the alignments to determine which transcriptome sequences best represent SOCS6. When interpreting the pairwise alignments from SkateBlast as in any BLAST tool, it is important to examine: a) alignment statistics; b) alignment coverage; and c) presence of protein domains that you may expect to be conserved. The alignment statistics are reported to ascertain whether you would expect the given alignment by chance or not. There are three key alignment statistics, the expectation (E)-value, percent identity and alignment length. The E-value represents the probability that you would expect an alignment with that alignment score or better by random chance, thus the lower the E-value, the better the alignment. Conversely, the greater the percent identity (percent identical sequence) and alignment length, the more similar the two sequences are assumed to be. Alignment coverage with respect to the query or subject sequence (alignment length divided by the length of the query or subject sequence) can also be an important consideration, as low coverage suggests that important regions of one or both sequences may not be represented in the alignment. Finally, there may be particular sequence features, such as protein domains, that you would expect to find in the alignment. If those domains are missing, then it suggests that you have a partial or misleading alignment.
Figure 5. Example of using SkateBase and NCBI resources to find transcriptome data for SOCS6.
A. SkateBLAST query form showing the four steps to align the UniProt sequence for human SOCS6 (O14544) against the skate embryonic transcriptome using tblastn. Step 1 is to enter the sequence in FASTA format. The second step is to choose the tblastn program that will align the query protein sequence against translated sequences in all six possible reading frames. The third step is to select the embryonic transcriptome as the sequence database to search. The fourth step is to launch the search. B. The complete BLAST output can be accessed by clicking the “Inspect BLAST output” link at the top of the summary report page. This is necessary to examine the sequence alignments. C. Four important fields in the output should be examined carefully to interpret the alignments and determine which returned alignment best represents the skate ortholog to SOCS6. First, the alignment score, E-value, alignment length and percent identity can be used to interpret the overall alignment significance. Alignment coverage with respect to the query protein sequence and the subject transcriptome sequence can be interpreted by comparing the alignment coordinates to the length of the query protein sequence and length of the transcriptome sequence. In this example, the entire query protein sequence is covered by this transcriptome sequence. D. The SkateBase Contig Lookup tool can be used to retrieve the transcriptome sequence found in the SOCS6 tblastn search in FASTA format. Sequences from the skate genome assembly or the skate, S. canicula or C. milii transcriptome assemblies can be retrieved using this tool. E. Output from the NCBI ORF Finder tool showing a 536aa ORF in the skate transcriptome contig that best represents SOCS6 (left). Alignment from blastx search of the skate transcriptome sequence (contig 15542) against human UniProt using NCBI BLAST to validate that the contig aligned best to human SOSC6 rather than another human gene.
Once a transcriptome sequence of interest, such as contig15542, is identified in the SkateBLAST results, you must do a reciprocal search of that sequence against a database of protein sequences to confirm that the sequence aligns best to your gene of interest. You can retrieve the full sequence directly from the BLAST tool or using the Skate Contig Lookup tool ( Figure 5D): a) specify the transcriptome that you had originally searched using SkateBLAST; b) enter the sequence identification or contig number is entered into the query box; and c) select the ‘GO’ button. The user can copy the returned sequence and use it for further exploration of sequence homology at NCBI or similar databases.
SkateBase classroom use case: teach concepts of gene and protein annotation
SkateBase includes valuable teaching resources derived from the project workshops on gene and protein annotation. Infrastructure for sequence annotation was developed and modules for use in teaching are available. Access to the teaching modules is through the Curator Access link from the homepage and permission is granted by request using the email link at the bottom of each page, info@SkateBase.org. Once successfully logged into the site, access to pre-computed blast results, guides and examples, annotation forms, and links to external tools helpful for sequence analysis are available. Gene annotation begins with a transcriptome contig identified through a SkateBlast search as illustrated above. The portion of the transcript that codes for protein is identified using an open reading frame or ORF finder tool. Annotation follows a workflow where complimentary sequences from the transcriptome and genome are aligned allowing annotation of both sequences using Sequence Ontology vocabulary 67. The evidence is recorded in an annotation form that records information about the annotator and sequences and includes a comment box for questions and comments between students and teachers or curators and annotators. The annotation form records the pairwise alignment of the transcriptome and genome contigs, notes concerning mismatches or gaps, as well as output from the ORF tool. The untranslated regions (UTR) at the beginning and end of each sequence, 5’UTR and 3’ UTR regions, as well as the intron/exon structure for the genomic contig and CDS for the transcriptomic contig are recorded in the Gene Annotation Form. When completing the Gene Annotation Form, the appropriate activity must be selected and can be customized to specify the user’s course ID, institution or workshop title to track annotation history. Protein annotation uses the rapid annotation interface for proteins, RACE-P, developed by the PIR. A UniProt accession number is required to initiate a new annotation form. The form is composed of 6 blocks of information, protein information, gene information, a bibliography, Gene Ontology (GO), computational analysis using tools such as Pfam 68, TMHMM 69, SignalP 70, COILS 71, NetPhos 72 and EMBOSS 73, and protein family evidence.
Discussion
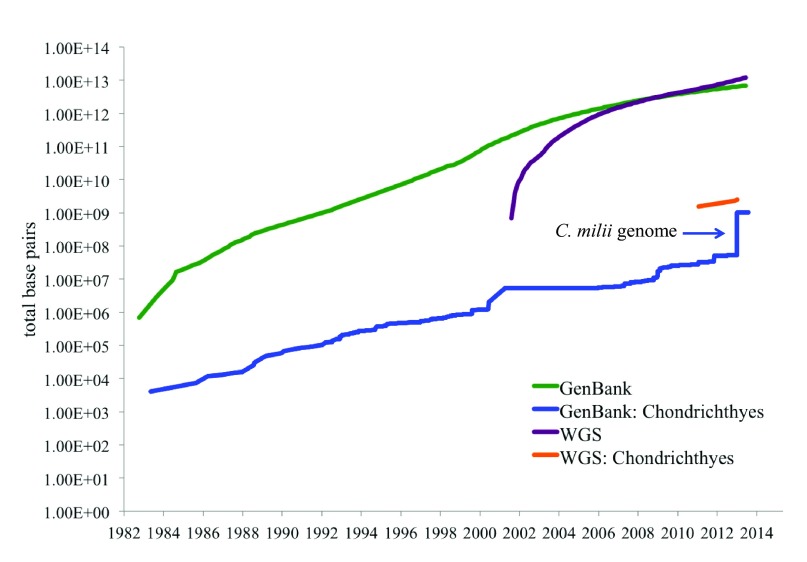
The volume of data in GenBank continues to grow exponentially, doubling nearly every 18 months. The first sequences for chondrichthyes appeared in 1983 and the overall data trend for chondrichthyans is similar to all of GenBank with three exceptions. First, the rate of increase is less than GenBank. Second, the number of sequences deposited during the first decade of the 21 st century was nearly stagnant in comparison. Third, a large spike is observed in late 2012 attributed to the Elephant Shark Genome Project data ( Figure 6). Molecular data is increasingly important for all aspects of research utilizing chondrichthyan fishes 74. It can be a forensic tool to understand species when fins are landed without carcass and ensure protected species and quotas are respected 75– 79. For migrating species molecular data serves as a surrogate to classical tagging data to understand population structure and range 80– 83. In studies of evolution, molecular data provides estimates of divergence time and supplements morphological and ecological traits as the basis for a phylogeny. The benefits and uses of molecular data for these fishes are limited only by the amount of data available. SkateBase provides the only genomic data publically available for an elasmobranch in addition to embryonic transcriptomes, data tools, and educational resources.
Figure 6. GenBank and WGS data trends for Chondrichthyes and all taxa.
GenBank is the National Institutes of Health (NIH) genetic sequence database and together with the DNA Databank of Japan (DDBJ) and the European Molecular Biological Laboratory (EMBL) comprise the International Nucleotide Sequence Database Collaboration (INSDC). The cumulative base pair total for all taxa as well as chondrichthyan only data are given versus time for GenBank and Whole Genome Shotgun (WGS) data. The Elephant Shark Genome Project is responsible for the spike in chondrichthyan GenBank in 2011. The little skate and elephant shark genome projects are currently the only two WGS datasets (yellow line).
Sequencing projects require significant funding and personnel commitments but generate a large amount of information that can be translated to knowledge by domain experts. The efficiency of this process is affected most by allowing the scientific community to access the data. The value of data sharing can be measured by the number of publications that result from its distribution. To date, 19 publications in peer-reviewed journals have used data derived from SkateBase ( http://skatebase.org/vitae). Molecular data are the means to investigate genes and develop reagents for gene expression studies by PCR or in situ hybridization. Small scale sequencing efforts that generate limited or fragmented data often get deposited to hard disks and remain ‘buried’ and out of reach. Efforts to deposit this data at public sequence repositories are encouraged to build the foundation of data required to describe this dynamic and ancient clade of fishes. We invite investigators to contact the authors in an effort to survey the volume of private data available for potential distribution through SkateBase.
The transcriptome data at SkateBase serves as a platform to teach molecular techniques, technologies, and bioinformatics in the context of studying elasmobranchs. As next generation sequencing (NGS) technologies evolve it is important for scientists and students to understand how the sequence was generated and caveats of workflow for each data type in order to recognize errors and customize analysis algorithms. The educational materials and infrastructure at SkateBase have been used by University of Delaware, Georgetown University, MDI Biological Laboratory, University of Maine at Machias, University of Rhode Island, and most recently the Virginia Institute of Marine Science to teach gene and protein annotation concepts. We invite and look forward to continued expansion of the SkateBase educational platform as we refine the infrastructure and expand the data available for investigation through continued sequencing efforts.
Acknowledgements
The authors thank the North East Bioinformatics Collaborative of the North East Cyberinfrastructure Consortium and Karl Steiner and Steven Stanhope of DE-INBRE, Patricia Hand of ME-INBRE, and Judith VanHouten of VT-INBRE. We thank the Delaware Biotechnology Institute (DBI) for hosting the website, database and NECC Shared Data Center. We thank Karol Miaskiewicz for systems administration support and Gang Li and Zhiwen Li for web development. We thank Bruce Kingham and the DBI Sequencing and Genotyping Center. Sylvie Mazan, Alistair Dove, Dave Ebert, Cecelia Arighi, Qinghua Wang, James Sulikowski and Eric Haenni contributed data, samples, artwork or editorial comments. We thank Gavin Naylor and the Chondrichthyan Tree of Life project team including Lindsay Marshall (illustrations), Jason Davies (database, computational work, and visualizations), Will White and Peter Last (maps and taxonomy), Shannon Corrigan and Lei Yang (gene capture data), and Callie Crawford and Thomas Fussell (CT scanning and anatomy) for providing a description of the project scope.
Funding Statement
This work was supported by a re-entry career award to JTW, National Institute of General Medical Sciences (NIGMS) IDeA Networks of Biomedical Research Excellence (INBRE) 3P20GM103446-12S1. Skate genome sequencing was funded by National Institutes of Health (NIH) National Center for Research Resources (NCRR) ARRA Supplements to 5P20RR016463-12 (MDIBL), 5P20RR016472-12 (UD), and 5P20RR16462 (UVM). The North East Cyberinfrastructure Consortium (NECC) is funded by NIH NCRR grants 5P20RR016463-12 (MDIBL), 5P20RR016472-12 (UD), 5 P20 RR16462 (UVM), 5P20RR016457-11 (URI), and 5P20RR030360-03 (UNH) and NIH NIGMS grants 8 P20 GM103423-12 (MDIBL), 8P20GM103446-12 (UD), 8P20GM103449 (UVM), 8 P20 GM103430-11 (URI), and 8P20GM103506-03 (Dartmouth), and NSF Experimental Program to Stimulate Competitive Research (EPSCoR) grants EPS-0904155 (UM), EPS-081425 (UD), EPS-1101317 (UVM), EPS-1004057 (URI), and EPS-1101245 (UNH).
v1; ref status: indexed
References
- 1.Inoue JG, Miya M, Lam K, et al. : Evolutionary origin and phylogeny of the modern holocephalans (Chondrichthyes: Chimaeriformes): a mitogenomic perspective. Mol Biol Evol. 2010;27(11):2576–86 10.1093/molbev/msq147 [DOI] [PubMed] [Google Scholar]
- 2.Ebert DA, Ho H, White WT, et al. : Introduction to the systematics and biodiversity of sharks, rays, and chimaeras (Chondrichthyes) of Taiwan.2013;3752(1):5–19 10.11646/zootaxa.3752.1.3 [DOI] [PubMed] [Google Scholar]
- 3.Stevens J, Bonfil R, Dulvy NK, et al. : The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci. 2000;57(3):476–94 10.1006/jmsc.2000.0724 [DOI] [Google Scholar]
- 4.García VB, Lucifora LO, Myers RA: The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc Biol Sci. 2008;275(1630):83–9 10.1098/rspb.2007.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field IC, Meekan MG, Buckworth RC, et al. : Chapter 4. Susceptibility of sharks, rays and chimaeras to global extinction. Adv Mar Biol. 2009;56:275–363 10.1016/S0065-2881(09)56004-X [DOI] [PubMed] [Google Scholar]
- 6.Springer S: Oviphagous Embryos of the Sand Shark, Carcharias taurus. Copeia. 1948;1948(3):153 Reference Source [Google Scholar]
- 7.Joung SJ, Chen CT, Clark E, et al. : The whale shark, Rhincodon typus, is a livebearer: 300 embryos found in one ‘megamamma’ supreme. Environ Biol Fishes. 1996;46(3):219–23 10.1007/BF00004997 [DOI] [Google Scholar]
- 8.Stevens J, Bonfil R, Dulvy NK, et al. : The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci. 2000;57(3):476–94 10.1006/jmsc.2000.0724 [DOI] [Google Scholar]
- 9.Liu SY, Chan CL, Lin O, et al. : DNA barcoding of shark meats identify species composition and CITES-listed species from the markets in Taiwan. PLoS One. 2013;8(11):e79373 10.1371/journal.pone.0079373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudgeon CL, Blower DC, Broderick D, et al. : A review of the application of molecular genetics for fisheries management and conservation of sharks and rays. J Fish Biol. 2012;80(5):1789–843 10.1111/j.1095-8649.2012.03265.x [DOI] [PubMed] [Google Scholar]
- 11.Pinhal D, Shivji MS, Nachtigall PG, et al. : A streamlined DNA tool for global identification of heavily exploited coastal shark species (genus Rhizoprionodon). PLoS One. 2012;7(4):e34797 10.1371/journal.pone.0034797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbuto M, Galimberti A, Ferri E, et al. : DNA barcoding reveals fraudulent substitutions in shark seafood products: The Italian case of “palombo” (Mustelus spp.). Food Res Int. 2010;43(1):376–81 10.1016/j.foodres.2009.10.009 [DOI] [Google Scholar]
- 13.Feldheim KA, Gruber SH, Dibattista JD, et al. : Two decades of genetic profiling yields first evidence of natal philopatry and long-term fidelity to parturition sites in sharks. Mol Ecol. 2014;23(1):110–7 10.1111/mec.12583 [DOI] [PubMed] [Google Scholar]
- 14.Schneider I, Aneas I, Gehrke AR, et al. : Appendage expression driven by the Hoxd Global Control Region is an ancient gnathostome feature. Proc Natl Acad Sci U S A. 2011;108(31):12782–6 10.1073/pnas.1109993108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillis JA, Dahn RD, Shubin NH: Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc Natl Acad Sci U S A. 2009;106(14):5720–4 10.1073/pnas.0810959106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillis JA, Modrell MS, Baker CVH: Developmental evidence for serial homology of the vertebrate jaw and gill arch skeleton. Nat Commun. 2013; ;4:1436 10.1038/ncomms2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hentschel DM, Bonventre JV: Novel non-rodent models of kidney disease. Curr Mol Med. 2005;5(5):537–46 10.2174/1566524054553469 [DOI] [PubMed] [Google Scholar]
- 18.Steele SL, Yancey PH, Wright PA: Dogmas and controversies in the handling of nitrogenous wastes: osmoregulation during early embryonic development in the marine little skate Raja erinacea; response to changes in external salinity. J Exp Biol. 2004;207(Pt 12):2021–31 10.1242/jeb.00959 [DOI] [PubMed] [Google Scholar]
- 19.Stolte H, Galaske RG, Eisenbach GM, et al. : Renal tubule ion transport and collecting duct function in the elasmobranch little skate, Raja erinacea. J Exp Zool. 1977;199(3):403–10 10.1002/jez.1401990314 [DOI] [PubMed] [Google Scholar]
- 20.Evans DH: A brief history of the study of fish osmoregulation: the central role of the Mt. Desert Island Biological Laboratory. Front Physiol. 2010;1:13 10.3389/fphys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyffels JT, Luer CA, Walsh CJ, et al. : In vivo exposure of clearnose skates, Raja eglanteria, to ionising X-radiation: acute effects on the peripheral blood, spleen, and epigonal and Leydig organs. Fish Shellfish Immunol. 2007;23(2):401–18 10.1016/j.fsi.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 22.Wyffels JT, Walsh CJ, Luer CA, et al. : In vivo exposure of clearnose skates, Raja eglanteria, to ionizing X-radiation: acute effects on the thymus. Dev Comp Immunol. 2005;29(4):315–31 10.1016/j.dci.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 23.Lutton BV, Callard IP: Morphological relationships and leukocyte influence on steroid production in the epigonal organ-ovary complex of the skate, Leucoraja erinacea. J Morphol. 2008;269(5):620–9 10.1002/jmor.10614 [DOI] [PubMed] [Google Scholar]
- 24.Walsh CJ, Luer CA, Bodine AB, et al. : Elasmobranch immune cells as a source of novel tumor cell inhibitors: Implications for public health. Integr Comp Biol. 2006;46(6):1072–81 10.1093/icb/icl041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eason DD, Litman RT, Luer CA, et al. : Expression of individual immunoglobulin genes occurs in an unusual system consisting of multiple independent loci. Eur J Immunol. 2004;34(9):2551–8 10.1002/eji.200425224 [DOI] [PubMed] [Google Scholar]
- 26.Anderson MK, Pant R, Miracle AL, et al. : Evolutionary origins of lymphocytes: ensembles of T cell and B cell transcriptional regulators in a cartilaginous fish. J Immunol. 2004;172(10):5851–60 10.4049/jimmunol.172.10.5851 [DOI] [PubMed] [Google Scholar]
- 27.Cai SY, Soroka CJ, Ballatori N, et al. : Molecular characterization of a multidrug resistance-associated protein, Mrp2, from the little skate. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R125–30 [DOI] [PubMed] [Google Scholar]
- 28.Clusin W, Spray DC, Bennett MVL: Activation of a voltage-insensitive conductance by inward calcium current. Nature. 1975;256(5516):425–7 10.1038/256425a0 [DOI] [PubMed] [Google Scholar]
- 29.Elger M, Hentschel H, Litteral J, et al. : Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14(6):1506–18 10.1097/01.ASN.0000067645.49562.09 [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Du Pasquier L, Hsu E: Shark IgW C region diversification through RNA processing and isotype switching. J Immunol. 2013;191(6):3410–8 10.4049/jimmunol.1301257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beard J: The origin and histogenesis of the thymus in Raja batis. Zool Jahrb Abt Anat Ontog Tiere. 1902;17:403–80 Reference Source [Google Scholar]
- 32.Luer CA, Walsh CJ, Bodine AB, et al. : The elasmobranch thymus: Anatomical, histological, and preliminary functional characterization. J Exp Zool. 1995;273(4):342–54 10.1002/jez.1402730408 [DOI] [Google Scholar]
- 33.Wyffels JT, Walsh CJ, Luer CA, et al. : In vivo exposure of clearnose skates, Raja eglanteria, to ionizing X-radiation: acute effects on the thymus. Dev Comp Immunol. 2005;29(4):315–31 10.1016/j.dci.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 34.Feldheim KA, Chapman DD, Sweet D, et al. : Shark virgin birth produces multiple, viable offspring. J Hered. 2010;101(3):374–7 10.1093/jhered/esp129 [DOI] [PubMed] [Google Scholar]
- 35.Chapman DD, Shivji MS, Louis E, et al. : Virgin birth in a hammerhead shark. Biol Lett. 2007;3(4):425–7 10.1098/rsbl.2007.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson DP, Baverstock W, Al-Jaru A, et al. : Annually recurring parthenogenesis in a zebra shark Stegostoma fasciatum. J Fish Biol. 2011;79(5):1376–82 10.1111/j.1095-8649.2011.03110.x [DOI] [PubMed] [Google Scholar]
- 37.Compagno LJV: Alternative life-history styles of cartilaginous fishes in time and space. Environ Biol Fishes. 1990;28(1–4):33–75 10.1007/978-94-009-2065-1_3 [DOI] [Google Scholar]
- 38.Luer CA, Gilbert PW: Mating behavior, egg deposition, incubation period, and hatching in the clearnose skate, Raja eglanteria. Environ Biol Fishes. 1985;13(3):161–71 10.1007/BF00000926 [DOI] [Google Scholar]
- 39.Luer CA, Walsh CJ, Bodine AB, et al. : Normal embryonic development in the clearnose skate, Raja eglanteria, with experimental observations on artificial insemination. Environ Biol Fishes. 2007;80(2–3):239–55 10.1007/s10641-007-9219-4 [DOI] [Google Scholar]
- 40.Masuda M, Izawa Y, Kametuta S, et al. : Artificial insemination of the cloudy catshark. J Japanese Assoc Zool Gard Aquariums. 2003;44(2):39–43 Reference Source [Google Scholar]
- 41.Castro JI, Bubucis PM, Overstrom NA, et al. : The Reproductive Biology of the Chain Dogfish, Scyliorhinus retifer. Copeia. 1988;1988(3):740 10.2307/1445396 [DOI] [Google Scholar]
- 42.Barrett T, Clark K, Gevorgyan R, et al. : BioProject and BioSample databases at NCBI: facilitating capture and organization of metadata. Nucleic Acids Res. 2012;40(Database issue):D57–63 10.1093/nar/gkr1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatesh B, Lee AP, Ravi V, et al. : Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505(7482):174–9 10.1038/nature12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatesh B, Kirkness EF, Loh YH, et al. : Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol. 2007;5(4):e101 10.1371/journal.pbio.0050101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo M, Kim H, Kudrna D, et al. : Construction of a nurse shark (Ginglymostoma cirratum) bacterial artificial chromosome (BAC) library and a preliminary genome survey. BMC Genomics. 2006;7:106 10.1186/1471-2164-7-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gwee PC, Tay BH, Brenner S, et al. : Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes. BMC Evol Biol. 2009;9:47 10.1186/1471-2148-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulley JF, Zhong YF, Holland PW: Comparative genomics of chondrichthyan Hoxa clusters. BMC Evol Biol. 2009;9:218 10.1186/1471-2148-9-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu C, Amemiya C, Dewar K, et al. : Molecular evolution of the HoxA cluster in the three major gnathostome lineages. Proc Natl Acad Sci U S A. 2002;99(8):5492–7 10.1073/pnas.052709899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattingly C, Parton A, Dowell L, et al. : Cell and molecular biology of marine elasmobranchs: Squalus acanthias and Raja erinacea. Zebrafish. 2004;1(2):111–20 10.1089/zeb.2004.1.111 [DOI] [PubMed] [Google Scholar]
- 50.Merson RR, Mattingly C, Planchart AJ: Tandem duplication of aryl hydrocarbon receptor (AHR) genes in the genome of the spiny dogfish shark (Squalus acanthias). Bull MDIBL. 2009;48:43–4 Reference Source [Google Scholar]
- 51.Oulion S, Debiais-Thibaud M, d’Aubenton-Carafa Y, et al. : Evolution of Hox gene clusters in gnathostomes: insights from a survey of a shark (Scyliorhinus canicula) transcriptome. Mol Biol Evol. 2010;27(12):2829–38 10.1073/pnas.052709899 [DOI] [PubMed] [Google Scholar]
- 52.King BL, Gillis JA, Carlisle HR, et al. : A natural deletion of the HoxC cluster in elasmobranch fishes. Science. 2011;334(6062):1517 10.1126/science.1210912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim CB, Amemiya C, Bailey W, et al. : Hox cluster genomics in the horn shark, Heterodontus francisci. Proc Natl Acad Sci U S A. 2000;97(4):1655–60 10.1073/pnas.030539697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravi V, Lam K, Tay BH, et al. : Elephant shark (Callorhinchus milii) provides insights into the evolution of Hox gene clusters in gnathostomes. Proc Natl Acad Sci U S A. 2009;106(38):16327–32 10.1073/pnas.0907914106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naylor GJP, Caira JN, Jensen K, et al. : Elasmobranch Phylogeny: A mitochondrial estimate based on 595 species. In: Carrier JC, Musick JA, Heithaus MR, editors. The Biology of Sharks and Their Relatives. CRC Press, Taylor & Francis Group.2012;31–56 Reference Source [Google Scholar]
- 56.Naylor GJP, Ryburn JA, Fedrigo O, et al. : Phylogenetic relationships among the Major Lineages of Modern Elasmobranchs.1995. Reference Source [Google Scholar]
- 57.Naylor GJP, Caira JN, Jensen K, et al. : A DNA Sequence–Based Approach To the Identification of Shark and Ray Species and Its Implications for Global Elasmobranch Diversity and Parasitology. Bull Am Museum Nat Hist. 2012;367:1–262 10.1206/754.1 [DOI] [Google Scholar]
- 58.Feijão PC, Neiva LS, de Azeredo-Espin AM, et al. : AMiGA: the arthropodan mitochondrial genomes accessible database. Bioinformatics. 2006;22(7):902–3 10.1093/bioinformatics/btl021 [DOI] [PubMed] [Google Scholar]
- 59.Li C, Hofreiter M, Straube N, et al. : Capturing protein-coding genes across highly divergent species. Biotechniques. 2013;54(6):321–6 [DOI] [PubMed] [Google Scholar]
- 60.UniProt Consortium. Activities at the Universal Protein Resource (UniProt). Nucleic Acids Res. 2014;42(Database issue):D191–8 10.1093/nar/gkt1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berman HM, Westbrook J, Feng Z, et al. : The Protein Data Bank. Nucleic Acids Res. 2000;28(1):235–42 10.1093/nar/28.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takechi M, Takeuchi M, Ota KG, et al. : Overview of the transcriptome profiles identified in hagfish, shark, and bichir: current issues arising from some nonmodel vertebrate taxa. J Exp Zool B Mol Dev Evol. 2011;316(7):526–46 10.1002/jez.b.21427 [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Arighi CN, King BL, et al. : Community annotation and bioinformatics workforce development in concert--Little Skate Genome Annotation Workshops and Jamborees. Database (Oxford). 2012;2012:bar064 10.1093/database/bar064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jennifer TW: Embryonic development of Chondrichthyan fishes – a review. In: W KY, Lue CA & Kapoor BG, editors. Development of Non-Teleost Fishes. Enfield: Science Publishers2009;1–103 10.1201/b10184-2 [DOI] [Google Scholar]
- 65.Deng W, Nickle DC, Learn GH, et al. : ViroBLAST: a stand-alone BLAST web server for flexible queries of multiple databases and user’s datasets. Bioinformatics. 2007;23(17):2334–6 10.1093/bioinformatics/btm331 [DOI] [PubMed] [Google Scholar]
- 66.Zadjali F, Pike AC, Vesterlund M, et al. : Structural basis for c-KIT inhibition by the suppressor of cytokine signaling 6 (SOCS6) ubiquitin ligase. J Biol Chem. 2011;286(1):480–90 10.1074/jbc.M110.173526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mungall CJ, Batchelor C, Eilbeck K: Evolution of the Sequence Ontology terms and relationships. J Biomed Inform. 2011;44(1):87–93 10.1016/j.jbi.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finn RD, Bateman A, Clements J, et al. : Pfam: the protein families database. Nucleic Acids Res. 2014;42(Database issue):D222–30 10.1093/nar/gkt1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krogh A, Larsson B, von Heijne G, et al. : Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305(3):567–80 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 70.Petersen TN, Brunak S, von Heijne G, et al. : SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–6 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 71.Lupas A, Van Dyke M, Stock J: Predicting coiled coils from protein sequences. Science. 1991;252(5009):1162–4 10.1126/science.252.5009.1162 [DOI] [PubMed] [Google Scholar]
- 72.Blom N, Gammeltoft S, Brunak S: Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294(5):1351–62 10.1006/jmbi.1999.3310 [DOI] [PubMed] [Google Scholar]
- 73.Rice P, Longden I, Bleasby A: EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16(6):276–7 10.1016/S0168-9525(00)02024-2 [DOI] [PubMed] [Google Scholar]
- 74.Heist E: Genetics of Sharks, Skates, and Rays. In: Musick J, Carrier J, Heithaus M, editors. Biology of Sharks and Their Relatives. CRC Press.2004;471–85 10.1201/9780203491317.ch16 [DOI] [Google Scholar]
- 75.Chapman D, Pinhal D, Shivji M: Tracking the fin trade: genetic stock identification in western Atlantic scalloped hammerhead sharks Sphyrna lewini. Endanger Species Res. 2009;9:221–8 10.3354/esr00241 [DOI] [Google Scholar]
- 76.Clarke SC, Magnussen JE, Abercrombie DL, et al. : Identification of shark species composition and proportion in the Hong Kong shark fin market based on molecular genetics and trade records. Conserv Biol. 2006;20(1):201–11 10.1111/j.1523-1739.2005.00247.x [DOI] [PubMed] [Google Scholar]
- 77.Liu SY, Chan CL, Lin O, et al. : DNA barcoding of shark meats identify species composition and CITES-listed species from the markets in Taiwan. PLoS One. 2013;8(11):e79373 10.1371/journal.pone.0079373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shivji MS: DNA Forensic Applications in Shark Management and Conservation. In: Carrier JC., Musick JA., Heithaus MR., editors. Sharks and Their Relatives II Biodiversity, Adaptive Physiology, and Conservation. CRC Press.2010;593–610 10.1201/9781420080483-c15 [DOI] [Google Scholar]
- 79.Rodrigues-Filho LF, Pinhal D, Sodré D, et al. : Applications and Impacts on Genetic Diversity. In: Caliskan M, editor. Analysis of Genetic Variation in Animals. InTech.2012;269–86 10.5772/35455 [DOI] [Google Scholar]
- 80.Chabot CL, Allen LG: Global population structure of the tope (Galeorhinus galeus) inferred by mitochondrial control region sequence data. Mol Ecol. 2009;18(3):545–52 10.1111/j.1365-294X.2008.04047.x [DOI] [PubMed] [Google Scholar]
- 81.Keeney DB, Heist EJ: Worldwide phylogeography of the blacktip shark (Carcharhinus limbatus) inferred from mitochondrial DNA reveals isolation of western Atlantic populations coupled with recent Pacific dispersal. Mol Ecol. 2006;15(12):3669–79 10.1111/j.1365-294X.2006.03036.x [DOI] [PubMed] [Google Scholar]
- 82.Schultz JK, Feldheim KA, Gruber SH, et al. : Global phylogeography and seascape genetics of the lemon sharks (genus Negaprion). Mol Ecol. 2008;17(24):5336–48 10.1111/j.1365-294X.2008.04000.x [DOI] [PubMed] [Google Scholar]
- 83.Ward RD, Holmes BH, White WT, et al. : DNA barcoding Australasian chondrichthyans: results and potential uses in conservation. Mar Freshw Res. 2008;59(1):57–71 10.1071/MF07148 [DOI] [Google Scholar]