Abstract
Melatonin, a hormone secreted mainly by pineal gland has been found to have antioxidant and anti-inflammatory properties in the oral cavity where it reaches through saliva. These properties have been found to be beneficial in certain oral pathologies including periodontal diseases, herpes viral infections and Candida, local inflammatory processes, xerostomia, oral ulcers and oral cancer. The objective of this review is to discuss the mechanism of action and potential role of melatonin as a preventive and curative agent for oral cancer. an extensive review of databases like pubmed, medline, science direct and Cochrane reviews was conducted to find articles related to beneficial actions of melatonin in human body with focus on cancers. Numerous studies both in-vitro and in-vivo had shown promising results regarding role of melatonin as anti-carcinogenic agent. Melatonin may play a role in protecting the oral cavity from tissue damage caused by oxidative stress. The experimental evidence suggests that melatonin may have utility in the treatment of several common cancers of the body. However, more specific studies are necessary to extend the therapeutic possibilities to oral carcinoma.
Keywords: Antioxidant, immunoenhancement, melatonin, oral carcinoma, proapoptosis
INTRODUCTION
Melatonin is N-acetyl-5-methoxytryptamine and is synthesized and secreted by the pineal gland and other organs. In the mouth, it is an antioxidant, anti-inflammatory agent, rather than a hormone. The effects of melatonin were described first in 1917, but it was not isolated and identified until 1958. Since its discovery, melatonin has been shown to have a variety of important functions in all species of the animal kingdom.[1] Pinealocytes, the major cells of the pineal gland, are responsible for producing and secreting melatonin into the blood. The mechanisms of melatonin synthesis are well known and have been described in numerous publications [Figure 1].[2,3]
Figure 1.
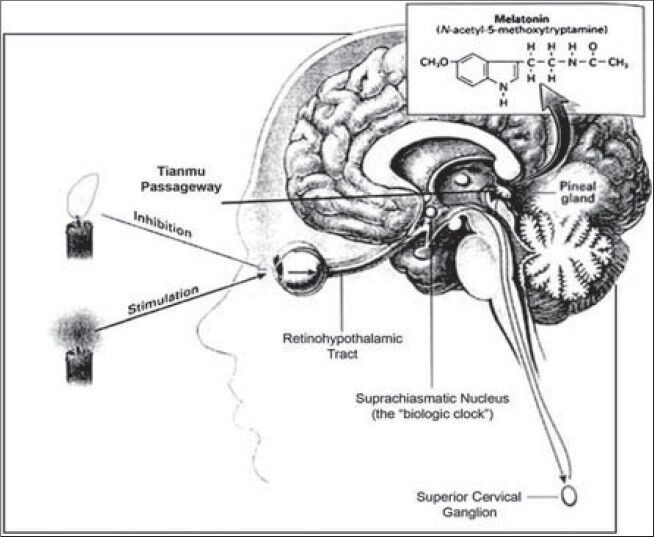
Physiology of melatonin secretion
Melatonin when released into oral cavity through saliva has been found to have protective actions against many oral conditions like periodontal diseases, herpes viral infections and Candida, local inflammatory processes, xerostomia, oral ulcers and oral cancer.[1] Most widely studied among them is its role in periodontal diseases which is related to anti-inflammatory and anti-oxidant properties of melatonin.[4,5] Important protective/beneficial actions of melatonin are listed in the table below Table 1.
Table 1.
Potential beneficial actions of melatonin in oral cavity

Our focus of interest in this review is role of melatonin in prevention and control of oral squamous cell carcinoma, because oral cancer is one of the few life threatening and poor survival rate diseases which a dentist encounters during his professional career. Therefore it is imperative that we find an agent which prevents, retards or controls the occurrence of this tumor.
THE PATHOGENESIS OF ORAL CANCER
An obvious feature of all oral cancers is excessive proliferation of oral keratinocytes. Initially, keratinization is confined to the epithelial compartment resulting in a thickened and disorganized epithelium. Eventually, the proliferating keratinocytes break through the epithelial basement membrane. Malignant epithelial masses then expand through the underlying connective tissue and invade lymph and blood vessels resulting in distant spread. In this context, oral cancer is a lesion characterized by dysregulated division of oral keratinocytes. Knowledge of normal DNA replication and keratinocyte division is the key to understanding abnormal cell division in oral cancer. Normally, oral keratinocyte division is stimulated by growth factors (EGF-Epidermal Growth Factor) binding the receptors (EGFr-Epidermal Growth Factor Receptor) on the surface of the basal keratinocytes. This activates a cascade which transmits the signal to the nucleus leading to DNA replication followed closely by cell division [Figure 2].
Figure 2.
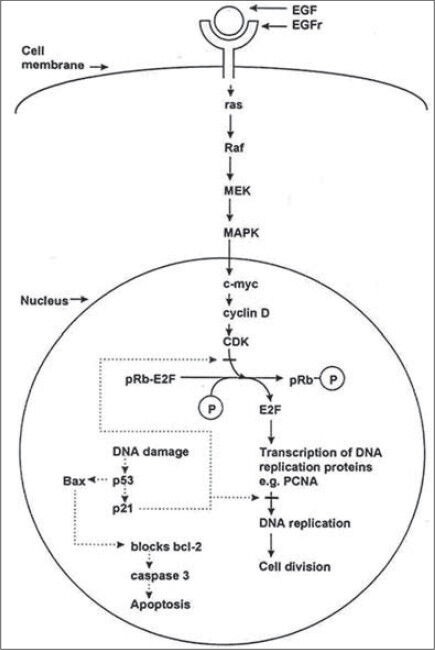
Mechanism of cell division and apoptosis in a normal cell
Most of the DNA replication proteins are degraded and must be newly transcribed with each round of cell division. Many of the proteins, which transmit the growth signal from the cell membrane to the nucleus, are encoded by oncogenes. Oncogene mutation may stimulate excessive keratinocyte proliferation in oral cancer.[6] The genetic hypothesis predicts a role for hyperactive oncogenes (growth promoting genes) in oral carcinogenesis. Highly reactive free radicals react with DNA to produce monomeric damage or strand breaks etc., Tumor suppressor protein under normal circumstances detects DNA damage and halts progression through the cell cycle. Both alleles of the tumor suppressor gene must be mutated for the tumor suppressor protein to become non-functional, up to 80% of oral squamous cell carcinomas are sporadic, and therefore both the alleles of the p53 gene in a single oral keratinocyte are lost by somatic mutation. Tumor suppressor gene mutations correlate with the stages of carcinogenesis from normal mucosa, to squamous hyperplasia (9p), to dysplasia (3p, 17p) to carcinoma in situ (11q, 13p, 14q) to invasive carcinoma (6p, 8 and 4q).[7]
Once oncogenes are activated, they may stimulate the production of an excessive amount of new genetic material through amplification or over expression of the involved gene. Oncogenes probably are involved in the initiation and progression of a wide variety of neoplasms, including oral squamous cell carcinoma. Tumor suppressor genes, on the other hand, allow tumor production indirectly when they become inactivated or mutated. Most authorities felt that an accumulation of several of these various genetic aberrations is necessary before the affected cell expresses a malignant phenotype.[8]
The most obvious immunologic change associated with head and neck cancer is a depression in the cell-mediated immune responses. The cellular responses remain depressed after surgical treatment of the tumor in head and neck carcinoma, but recover in adenocarcinomas, melanomas, and sarcomas. Therefore it is possible that the immune defect in those with head and neck cancer is a primary event.[9] In vitro tests of cell-mediated immunity confirm impaired responses in patients with head and neck cancer. T-lymphocyte numbers are reduced in patients with head and neck and oral cancer.[10] The percentage of the total lymphocyte count is also reduced, particularly as the tumor advances.[11] Therefore a substance that has antioxidant property, proapoptotic characteristics, reduces uptake of growth factors by the cell surface receptors or/and enhances the immune mechanisms would be helpful in preventing and treating oral cancer. Melatonin has been found to exhibit these features to varied extent as discussed below.
ANTI-CARCINOGENIC ACTION OF MELATONIN
There are several mechanisms by which melatonin, at greater than physiological concentrations, can exert its oncostatic actions: (a) by its antioxidant actions; (b) by its direct proapoptotic, gene-mediated actions on tumor cells; (c) by reducing the uptake of key factors for tumor growth and tumor growth signaling molecules (eg, linoleic acid); and (d) by enhancing immune mechanisms in the body [Figure 3].
Figure 3.
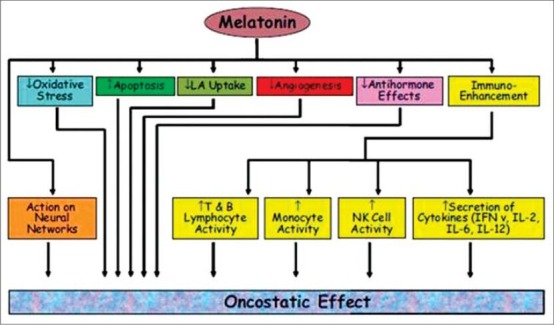
Potential oncostatic effects of melatonin
Melatonin as an anti-oxidant
Before discussing anti-oxidant effect of melatonin we must understand the role of free radicals or reactive oxygen species (ROS) in causing cancer. Free radicals are molecules or portion of molecules that possess an unpaired electron in their valence orbital. This electron-deficient state makes these agents highly reactive, and, as a consequence, they damage adjacent molecules by abstracting an electron from or donating an electron to them.[12] Because of their high reactivity, free radicals and related reactants damage small biomolecules (i.e., vitamins, amino acids, carbohydrates, and lipids) as well as macromolecules (i.e., proteins, lipids, nucleic acids); the latter can lead to destruction of supramolecular elements (i.e., cell membranes, mitochondria, and lipoproteins). In particular, their attack on fats causes lipid peroxidation, which, in turn, brings about the formation of additional lipid radicals and toxic metabolites; hence, the initiation of lipid peroxidation sets in motion a chain of events that can lead to extensive cellular damage. Free radicals often cause damage to the nitrogen bases of DNA as well as to proteins. Ultimately, the damage may contribute to diseases, such as cancer, neurodegeneration, and autoimmune conditions.[13] When the balance between free radicals (oxidants) and antioxidant defense systems is disrupted, a condition of oxidative stress occurs. Reversal of this oxidative state is important to protect the body from its ill effects as explained above. Various anti oxidants like vitamin A, C and E have been tried but they all fail to provide adequate protection against ROS in majority of clinical trials, thus, the current focus is to find out a natural antioxidant e.g., derived from plants, endogenous oxidative enzymes.[14] Melatonin is considered an effective cancer protective agent and this property is mainly due to its free radical scavenging activity and its indirect antioxidant actions.[15]
Aging is associated with increased incidence of neoplastic diseases because of the accumulated molecular damage inflicted by lifetime ROS exposure. Because melatonin production also wanes with age, its deficiency has been suggested as one of the probable causes for increased incidence of cancer cases among the elderly. It is well-known that glutathione exists at high concentration intracellularly and protects cells from free radicals and oxidative stress. Melatonin regulates the production of reduced glutathione by stimulating its rate limiting enzyme γ- glutamylcysteine synthase. This action in turn is essential for reducing the generation of hydrogen peroxide (H2O2) and hydroxyl (-OH) radicals within the cell. This interaction may explain the oncostatic effect of melatonin on cancer cells transformed by oxidative stress.[16]
Melatonin's effect on angiogenesis in cancer
Angiogenesis is an essential step in the development of primary tumors. Endothelin-1 synthesis in blood vessels is considered as one of the main stimulants of angiogenesis in primary tumors. Endothelin-1 directly stimulates endothelial as well as perivascular cells by releasing proangiogenic substances such as vascular endothelial growth factor. These effects are arrested by melatonin which suppresses the formation of endothelin-1 by inhibiting endothelin-converting enzyme-1. Further characterization of this effect may provide insights about the molecular mechanisms by which melatonin causes vacuolization of tumors and will contribute significantly to the understanding of its antitumor effects.[15]
Melatonin and apoptosis in cancer cells
The role of melatonin in regulating apoptosis has been examined. Several studies have shown melatonin's antiapoptotic effect in immune cells, mainly through direct and indirect mechanisms.[17] In contrast, in vitro melatonin has been shown to promote apoptosis in breast and colon cancer cells. Melatonin induced apoptosis on MCF-human breast cancer cells by increasing the expression of p21 and p53 proteins that are related to cell cycle control.[18] This is a new promising field of study where melatonin's proapoptotic effect on tumor cells may have a wide range of therapeutic applications.
Melatonin as immunoenhancer
Immunosurveillance is one of the major mechanisms by which cancerous cells are detected and destroyed and natural killer (NK) cells play an important role in immunosurveillance against neoplastic growth.[19] The decline in immune function with aging has been shown to increase the risk for cancerous growth.[20] Melatonin's oncostatic effects can be attributed to its stimulatory potential on lymphocytes, monocytes/macrophages, and NK cells. Melatonin enhances the production of IL-1, IL-6, tumor necrosis factor (TNF), and IL-12 from the monocytes and enhances the production of IL-2, IFN-Y and IL-6 from cultured human peripheral blood mononuclear cells.[21] In addition to stimulating the production of several cytokines that regulate immune function, melatonin enhances immune function by directly stimulating polymorphonuclear cells, macrophages, NK cells, and lymphocytes.[22] Because NK cells are effective against a variety of tumors, especially leukemias and lymphomas, the regulation of NK cell activity and the enhancement of the cytolytic function of NK cells by melatonin have considerable significance for possible therapeutic applications.
DISCUSSION
The oncostatic and/or cytotoxic effects of melatonin against a variety of cancer cells have been investigated in cell culture models. The data suggest that melatonin possesses antiproliferative effects against a variety of cancer cells. However, a marked variability has been observed among the findings of these investigations, possibly due to the differences in the study parameters such as plating densities, culture conditions, and melatonin concentrations. These studies show varying results ranging from the oncostatic effects of melatonin to no effect at all, albeit under different experimental conditions, such as doses, time of dosage, and length of treatment. A common finding of most of these in vivo studies was that melatonin does not have any unfavorable side effects.[23]
Next stage of investigation is epidemiologic studies, several studies have found an increase in breast cancer risk among subjects who frequently did not sleep during the period of the night when melatonin levels were typically at their highest, such as those women who worked the graveyard shift.[24,25] A significant correlation has been found between melatonin plasma levels and the presence of endometrial cancer, suggesting that decreasing melatonin levels may be an indicator of endometrial cancer.[26] Another epidemiologic study investigated women who worked 1 to 14 years on night shift or 15 or more years rotating compared with women who never worked night shift found that women working a rotating shift at least three nights monthly for 15 or more years may have an increased risk of colorectal cancer.[27] However, some studies conducted have found no correlation between various cancer types and melatonin levels.[28]
Several clinical studies have been conducted to investigate the effects of melatonin against certain cancer types, including cancers of the breast, skin, kidney, and solid tumors with brain metastasis. The results have been variable from no effect to considerable improvement in patient's condition. Mills et al.,[29] conducted a systematic review of randomized controlled trials (RCTs) of melatonin in solid tumor cancer patients and its effect on survival at 1 year. The results showed that Melatonin reduced the risk of death at 1 yr (relative risk: 0.66). Effects were consistent across melatonin dose, and type of cancer. No severe adverse events were reported. The authors reiterated that substantial reduction in risk of death, low adverse events reported and low costs related to this intervention suggest great potential for melatonin in treating cancer but they also added that confirming the efficacy and safety of melatonin in cancer treatment will require completion of blinded, independently conducted RCTs.
CONCLUSIONS
Oral diseases like dental caries and periodontal diseases are highly prevalent throughout the world cutting across barriers of race, gender, cultural and social factors. Much of dental research resources is utilized in search of means for prevention and control of these diseases and dental professionals tend to sideline the importance of preventing a potentially life threatening condition i.e., oral cancer. We still haven’t discovered agents to control oral squamous cell carcinoma except advising/counselling people not to consume tobacco or alcohol or prescribing anti oxidants like vitamin A and E at precancerous stages. The effects of these measures in a population are still debatable and require further research. Oral cancer is among the ten most common cancers affecting human race and has one of the poorest five year survival rate among all cancers. As prevention is better than cure there is an urgent need to discover an agent which can help in preventing oral cancer.
Melatonin has important physiological functions that have not been exploited in management of oral diseases. The actions of melatonin, as described herein, may have clinical applications in preventing and treating oral cancer. For high risk groups like chronic tobacco consumers, elderly etc., the administration of melatonin, in local or systemic form, might be indicated with the goal to protect their mouth against tumors. It can be used as an adjunct in management of oral cancer patients to retard the growth of tumor and enhance the immunity of patients. Further research must be carried out by dental researchers to provide better evidence of role of melatonin in management and prevention of oral cancer.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Cutando A, Gomez-Moreno G, Arana C, Acuna-Castroviejo D, Reiter RJ, Acuna-Castroviejo D, Reiter RJ. Melatonin: Potential Functions in the Oral Cavity. J Periodontol. 2007;78:1094–102. doi: 10.1902/jop.2007.060396. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod J. The pineal gland: A neurochemical transducer. Science. 1974;184:1341–8. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RJ. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–80. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 4.Cutando A, Gómez-Moreno G, Villalba J, Ferrera MJ, Escames G, Acuña-Castroviejo D. Relationship between salivary melatonin levels and periodontal status in diabetic patients. J Pineal Res. 2003;35:239–44. doi: 10.1034/j.1600-079x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 5.Cutando A, Galindo P, Gómez-Moreno G, Arana C, Bolaños J, Acuña-Castroviejo D, et al. Relationship between salivary melatonin and severity of periodontal disease. J Periodontol. 2006;77:1533–8. doi: 10.1902/jop.2006.050287. [DOI] [PubMed] [Google Scholar]
- 6.Sugerman PB, Savage NW. Current concepts in oral cancer. Aust Dent J. 1999;44:147–56. doi: 10.1111/j.1834-7819.1999.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 7.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic Progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996;26:2488–92. [PubMed] [Google Scholar]
- 8.Neville BW, Damm DD, Allen CM, Bouquot JE. 2nd ed. London: W.B. Saunders Company; 2002. Oral and Maxillofacial pathology; p. 338. [Google Scholar]
- 9.Lichtenstein A, Zighelboim J, Dorey F, Brossman S, Faley IL. Comparison of immune derangements in patients with different malignancies. Cancer. 1980;45:2090–5. doi: 10.1002/1097-0142(19800415)45:8<2090::aid-cncr2820450816>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Eastham RJ, Mason JM, Jennings BR, Belew PW, Maguda TA. T-cell rosette test in squamous cell carcinoma of the head and neck. Arch Otolaryngol. 1976;102:596–600. doi: 10.1001/archotol.1976.00780080093013. [DOI] [PubMed] [Google Scholar]
- 11.Deegan MJ, Coulthard SW. Spontaneous rosette formation and rosette inhibition assays in patients with squamous cell carcinoma of the head and neck. Cancer. 1977;39:2137–41. doi: 10.1002/1097-0142(197705)39:5<2137::aid-cncr2820390530>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9:526–33. [PubMed] [Google Scholar]
- 13.Zhang L, Wei W, Xu J, Min F, Wang L, Wang X, et al. Inhibitory effect of melatonin on diquat-induced lipid peroxidation in vivo as assessed by the measurement of F2-isoprostanes. J Pineal Res. 2006;40:326–31. doi: 10.1111/j.1600-079X.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 14.Czesnikiewicz-Guzik M, Konturek SJ, Loster B, Wisniewska G, Majewski S. melatonin and its role in oxidative stress related diseases of oral cavity. J Physiol Pharmocol. 2007;58(suppl 3):5–19. [PubMed] [Google Scholar]
- 15.Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Therapeutic Actions of Melatonin in Cancer: Possible Mechanisms. Integr Cancer Ther. 2008;7:189–203. doi: 10.1177/1534735408322846. [DOI] [PubMed] [Google Scholar]
- 16.Blask DE, Wilson ST, Lemus-Wilson AM. The oncostatic and oncomodulatory role of the pineal gland and melatonin. Adv Pineal Res. 1994;7:235–41. [Google Scholar]
- 17.Yu Q, Miller SC, Osmond DG. Melatonin inhibits apoptosis during early B-cell development in mouse bone marrow. J Pineal Res. 2000;29:86–93. doi: 10.1034/j.1600-079x.2000.290204.x. [DOI] [PubMed] [Google Scholar]
- 18.Cos S, Mediavilla MD, Fernández R, González-Lamuño D, Sánchez-Barcheló EJ. Does melatonin induce apoptosis in MCF-7 human breast cancer cells in vitro? J Pineal Res. 2002;32:90–6. doi: 10.1034/j.1600-079x.2002.1821.x. [DOI] [PubMed] [Google Scholar]
- 19.Herberman RB, Ortaldo JR. Natural killer cells: Their roles in defenses against disease. Science. 1981;214:24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- 20.Stanta G, Campagner L, Cavallieri F, Giarelli L. Cancer of the oldest old. What we have learned from autopsy studies. Clin Geriatr Med. 1997;13:55–68. [PubMed] [Google Scholar]
- 21.Garcia-Mauriño S, Gonzalez-Haba MG, Calvo JR, Rafii-El-Idrissi M, Sanchez-Margalet V, Goberna R, et al. Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+cells: A possible nuclear receptor mediated mechanism involving T helper type 1 lymphocytes and monocytes. J Immunol. 1997;159:574–81. [PubMed] [Google Scholar]
- 22.Currier NL, Sun LZ, Miller SC. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J Neuroimmunol. 2000;104:101–8. doi: 10.1016/s0165-5728(99)00271-4. [DOI] [PubMed] [Google Scholar]
- 23.Vijayalaxmi, Thomas CR, Jr, Reiter RJ, Herman TS. Melatonin: From basic research to cancer treatment clinics. J Clin Oncol. 2002;20:2575–601. doi: 10.1200/JCO.2002.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 25.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 26.Grin W, Grunberger W. A significant correlation between melatonin deficiency and endometrial cancer. Gynecol Obstet Invest. 1998;45:62–5. doi: 10.1159/000009926. [DOI] [PubMed] [Google Scholar]
- 27.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Nightshift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–8. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 28.Jung B, Ahmad N. Melatonin in Cancer Management: Progress and Promise. Cancer Res. 2006;66:9789–93. doi: 10.1158/0008-5472.CAN-06-1776. [DOI] [PubMed] [Google Scholar]
- 29.Mills E, Wu P, Seely D, Guyatt G. Melatonin in the treatment of cancer: A systematic review of randomized controlled trials and meta-analysis. J Pineal Res. 2005;39:360–6. doi: 10.1111/j.1600-079X.2005.00258.x. [DOI] [PubMed] [Google Scholar]


