Abstract
Precise coordination among organs is required to maintain homeostasis throughout hibernation. This is particularly true in balancing bone remodeling processes (bone formation and resorption) in hibernators experiencing nutritional deprivation and extreme physical inactivity, two factors normally leading to pronounced bone loss in non-hibernating mammals. In recent years, important relationships between bone, fat, reproductive, and brain tissues have come to light. These systems share interconnected regulatory mechanisms of energy metabolism that potentially protect the skeleton during hibernation. This review focuses on the endocrine and neuroendocrine regulation of bone/fat/energy metabolism in hibernators. Hibernators appear to have unique mechanisms that protect musculoskeletal tissues while catabolizing their abundant stores of fat. Furthermore, the bone remodeling processes that normally cause disuse-induced bone loss in non-hibernators are compared to bone remodeling processes in hibernators, and possible adaptations of the bone signaling pathways that protect the skeleton during hibernation are discussed. Understanding the biological mechanisms that allow hibernators to survive the prolonged disuse and fasting associated with extreme environmental challenges will provide critical information regarding the limit of convergence in mammalian systems and of skeletal plasticity, and may contribute valuable insight into the etiology and treatment of human diseases.
Introduction
Excessive accumulation of fat via hyperphagia prior to hibernation and reduced metabolic rate during hibernation allows mammalian hibernators (e.g., Ursus and Marmota) to survive periods of prolonged famine and extreme ambient temperatures (Ta) with little, if any, adverse effects to tissues and organs. This is likely accomplished in part by neural, hormonal, and metabolic changes that promote energy conservation and tissue and organ maintenance. Precise coordination between many organs is required to maintain homeostasis for the duration of the hibernation season. In recent years, important relationships between bone, fat, reproductive, and brain tissues in mammals have come to light; all four systems share interconnected regulatory mechanisms of energy metabolism. Understanding the biological mechanisms by which hibernators can survive the prolonged disuse and fasting associated with extreme environmental challenges will provide critical information regarding the limit of convergence in mammalian systems and of skeletal plasticity, and may contribute valuable insight into human disease.
Several possible strategies are available to mammals for surviving prolonged periods of harsh environmental conditions with limited or no food: migration to food-rich environments, storage of food in caches, storage of energy in the form of fat, reduction in expenditure of metabolic energy, or a combination of these strategies. Hibernating animals have the ability to lower endogenous energy demands by lowering body temperature (Tb) and metabolism for multiple days (torpor bouts) (Carey et al. 2003), and thus are able to survive long periods of fasting (i.e., during winter) without changing their environmental location. Food-storing hibernators (e.g., Glis) are more active and continue to eat food throughout the winter, unlike fat-storing hibernators. Excessive accumulation of fat by small hibernating rodents (<400 g) would limit their mobility and increase the risk of predation, but with food-storage strategies they are capable of collecting large hoards of food exceeding their body size (Humphries et al. 2003). Fat-storing hibernators, such as marmots (Marmota flaviventris) and some ground squirrels (Callospermophilus), on the other hand, must forage enough to fatten prior to winter (maximum fat reserves are 40–50% body mass). In these animals, large amounts of triglycerides are deposited in white adipose tissue during preparation for hibernation (Dark 2005). During torpor, fat-storing hibernators switch from carbohydrate catabolism to lipid-based metabolism (Storey and Storey 2010). Stores of fat and oxidation of lipids are used almost exclusively to maintain cellular processes and survive the winter when food is absent (Galster and Morrison 1975). Indeed, the metabolic switch to lipid hydrolysis characteristic of animals that are capable of torpor has been extensively studied and is quite complex (Carey et al. 2003; Melvin and Andrews 2009). Thus, hibernation is essentially the conservation of energy during seasonal periods of scarcity of food (Melvin and Andrews 2009). As such, hibernating animals experience dramatic reduction in their basal metabolic rate (as low as 2–5% of summer levels) by going from a homeothermic (∼37°C) state to one of heterothermy (torpor), during which Tb can vary between euthermia and near Ta (Florant and Heller 1977; Buck and Barnes 2000; Geiser 2004, 2013; Storey and Storey 2010). Such fluctuations are indicative of extreme changes in the regulation of organ function and cellular processes.
The skeleton and homeostasis of calcium during hibernation
Disuse in non-hibernators, including humans, causes unbalanced bone remodeling and bone loss (Zerwekh et al. 1998; Shackelford et al. 2004; Li et al. 2005). Imbalances in bone remodeling typically lead to hypercalcemia and increased excretion of calcium. However, hibernating bears, woodchucks (Marmota monax), and marmots maintain balanced bone remodeling and are capable of limiting loss of muscle and skeletal mass (Floyd et al. 1990; Harlow et al. 2001; Pardy et al. 2004; Lohuis et al. 2007; McGee et al. 2008; McGee-Lawrence et al. 2009a, 2009b; Doherty et al. 2012; Doherty 2013). In fact, grizzly bears (Ursus arctos horribilis) reduce cortical bone remodeling during hibernation to 25% of summer levels (McGee et al. 2008), which is similar to the overall reduction in metabolism in American black bears (Ursus americanus) (Tøien et al. 2011). Balanced bone remodeling during hibernation is likely driven by the need to maintain eucalcemia during seasonal anorexia and anuria. Indeed, calcium regulatory hormones (e.g., parathyroid hormone [PTH]) may tightly control calcium concentrations in the serum during hibernation (Donahue et al. 2006a). Furthermore, the consequences to the skeleton from lack of an adequate diet suggest that hibernating animals would experience significant bone loss from nutritional deprivation in addition to the loss from physical inactivity. Non-hibernating mammals, in contrast, experience severe negative consequences with nutritional deficiency. In humans, “anorexia nervosa” increases resorption of bone and decreases bone formation to eventually result in osteoporosis (Powers 1999). Thus, it is believed that reduced bone turnover contributes to the conservation of energy during hibernation and balanced bone resorption/formation prevents osteoporosis.
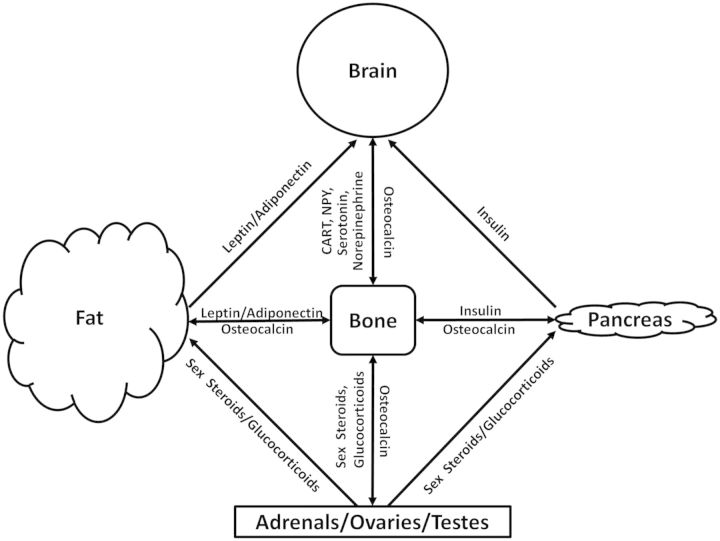
Excessive seasonal accumulation of fat by hibernators has several implications for the skeletal health of these animals. There is a high degree of cross-talk among adipokines, sex hormones, and energy/bone metabolism as evidenced by the involvement of endocrine factors (e.g., leptin) in multiple systems. Hormones derived from fat negatively influence skeletal regulation by favoring bone resorption relative to bone formation in mice (Ducy et al. 2000). Thus, the processes controlling and balancing the body systems involved in the metabolism of fat and bone are complex (Fig. 1). Indeed, the metabolic syndrome characteristic of high abdominal adiposity, fatty diet, and sedentary lifestyle increases the risk of developing severe cardiovascular disease, type II diabetes, hypertension, and a number of other metabolic disorders (Cornier et al. 2008). Furthermore, some bone fractures may be attributed to obesity (Mathey et al. 2002; Goulding et al. 2005; Hsu et al. 2006; Cao et al. 2010; Patsch et al. 2011).
Fig. 1.
The interrelationships of bone. Energy homeostasis involves regulation of bone by adipose tissue and the pancreas. The gonads and brain also contribute significantly to bone metabolism. There is considerable cross-talk among systems, including the endocrine signaling of bone in fat-deposition, reproduction, and neuroprotective functions.
The purpose of this article is to review the metabolism of energy as regulated by the interactions of fat with the skeleton through endocrine and neuroendocrine pathways and to discuss these mechanisms as they pertain to hibernators (Tables 1–4). Data are grouped by body system in Tables 1, 2, 3, and 4, and some examples of each metabolic factor are given in the tables and text. The values in Tables 1–4 were estimated from graphs and charts when numerical values were not reported. The hormones listed in the tables are not exhaustive of the extensive literature on these topics, and individual differences among studies should be kept in mind. Furthermore, studies of serum are preferentially presented for maintaining consistency of the data, and for that reason tissue homogenates or histological data typically are not reported here. An additional confounding factor potentially contributing to variation in data between studies is likely because of different time points of sample collection (i.e., seasonal sample collections are not necessarily comparable). It is becoming increasingly apparent that seasonal groupings (such as pre-hibernation, hibernation, and post-hibernation) do not necessarily capture physiological changes in short-term transitional stages (e.g., the transition periods from pre-hibernation to hibernation and from hibernation to post-hibernation). Therefore, researchers need to define these seasonal categories in a consistent way. With these caveats in mind, this article reviews the physiological processes that may impact bone in hibernators.
Table 1.
Summary of some of the reported metabolic factors influencing bone metabolism in hibernatorsa
| Hormone(s) | Species | Seasonal concentrations |
References | |||||
|---|---|---|---|---|---|---|---|---|
| Fall (pre-hibernation) | Winter (hibernation) |
Spring (post-hibernation) | Summer (active) | |||||
| Early hibernation | Late hibernation | |||||||
| Leptin | American Black Bear (U. americanus) | 4.0 ng/ml | 3.66–3.8 ng/ml | 2.08–3.1 ng/ml | Donahue et al. (2006a, 2006b) and Seger (2008) | |||
| Woodchuck (M. monax) | 1.11 ng/ml | 0.035–0.672 ng/ml | 0.888–0.1 ng/ml | 0.48–1.0 ng/ml | Doherty (2013) and Concannon et al. (2001) | |||
| Marmot (M. flaviventris) | 9.7 ng/ml | 10.7 ng/ml | 4.3 ng/ml | 3.2 ng/ml | Florant et al. (2004) | |||
| Golden-mantled Ground Squirrel (C. lateralis) | 12.7 ng/ml | 6.25 ng/ml | 2.7 ng/ml | 2.5 ng/ml | 3.1 ng/ml | Florant et al. (2012) and Healy and Florant (2012) | ||
| Greater Asiatic Yellow Bat (S. heathi) | 140 pmol/l | 230 pmol/l | 125 pmol/l | 112 pmol/ml | 125 pmol/l | Srivastava and Krishna (2007) | ||
| Insulin | American Black Bear | 8.5–53 µU/ml | 6.4–17 µU/ml | 5.7–32 µU/ml | 7.47 µU/ml | 14 µU/ml | Bradford et al. (2010) and Palumbo et al. (1983) | |
| Marmot | 12.43–37.2 µU/ml | 20.1 µU/ml | 13.0 µU/ml | 4.31–10.3 µU/ml | 4.93 µU/ml | Florant et al. (1991) and Tokuyama et al. (1991) | ||
| Hedgehog (E. europaeus) | 3.15 µU/ml | 2.1–10.0 µU/ml | 3.27–45.0 µU/ml | Laurila and Suomalainen (1974) and Hoo-Paris et al. (1978) | ||||
| Garden dormouse (E. quercinus) | 17 µU/ml | 18.5 µU/ml | 20.5 µU/ml | Agid et al. (1978) | ||||
| Little Brown Bat (M. lucifugus) | 7.29 µU/ml | 16.05 µU/ml | 27.43 µU/ml | 5.07 µU/ml | Bauman (1990) | |||
| Glucose | American Black Bear | 71–165.26 mg/dl | 60–120 mg/dl | 136 mg/dl | 74 mg/dl | Bradford et al. (2010) and Palumbo et al. (1983) | ||
| Marmot | 154 mg/dl | 135 mg/dl | 131 mg/dl | 137 mg/dl | Tokuyama et al. (1991) | |||
| Golden-mantled Ground Squirrel | 59 mg/dl | 57 mg/dl | 130 mg/dl | 152 mg/dl | 166–220 mg/dl | Galster and Morrison (1975) | ||
| Little Brown Bat | 28 mg/dl | 155 mg/dl | Bauman (1990) | |||||
| Ghrelin | Grizzly Bear (U. arctos horribilis) | 110 pg/ml | 95 pg/ml | 160 pg/ml | Gardi et al. (2011) | |||
| Marmot | 4.05 ng/ml | 8.20 ng/ml | 9.86 ng/ml | Doherty et al.; unpublished data (2013) | ||||
| Golden-mantled Ground Squirrel | 8.0–4.5 ng/ml | 3.0–3.8 ng/ml | 1.0–3.0 ng/ml | 3.6–5.4 ng/ml | Healy et al. (2010) | |||
| Adiponectin | American Black Bear | 1912 ng/ml | 1333 ng/ml | 1906 ng/ml | Bradford et al. (2010) | |||
| Marmot | 1.2b | 1.0 | 0.7 | 1.2 | Florant et al. (2004) | |||
| Little Brown Bat | 3–4 ng/ml | Townsend et al. (2008) | ||||||
| Thyroid (T4)c | American Black Bear | 1.2 µg/100 ml | 0.8 µg/100 ml | 1.6 µg/100 ml | Nelson et al. (1973) | |||
| Woodchuck | 7.5 µg | 11 µg | 4.5 µg | Wenberg and Holland (1973) | ||||
| Glucocorticoids | American Black Bear | 4.3–7.4 µg/dl | 5.6–7.7 µg/dl | 1.5–3.8 µg/dl | 1.8–5.4 µg/dl | Palumbo et al. (1983), Donahue et al. (2003a, 2003b) and Hellgren et al. (1993) | ||
| Woodchuck | 9.725 ng/ml | 7.325 ng/ml | Florant and Weitzman (1980) | |||||
| Marmot | 10 ng/ml | 54 ng/ml | Kastner et al. (1977) and Kastner et al. (1978) | |||||
| European Ground Squirrel (S. citellus) | 141.2 nmol/l | 49.97 nmol/l | 138.3 nmol/l | 170 nmol/l | 225.4 nmol/l | Shivatcheva et al. (1988) | ||
| Little Brown Bat | 0.435 µg/dl | 0.87 µg/dl | 0.525 µg/dl | 0.41 µg/dl | Gustafson and Belt (1981) | |||
aThis table reflects a summary of key studies in hibernation with potential insight into bone metabolism. The table is not intended to be all inclusive and the ranges given do not highlight seasonal changes that may be found in particular studies.
bProtein expression of serum adiponectin.
cT4 concentration is reported for comparison between studies because T3 was not investigated in both studies.
Table 2.
Summary of two neuroendocrine factors influencing bone and fat metabolism in hibernatorsa
| Hormone | Species | Seasonal concentrations |
References | |||||
|---|---|---|---|---|---|---|---|---|
| Fall (pre-hibernation) | Winter (hibernation) |
Spring (post-hibernation) | Summer (active) | |||||
| Early hibernation | Late hibernation | |||||||
| NPY | American Black Bear (U. americanus) | 211.9 pmol/l | 226.17 pmol/l | 192.49 pmol/l | Bradford et al. (2010) | |||
| Daurian Ground squirrel (S. dauricus) | 0.70RUb | 0.75RU | 1.0RU | 0.56RU | Xing et al. (2012) | |||
| Greater Egyptian Jerboa (J. orientalis) | 23.9RU | 10.4RU | El Ouezzani et al. (2001) | |||||
| Greater Mouse-tailed Bat (R. microphyllum) | 2.8 pg/mgb | 1.25 pg/mg | 3.5 pg/mg | Levin et al. (2012) | ||||
| Serotonin | American Black Bear | 753.1 ng/ml | 600.95 ng/ml | 689.55 ng/ml | Bradford et al. (2010) | |||
| Woodchuck (M. monax) | 5.0 ng/mgc | 5.1 ng/mg | 5.3 ng/mg | Young et al. (1979) | ||||
| Marmot (M. flaviventris) | 10.4 nM | 1.1 nM | Reid et al. (1992) | |||||
| Golden-mantled Ground Squirrel (C. lateralis) | 0.30–0.40 µg/g | 0.40–0.70 µg/g | Spafford and Pengelley (1971) | |||||
| Little Brown Bat (M. lucifugus) | 4.15 µg/gc | 11.44 µg/g | 4.94 µg/g | 4.05 µg/g | Haymovits et al. (1976) | |||
aThis table reflects a summary of key studies in hibernation with potential insight into bone metabolism. The table is not intended to be all inclusive and the ranges given do not highlight seasonal changes that may be found in particular studies.
bDenotes RNA gene expression.
cProtein expression of serum adiponectin.
Table 3.
Summary of some of the skeletal factors influencing energy and fat metabolism in hibernatorsa
| Hormone | Species | Seasonal concentrations |
References | |||
|---|---|---|---|---|---|---|
| Fall (pre-hibernation) | Winter (hibernation) | Spring (post-hibernation) | Summer (active) | |||
| Osteocalcin | American Black Bear (U. americanus) | 16.9 ng/ml | 27.7–70.4 ng/ml | 49.3 ng/ml | 51.2 ng/ml | Donahue et al. (2006a, 2006b) and Vestergaard et al. (2011) |
| Woodchuck (M. monax) | 93.1 ng/ml | 139.72 ng/ml | 192.13 ng/ml | Doherty (2013) | ||
| PTH | American Black Bear | 14.6 pg/ml | 25.3–36.66 pg/ml | 41.4 pg/ml | 41.36 pg/ml | Donahue et al. (2006a, 2006b) and Vestergaard et al. (2011) |
| 1,25(OH)2D | American Black Bear and Brown Bear (U. arctos) | 71.97–88.6 pmol/l | 103.09–192.5 pmol/l | Vestergaard et al. (2011) and Seger et al. (2011) | ||
aThis table reflects a summary of key studies in hibernation with potential insight into bone metabolism. The table is not intended to be all inclusive and the ranges given do not highlight seasonal changes that may be found in particular studies.
Table 4.
Summary of some reproductive factors influencing bone and fat metabolism in hibernatorsa
| Hormone | Species | Seasonal concentrations |
References | ||||
|---|---|---|---|---|---|---|---|
| Fall (pre-hibernation) | Winter (hibernation) |
Spring (post-hibernation) | Summer (active) | ||||
| Early hibernation | Late hibernation | ||||||
| Estrogens | American Black Bear (U. americanus) | 19–25 pg/ml | 25–27 pg/ml | 20–44 pg/ml | 44–70 pg/ml | Tsubota et al. (1997) | |
| European Ground Squirrel (S. citellus) | 120 pg/ml | 198 pg/ml | 330 pg/ml | Millesi et al. (2008) | |||
| Greater Asiatic Yellow Bat (S. heathi) | 250.43 pg/ml | 197.9 pg/ml | 51.9 pg/ml | 182.1 pg/ml | 119.05 pg/ml | Krishna and Abhilasha (2000) | |
| Prolactin | Japanese Black Bear (U. thibetanus japonicas) | 2.24 ng/ml | 7.96 ng/ml | 8.14 ng/ml | 8.4–9.1 ng/ml | 8.67 ng/ml | Sato et al. (2001) and Howell-Skalla et al. (2000) |
| Woodchuck (M. monax) | 0.2–1.3 ng/ml | 2.35 ng/ml | 7.0–20.7 ng/ml | Concannon et al. (1999) | |||
| Testosterone | American Black Bear | 0.1–2.0 ng/ml | 1.0 ng/ml | 2.4 ng/ml | 0.6–12.4 ng/ml | 12.4–63.2 ng/ml | Tsubota et al. (1997) and Tsubota et al. (1999) |
| Woodchuck | 0.1–0.2 ng/ml | 1.0 ng/ml | 3.4–6.6 ng/ml | 0.6–1.0 ng/ml | 0.1–0.2 ng/ml | Concannon et al. (1999) and Baldwin et al. (1985) | |
| Hedgehog (E. europaeus) | 1.33 pg/ml | 17.9 pg/ml | 17.9 pg/ml | Saboureau (1986) | |||
| Golden-mantled Ground Squirrel (C. lateralis) | 0.061 ng/ml | 0.063 ng/ml | 8.824 ng/ml | Barnes (1986) | |||
aThis table reflects a summary of key studies in hibernation with potential insight into bone metabolism. The table is not intended to be all inclusive and the ranges given do not highlight seasonal changes that may be found in particular studies.
Regulators of fat, bone, and energy metabolism in hibernating mammals
Leptin
Leptin plays a role in energy metabolism by controlling appetite and body mass. Long before its genetic identification, leptin was identified as the satiety factor because of its negative effects on long-term intake of food and maintenance of a lean-mass phenotype (Coleman 1978). Mice that are deficient in leptin (ob/ob) and leptin receptor (db/db) are obese, hyperphagic, and experience high serum levels of insulin and glucose (Coleman 1978; Zhang et al. 1994; Tartaglia et al. 1995). Leptin is produced by white and brown adipocytes and circulates in plasma to signal, primarily to the hypothalamus, the amount of stored body fat (Maffei et al. 1995).
Diets high in fat not only result in obesity and high levels of circulating leptin but also an inability to decrease the intake of food (Frederich et al. 1995). This resistance to the effects of leptin, or hyperleptinemia, is attributed to the inability of leptin to induce the signaling cascades responsible for inhibiting appetite. Leptin is not transported across the blood–brain barrier (BBB) during induced peripheral hyperleptinemia (Banks et al. 1996, 2006; Caro et al. 1996; Halaas et al. 1997; Van Heek et al. 1997; Tu et al. 2008). The short, soluble leptin receptor (ObR) normally transports leptin across the BBB (Lee et al. 1996; Kastin et al. 1999; Hileman et al. 2002; Tu et al. 2008) and stimulates various hypothalamic nuclei to initiate multiple signaling cascades (Lee et al. 1996). This includes regulating hypothalamic neuropeptides important in feeding and maintenance of energy. Low levels of circulating leptin promote the production of neuropeptide-Y (NPY) and agouti-related protein (AGRP) which increase the intake of food and decrease energy expenditure (Stanley et al. 1986; Billington et al. 1991; Shutter et al. 1997; Rossi et al. 1998). Alternatively, high levels of leptin inhibit NPY/AGRP while stimulating pro-opiomelanocortin (POMC) and cocaine-amphetamine regulated transcript (CART) to reduce food intake and increase energy expenditure (Schwartz et al. 1997; Kristensen et al. 1998). Furthermore, leptin works in association with insulin and glucocorticoids in regulating food intake and body mass (Bornstein et al. 1997; Inui 1999).
Leptin is also a regulator of bone remodeling through multiple pathways (Lee and Karsenty 2008). To influence bone remodeling, leptin acts primarily through the ventromedial nuclei to stimulate osteoblastic β2-adrenergic receptors (Adrβr), and thereby up-regulate osteoclastogenesis and bone resorption via receptor activator of nuclear factor-Кβ ligand (RANKL) (Takeda et al. 2002). Leptin also acts through the arcuate nuclei in a separate pathway to induce production of CART which inhibits bone resorption (Elefteriou et al. 2005). The role of leptin on bone, therefore, is complex and may contribute to the regulation of balanced bone remodeling during hibernation.
To prepare for hibernation, food intake is increased in the summer to build fat stores. Hibernators gradually reduce feeding and metabolic rate during the summer-to-fall transition to prepare for hibernation (Davis 1976; Ward and Armitage 1981; Hissa et al. 1998). Typically, serum leptin peaks in the fall and hibernators are believed to experience hyperleptinemia before they stop eating to allow sufficient accumulation of fat for the winter (Kronfeld-Schor et al. 2000; Florant et al. 2004). Well into the hibernation season (November and December), the level of leptin begins to drop, although in marmots it remains elevated above spring concentrations until late February (Florant et al. 2004). High leptin concentrations during pre-hibernation and early hibernation is found across various species, including bears, woodchucks, marmots, and ground squirrels (Callospermophilus lateralis) (Concannon et al. 2001; Florant et al. 2004, 2012; Donahue et al. 2006b; Seger et al. 2011; Healy and Florant 2012; Doherty 2013). Little brown bats (Myotis lucifugus) decrease leptin and ObR expression transiently during periods of greatest gain of fat in the summer, but these subsequently rise in the pre-hibernation season (Townsend et al. 2006). This pattern has also been reported in greater Asiatic yellow bats (Scotophilus heathi) with the highest leptin concentrations reached in late fall to early winter (Srivastava and Krishna 2007). Leptin protein isoforms appear to be structurally different between hibernating and active bats, indicating that leptin may have an altered role during hibernation (He et al. 2010).
Considering that obesity leads to high circulating leptin, hibernators would seem to be at risk for fragile bones and fractures with repeated seasonal obesity. However, impairment of leptin signaling during hyperleptinemia in the fall may provide some protection against bone loss in hibernators. Indeed, it has been suggested that resistance to leptin and the subsequent inability of leptin to elicit bone remodeling is a protective factor for bone in obese individuals (Ducy et al. 2000; Schilling et al. 2001; Takeda et al. 2002). A potential mechanism through which leptin could exert a positive influence on bone during hyperleptinemia and impaired transport across the BBB is through arcuate projections into circulation which express ObR (Cheunsuang and Morris 2005). Activation of CART through this route could reduce bone resorption by decreasing production of osteoblastic RANKL (Elefteriou et al. 2005).
Insulin and glucose
Mammalian hibernators have high insulin levels in their serum in the fall relative to summer (Florant and Bauman 1984; Florant et al. 1985, 1991; Mrosovsky and Boshes 1986). These animals experience seasonal hyperinsulinemia, hyperglycemia, and peripheral resistance to insulin. High circulating insulin concentration is related to the reduced ability of fat and muscle cells to take up glucose, causing further stimulation of pancreatic β-cells to release insulin (Florant and Bauman 1984; Hoo-Paris et al. 1984), similar to insulin resistance observed in humans (Flier et al. 1982). Insulin’s reduced ability to promote glucose uptake in hibernators in the fall may be related to changes in muscle and fat-cell processes that involve glucose transporter function (Hoehn et al. 2004). The reasons why hibernators do not become overtly diabetic most likely reside in their ability to stop eating for prolonged periods.
In greater Asiatic yellow bats, melatonin has been shown to enhance glucose clearance in the blood during pre-hibernation when the animals are at their maximum fat mass, and it may also protect the animal from hypoglycemia during hibernation (Srivastava and Krishna 2010). Furthermore, increased melatonin concentrations during hibernation compared to summer levels in brown bears (U. arctos) suggest that this hormone may play a role in metabolic suppression during the winter (Ware et al. 2013). What turns off a hibernator’s appetite and thus reduces circulating glucose to basal levels is unknown, but recent studies suggest that AMPK, NPY, and NPY-Y1 receptors, in addition to melatonin, may be involved in the mechanism (Dark 2005; Dark and Pelz 2008; Swoap 2008; Florant et al. 2010). Interestingly, melatonin decreases RANKL-mediated osteoclastogenesis to result in increased bone mass (Koyama et al. 2002); however, the role of melatonin on bone in hibernating animals has not been investigated.
During hibernation, insulin concentrations are low in hedgehogs (Erinaceus europaeus) and 13-lined ground squirrels (Spermophilus tridecemlineatus; Laurila and Suomalainen 1974; Agid et al. 1978; Hoo-Paris et al. 1978). Marmots’ insulin levels are highest in the plasma in September through October and begin to decline throughout hibernation to reach low concentrations in the spring (Florant et al. 1991; Tokuyama et al. 1991). However, insulin levels remain unchanged from active periods to torpor in garden dormice (Eliomys quercinus) and edible dormice (Glis glis; Agid et al. 1978; Castex et al. 1984). In the American black bear, insulin decreased during hibernation (Bradford et al. 2010) or did not change compared to active seasons (Palumbo et al. 1983). Only bats (M. lucifugus and S. heathi) experienced increasing insulin during the early phase of winter dormancy (Bauman 1990; Srivastava and Krishna 2007).
In skeletal signaling, insulin binds its receptor on osteoblasts, decreasing osteoprotegerin (OPG) expression, which allows increased binding of RANKL and promotion of osteoclastic bone resorption (Elefteriou et al. 2005; Ferron et al. 2010). The acidic environment of resorption lacunae (pH 4.5) effectively decarboxylates osteocalcin (ucOC) to release it from the bone matrix. This biologically active ucOC increases adiponectin, proliferation of β-cells, production of insulin, and a sensitivity to insulin that causes a positive feedback loop between insulin and ucOC (Ferron et al. 2010). In mice, ucOC stimulates the secretion and sensitivity of insulin and increases energy expenditure by muscles (Lee and Karsenty 2008).
Paradoxically, hibernating American black bears have increased total serum osteocalcin, but decreased levels of serum insulin (Bradford et al. 2010). Woodchucks also experience high total osteocalcin levels throughout the hibernation season and spring (Doherty 2013). However, a separate study found the opposite in brown bears and reported decreased osteocalcin concentrations during hibernation (Vestergaard et al. 2011). Varying osteocalcin concentrations in different studies may be a result of unidentified aspects of hibernation physiology, species-specific differences in osteocalcin carboxylation, and/or difficulties in identifying osteocalcin fragments (Booth et al. 2013). Low levels of serum insulin in bears are likely the result of their fasting state during hibernation. Since ucOC regulates insulin sensitivity independently of its effect on the secretion of insulin (Lee et al. 2007), bears may compensate for lower insulin levels by increasing their sensitivity to insulin via increased osteocalcin. Because osteocalcin is eliminated through renal degradation and urinary removal (Delmas et al. 1983), high levels of serum osteocalcin is likely caused by reduced renal function while bears are anuric during hibernation.
Given varying concentration patterns in different species during hibernation, insulin may play different roles in regulating bone in hibernators. Reduction of insulin during hibernation may provide a protective function against bone resorption in bears, hedgehogs, marmots, and ground squirrels. However, unchanging levels in other hibernating species, especially during periods of reduced serum glucose, suggest bone resorption would be favored over bone formation. Therefore, insulin may have alternate roles in bone metabolism in different hibernating species.
Ghrelin
Ghrelin is an orexigenic hormone working in opposition to leptin (Hewson et al. 2002; Kim et al. 2004). Fasting increases ghrelin expression from the stomach (Toshinai et al. 2001), although it is produced in a number of other tissues (Kojima et al. 1999). Peripheral and intercerebroventricular administration of exogenous ghrelin increase food intake and weight gain (Tschop et al. 2000; Keen-Rhinehart and Bartness 2005), but ghrelin action can be overridden by central injection of leptin or insulin (Hewson et al. 2002). Ghrelin acts through the hypothalamus to stimulate production of NPY and AGRP while inhibiting POMC to balance energy homeostasis (Cowley et al. 2003; Chen et al. 2004). Considering its production by multiple tissues and its close association with leptin and insulin, it is not surprising that ghrelin has a role in coupling bone and energy metabolism (Delhanty et al. 2014). In fact, osteoblasts themselves produce ghrelin (Delhanty et al. 2006). In the skeleton, ghrelin promotes osteoblast proliferation by stimulating the phosphoinositide-3 kinase and mitogen-activated protein kinase pathways through an unknown mechanism (Delhanty et al. 2006). Central and peripheral administration of ghrelin increase bone mineral density in rats (Fukushima et al. 2005; Choi et al. 2013), and osteoblast apoptosis is suppressed by ghrelin in vitro (Kim et al. 2005), indicating that ghrelin positively regulates bone formation.
In hibernating grizzly bears (U. arctos horribilis) ghrelin levels in the serum are significantly lower than during summer (Gardi et al. 2011). Low circulating ghrelin is also documented in hibernating marmots and golden-mantled ground squirrels (Healy et al. 2010; Doherty et al. unpublished data 2013). The highest levels of plasma ghrelin in golden-mantled ground squirrels occurs during the hyperphagic period in the fall, indicative of the gain in fat observed in the fall in these animals (Healy et al. 2010). Furthermore, low concentrations of ghelin in the plasma during the winter are hypothesized to contribute to suppression of appetite throughout hibernation. Peripheral administration of ghrelin to golden-mantled ground squirrels during hibernation increases food intake and physical activity (Healy et al. 2011). Considering that the level of circulating ghrelin is significantly lower during hibernation in bears, marmots, and ground squirrels, it is unlikely that ghrelin has a prominent role in maintaining bone through the winter when these animals are physically inactive. However, the direct effects of ghrelin on bone in a hibernating animal remain to be investigated.
Adiponectin
Like leptin, adiponectin is a fat-derived hormone that increases expenditure of energy and heightens sensitivity to insulin, thereby reducing body weight (Berg et al. 2002; Qi et al. 2004). Obese and insulin-resistant animals experience decreased adiponectin levels (Havel 2002), indicating that adiponectin works in concert with leptin and insulin to regulate energy metabolism. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) have been identified in osteoblasts and osteoclasts, suggesting paracrine and/or autocrine action (Berner et al. 2004; Shinoda et al. 2006). Adiponectin-transfected mice fed a normal diet experienced an increase in trabecular bone mass and suppressed osteoclastogenesis while plasma glucose and insulin concentrations did not change (Oshima et al. 2005). Thus, adiponectin may affect bone independent of insulin and energy homeostasis. Moreover, adiponectin is structurally similar to RANKL, OPG, and TNFα and inhibits activation of the cAMP-PKA pathway by NF-КB (Ouchi et al. 2000; Berner et al. 2004), further supporting its negative effects on osteoclastogenesis. Osteocalcin also stimulates production of adiponectin by adipocytes, thereby promoting insulin sensitivity in a positive feed-back loop further linking bone and energy metabolism (Lee et al. 2007).
Animals seeking to gain significant body mass for hibernation (e.g., marmots and bats) demonstrate high adiponectin levels in the summer and decreased levels in the fall when leptin and insulin levels are highest (Florant et al. 2004; Townsend et al. 2008). This pattern occurs in concert with the accumulation of lipid in the fall until the intake of food ceases entirely and lipolysis begins. American black bears experience low adiponectin concentrations during hibernation (Bradford 2010). Low adiponectin levels are consistent with the requirement for conserving energy during hibernation; therefore, any protective function adiponectin may have on bone is likely second to the metabolic actions of this hormone.
Thyroid hormone
Thyroid hormone has multiple effects in the body across several systems beyond the scope of this review. However, one of the most prominent effects of thyroid hormone is on metabolism in which oxygen consumption is increased, thereby stimulating the turnover of glucose and lipid, as well as the promotion of heat production (Berne et al. 2004). Thyroid hormone is important in the growth and maturation of bone and influences bone remodeling in non-hibernating adults. Triiodothyronine (T3), the most biologically active of the thyroid compounds, regulates both bone formation and bone resorption through the downstream release of IGF-1 and interleukin 6 and 8, respectively (Berne et al. 2004).
The metabolic and Tb functions of thyroid hormone make it a critical factor in hibernation studies. Its relation to bone remodeling bolsters the link between fat and skeletal metabolism. In the American black bear, T3 and thyroxine (T4) serum concentrations are significantly lower during hibernation (Nelson et al. 1973; Azizi et al. 1979; Tomasi et al. 1998). This pattern is different, however, in woodchucks which experience increased serum T4 levels during hibernation compared to pre-hibernation and post-hibernation seasons (Wenberg and Holland 1973). In support of these findings, plasma T4 and T3 concentrations are lowest during the early summer months in woodchucks and begin to increase in the pre-hibernation season (Young 1984). Thus, the action of thyroid hormone on the skeleton of hibernators may function differently among species and requires further investigation.
Cortisol
Endogenous glucocorticoids serve a number of important functions including increasing protein catabolism and gluconeogenesis in the liver, producing and reserving carbohydrates during periods of fasting, and promoting conversion of glucose and mobilization of fat (McCarthy et al. 1990; Canalis 2005; Peckett et al. 2011). Direct effects of glucocorticoids on bone include stimulating RANKL while inhibiting OPG production by osteoblasts (Hofbauer et al. 1999), effectively increasing osteoclastogenesis and osteoclast activity. In contrast, glucocorticoids also mediate apoptosis of osteoclasts (Tobias and Chambers 1989; Dempster et al. 1997) and reduce their number (Lindgren et al. 1983); however, long-term glucocorticoid treatment associated with bone loss is well documented (Kanis et al. 2007).
Cortisol is the primary glucocorticoid produced in the adrenal cortex in many species, including marmots and bears (Kastner et al. 1977; Donahue et al. 2003b). In bears, cortisol increases significantly during hibernation (Palumbo et al. 1983; Harlow et al. 1990; Hellgren et al. 1993; Donahue et al. 2003a, 2003b). Wild American black bears have 28–39% higher average cortisol levels in the winter and spring compared to the summer and fall (Harlow et al. 1990). Hibernating little brown bats also have high circulating cortisol concentrations compared to active bats (Gustafson and Belt 1981). Unlike bears and bats, however, plasma cortisol decreased in marmots and European ground squirrels (Spermophilus citellus) during hibernation (Kastner et al. 1978; Shivatcheva et al. 1988; Tokuyama et al. 1991), but woodchucks did not experience changing cortisol levels between seasons despite a significant circadian rhythm (Florant and Weitzman 1980).
High levels of glucocorticoids during hibernation may contribute to a reduced number of osteoclasts by promoting apoptosis. This promotes reduced and balanced bone remodeling by decreasing resorption; however, glucocorticoid stimulation of RANKL in non-hibernating animals suggests that bone loss would be the primary outcome of increased cortisol during hibernation. Different seasonal patterns of cortisol concentrations among hibernators indicate that this hormone and its derivatives may have species-specific bone-related functions.
Neuropeptide-Y
NPY positively regulates the intake of food and the storage of fat. In bone marrow, NPY is produced by megakaryocytes, and NPY-immunoreactive fibers have been identified in bone lining and marrow cells. Similarly, NPY is expressed in osteoblasts, osteocytes, and potentially in osteoclasts (Igwe et al. 2009; Khor and Baldock 2012) in addition to a number of peripheral organs (e.g., pancreas and adrenal medulla). Furthermore, the distribution of NPY receptors spans multiple tissues, indicating a pronounced circulating role for NPY (Khor and Baldock 2012). NPY acts downstream of leptin and its production is inhibited by central leptin signaling which contributes to the homeostatic response to stimulate appetite and conserve energy (Allison et al. 2007). However, NPY acts independently of leptin in regulating bone metabolism (Ducy et al. 2000).
Like many neuroendocrine factors, the importance of NPY on the regulation of bone is complex but becoming increasingly apparent. When NPY is centrally over-expressed in mice, the volume and mass of vertebral bone decrease significantly (Ducy et al. 2000). In knockout mice, both NPY−\− and Y2−\− receptor in the hypothalamus, cause an increase in the formation and mineralization of bone (Allison and Herzog 2006; Allison et al. 2007; Baldock et al. 2009). mRNA levels for the Y2 receptor were not detected in bone suggesting a centrally mediated effect (Allison and Herzog 2006), however, NPY Y1 receptors on osteoblasts suggest that peripheral NPY may mediate bone metabolism (Baldock et al. 2007; Lundberg et al. 2007).
In grizzly bears, plasma NPY concentrations remained the same between hibernation and periods of activity (Gardi et al. 2011). Similarly, NPY mRNA expression patterns in the brains of hibernating and active ground squirrels (S. tridecemlineatus, Spermophilus richardsonii, and Spermophilus dauricus) are not different (Reuss et al. 1990; Xing et al. 2012). In greater mouse-tailed bats (Rhinopoma microphyllum) increased hypothalamic NPY mRNA expression corresponded with a high demand for increased mass of fat during summer (Levin et al. 2012). Interestingly, increased NPY mRNA expression was hypothesized to stimulate food intake during seasonal resistance to leptin associated with pre-hibernation. In a separate study in little brown bats, NPY expression remained elevated throughout hibernation (Laemle and Cotter 1992). Increased NPY expression (mRNA) was also found in hibernating jerboas (Jaculus orientalis) (El Ouezzani et al. 2001) and the serum of American black bears (Bradford 2010) in comparison to animals in summer. Of additional significance, NPY receptor Y1 may induce reduction of Tb associated with torpor (Dark and Pelz 2008). Thus, NPY may have different bone-related functions in different hibernating species and requires further investigation.
Serotonin
Peripheral serotonin is primarily produced in the gut by enterochromaffin cells and is involved in numerous aspects of digestion, food intake, and a host of other metabolic functions (Berger et al. 2009). Central serotonin acts to decrease appetite and inhibit gain in weight (Blundell 1984). Functional serotonin receptors exist in osteoblasts and osteocytes (Westbroek et al. 2001), and gut-derived serotonin may play a negative role in the maintenance of bone (Yadav et al. 2009). On the other hand, brain-derived serotonin cannot cross the BBB, and it acts on ventromedial hypothalamic neurons to promote bone formation in the absence of leptin (Yadav et al. 2009).
It is unknown if hyperleptinemia coupled with endogenous levels of serotonin during hibernation would be able to elicit changes in bone despite hibernation. Serum serotonin in American black bears is highest during pre-hibernation, decreases in hibernation, and begins to increase again in the post-hibernation season (Bradford et al. 2010). In woodchucks, however, serotonin concentrations in cerebral spinal fluid do not change significantly between seasons (Young et al. 1979), yet serotonin in cerebral spinal fluid in marmots is reported to be significantly higher during hibernation (Reid et al. 1992). Serotonin levels in the brain are lowest during entrance into hibernation in golden-mantled ground squirrels (Spafford and Pengelley 1971) compared to decreased serotonin protein expression in late hibernation experienced by little brown bats (Haymovits et al. 1976). Low levels of gut-derived serotonin during hibernation may result from decreased intestinal activity and may reduce the negative influence of peripheral serotonin on bone to contribute to the protection of bone during hibernation.
Parathyroid hormone
A calcitropic hormone, PTH works in conjunction with calcitonin to maintain serum calcium levels within a narrow physiological range. PTH promotes mobilization of calcium from bone by increasing bone resorption and the kidney’s production of 1,25-dihydroxyvitamin D in conditions of serum hypocalcemia (de Paula and Rosen 2010). Both single-dose and intermittent PTH treatments stimulate lipolysis of white adipose tissue and the production of glycerol in a dose-dependent manner, also linking PTH and adiposity (Gozariu et al. 1974; Sinha et al. 1976). Intermittent PTH treatment inhibits the differentiation of adipocytes (Rickard et al. 2006; Kulkarni et al. 2007). Evidence suggests that high PTH levels may contribute to decreased sensitivity to insulin (Kumar et al. 1994; Chiu et al. 2000), although other studies found no relationship between these hormones (Anastasilakis et al. 2008). Regardless, individuals with high body mass have high circulating PTH (Snijder et al. 2005; Ishimura et al. 2013).
In American black bears, PTH levels increased during hibernation and peaked in the post-hibernation season relative to pre-hibernation (Donahue et al. 2006a). Increased PTH during hibernation and post-hibernation would be expected to signal the release of calcium from the bone matrix, but bone resorption decreases (McGee et al. 2008; Bradford 2010) and serum calcium is unchanged (Floyd et al. 1990; Bradford 2010). However, the PTH assay used in these studies detects large C-terminal PTH (C-PTH) fragments in addition to the full protein (PTH 1–84). Large C-PTH fragments (e.g., 7–84) may have anti-resorptive effects on bone (Divieti et al. 2002; Langub et al. 2003). Considering that C-PTH fragments are produced in greater proportion than intact PTH (1–84) during renal failure in humans (Brossard et al. 1996), they may provide a protective function to bone by reducing bone resorption during hibernation (McGee et al. 2008).
Patients with primary hyperparathyroidism experience significant reductions in the cortical thickness of the iliac crest, while trabecular number increases compared to controls (Bilezikian et al. 1991). However, mild hyperparathyroidism may have a protective effect against post-menopausal osteoporosis (Dempster et al. 1999). Thus, PTH may act as an anabolic agent that helps preserve bone during hibernation in a similar manner as in post-menopausal women with mild hyperparathyroidism. PTH also promotes renal reabsorption of calcium; this may contribute to eucalcemia. Other cross-sectional and longitudinal studies in bears, however, indicate that there is no difference between seasonal PTH concentrations (Seger 2008; Seger et al. 2011; Vestergaard et al. 2011).
Vitamin D
Vitamin D3 is obtained through diet or from exposure of skin or fur to ultraviolet light (Vieth 1999; Hymoller and Jensen 2010). Vitamin D3 is converted into the active form of vitamin D, calcitriol, or 1,25-dihydroxyvitamin D (1,25(OH)2D), by hydroxylation of 25(OH)D in the kidney. Calcitriol is responsible for homeostasis of calcium and phosphate, maintaining normal bone turnover, and mineralization of the organic matrix by controlling gastrointestinal absorption of calcium, excretion of renal calcium and bone turnover. Vitamin D is also inversely associated with adiposity (Snijder et al. 2005).
Lower levels of 1,25(OH)2D are expected during hibernation because of reduced exposure to sun. The serum concentration of 25(OH)D was reported to be higher or unchanged in hibernating American black bears compared to non-hibernating bears (Donahue et al. 2006a; Seger 2008). However, bears’ concentrations of serum calcitriol were higher post-hibernation compared to hibernation (Seger et al. 2011; Vestergaard et al. 2011). Increased calcitriol is likely a consequence of the need for increased intestinal absorption of calcium when feeding resumes following hibernation. The low levels of calcitriol during hibernation may contribute to lower rates of bone remodeling (i.e., bone formation and resorption).
Role of sex hormones in bone and fat metabolism
Bone and adipose tissues also are greatly influenced by hormones of reproduction. Estrogen and androgen receptors (ER and AR, respectively) are found throughout bone and fat tissue and mediate the effects of sex hormones in conjunction with other endocrine factors (e.g., PTH), resulting in highly complex regulation of interrelated systems. Of considerable importance is the task of determining different mechanisms of these systems in mammalian hibernators compared to non-hibernating species as an evolutionary means of successfully surviving prolonged shortages in resources and extreme temperatures, and then be able to reproduce after hibernation. The literature covering sex hormones in hibernators is quite extensive and this review touches only on key points that relate to skeletal regulation.
Estrogens
Estradiol 17β is an estrogen produced by the sex organs, adrenal glands, or converted from testosterone by the P450 aromatase enzyme (Venken et al. 2008). Estrogen deficiency (e.g., ovariectomy) increases the mass of fat and the concentration of hypothalamic NPY, while decreasing sensitivity to leptin (Ainslie et al. 2001). Treatment with exogenous estrogen returned NPY levels to normal, reduced bone loss, and reduced the accumulation of fat (Malluche et al. 1986; Hill et al. 2008; Zengin et al. 2010). Effects of estrogen deficiency in bone include increased bone remodeling and decreased bone mineral density (Tozum et al. 2004). Additional osteoclast progenitors may result from increased production of osteoclastogenic cytokines that are normally suppressed by estrogens (Di Gregorio et al. 2001). Estradiol 17β can suppress osteoclastogenesis by enhancing the production of OPG and by decreasing receptor activator of RANKL in osteoblasts (Venken et al. 2008).
Estradiol 17β in male American black bears decreased from December to March (Tsubota et al. 1999), which may be the result of decreased testosterone production during denning and less testosterone being converted to estradiol. Estradiol 17β also has been measured in 23 captive adult female Japanese black bears (Ursus thibetanus japonicus), eight of which gave birth (Sato et al. 2000). The lowest levels of estradiol were observed in December with rises in January when most bears gave birth. Thus, low concentrations of estrogen would seemingly cause these animals to experience increased resistance to leptin, NPY levels, and bone resorption during hibernation. These patterns are not seen in association with the low estrogen levels reported during hibernation, but rather in the pre-hibernation season when hibernators are gaining fat in preparation for winter. European ground squirrels experience the highest levels of estradiol post-lactation, indicative of two annual estrus cycles (Millesi et al. 2008). Greater Asiatic yellow bats have low concentrations of estradiol during the ovulatory delay experienced during winter dormancy, December through mid-February (Krishna and Abhilasha 2000). This, however, was preceded and followed by high estradiol levels associated with recrudescence (October–November) and renewal of ovarian activity (mid-February through early March). Therefore, the effects of estrogen may be seasonally uncoupled from its influence on the metabolism both of bone and fat in hibernating mammals, as indicated by species-specific reproductive cycles.
Prolactin
Prolactin is a hormone that is produced by the lactotrope cells of the anterior pituitary gland (Seriwatanachai et al. 2009). It is responsible for the production of breast milk in postpartum women, although it also has an active role in the inhibition of adipocyte production of leptin and lipolysis in male and female rats (Brandebourg et al. 2007; Seriwatanachai et al. 2009). During pregnancy, prolactin causes a high turnover of bone that provides calcium for fetal growth and for production of breast milk (Seriwatanachai et al. 2008). Continued exposure to prolactin (e.g., during lactation), increases bone turnover, loss of calcium, osteopenia, and osteoporosis (Seriwatanachai et al. 2008).
The projected effects of prolactin on the health of bones are potentially significant in seasonally inactive, hibernating, female animals. The concentration of prolactin increases in December, 1 month prior to parturition (January to early February), relative to pre-hibernation in pregnant Japanese black bears and continues to rise post-parturition (Sato et al. 2001). Interestingly, prolactin is also high in the spring compared to other seasons in non-pregnant female bears. American male black bears experience little change in prolactin levels during hibernation (Tsubota et al. 1999); however, they increase significantly March through June (Howell-Skalla et al. 2000). Changes in prolactin concentrations in male marmots are linked to seasonal changes in photoperiod (Concannon et al. 1999). Increased prolactin may contribute to increased bone resorption in pregnant and lactating females bears during hibernation and to increased resorption in all bears, including males, following hibernation.
Testosterone
Testosterone is a sex hormone produced in the testicles of males and in the ovaries of females. In addition to its role as a sex hormone, testosterone has profound effects in fat and bone. Low testosterone contributes to the metabolic syndrome by influencing metabolic hormones, such as insulin, leptin, adiponectin, and ghrelin, to favor increasing adiposity (De Maddalena et al. 2012). To impact bone remodeling, testosterone is aromatized to estrogen and acts to increase the lifespan of osteoblasts and osteoclasts by preventing apoptosis (Riggs et al. 2002). In addition, there are receptors for testosterone on osteoblasts and osteoclasts (Vanderschueren and Vandenput 2000; Kung 2003). In vitro testosterone increases the proliferation and differentiation of osteoblast-like cells (Kung 2003), and stimulation of ARs in osteoblastic stromal cells suppresses osteoclast differentiation in bone marrow (Vanderschueren and Vandenput 2000). Testosterone also increases periosteal bone formation to enhance the overall mechanical strength of bone (Vanderschueren and Vandenput 2000; Riggs et al. 2002; Kung 2003). Interestingly, biosynthesis of testosterone is promoted by the osteocalcin receptor, GPRC6A, found in testes (Oury et al. 2011).
Hibernating male rodents experience involution of the testes throughout the dormant period. As hibernators emerge from hibernation, testicular activity resumes in preparation for mating (McMillin et al. 1976; Baldwin et al. 1985; Barnes 1986; Saboureau 1986; Barnes et al. 1988; Concannon et al. 1999). In American black bears, testicular recrudescence is experienced January through May, reflecting the later breeding season (May through July) in these animals (Howell-Skalla et al. 2000). Serum testosterone in sexually mature, male, captive and free-ranging American black bears remains rather constant year-round except for peaks in the spring (Tsubota et al. 1997, 1999; Howell-Skalla et al. 2000). The increase in testosterone in male animals in the spring is expected to have positive impacts on bone; however, there does not appear to be a large difference between the sexes in the properties of bone in most hibernating animals. Therefore, the biological role of testosterone in bone metabolism of hibernating animals remains to be defined for both sexes.
Summary and conclusions
It is apparent that, at least for bears, marmots, woodchucks, and ground squirrels, a general pattern of bone maintenance and eucalcemia can be explained by the lack of an increase in bone resorption that would otherwise be expected in an obese and physically inactive non-hibernating animal. The lack of a mechanical loading signal in non-hibernating animals results in the inhibition of nitric oxide (NO) which stimulates increased production of osteoblastic RANKL that in turn increases osteoclastogenesis, osteoclast activity, and bone resorption (Fig. 2A) (Rubin et al. 2000, 2003). The skeleton may fail to perceive the lack of loading associated with hibernation (Seger et al. 2011). This may occur through continued production of NO and subsequent inhibition of RANKL production (Fig. 2B). Furthermore, anabolic PTH mechanisms and C-terminal PTH anti-resorptive effects may contribute to preservation of bone during hibernation (Fig. 2B).
Fig. 2.
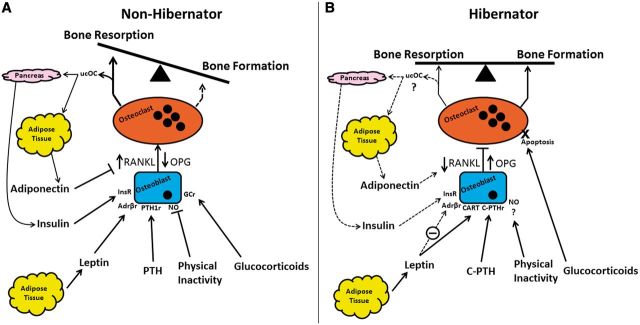
Some of the factors that influence osteoclastogenesis, osteoclast activity, and bone resorption in non-hibernators (A) compared to hibernators (B). InsR, insulin receptor; C-PTHr, C-PTH fragment receptor; GCr, glucocorticoid receptor; ucOC, uncarboxylated osteocalcin. Dashed lines represent reduced effects on bone in each pathway due to lowered bioactivity (e.g., adiponectin), impaired function (e.g., hyperinsulinemia, hyperleptinemia), or unbalanced processes (e.g., bone formation in non-hibernators during periods of high bone resorption). Arrows: activation; blunted arrows: blocked action; circled dash: impaired function; ?: mechanism still unknown; X: cell death.
The effects of seasonal adiposity on the skeleton of hibernators have considerable implications for the plasticity of the regulatory signaling pathways linking fat and bone. Leptin, insulin, and glucocorticoids, all acting through their individual receptors, primarily increase bone resorption through the RANKL/OPG pathway (Fig. 2A). Each agent, however, may have a unique role in regulating bone in hibernators. For instance, leptin, which is elevated for some time during the hibernation season in many hibernators, may fail to transport across the BBB and exert its catabolic effects on bone. Instead, it may act through hypothalamic arcuate projections to stimulate production of CART and inhibition of RANKL (Fig. 2B). Resistance may also occur with insulin and block insulin-induced bone resorption and uncarboxylation of osteocalcin from the bone matrix in animals that do not lower insulin concentration during hibernation. High circulating levels of glucocorticoids for extended periods of time may also promote osteoclastic apoptosis, resulting in the reduction of available cells to degrade the surface of the bone and slow the overall rate of bone loss (Fig. 2B); although additional investigation of the effects of glucocorticoids on the skeleton during hibernation is required.
The role of osteocalcin in energy balance provides a strong link in the cross-talk between the skeleton and adipose tissue in mice. This positive feed-back loop between insulin, uncarboxylated osteocalcin, and adiponectin demonstrates a mechanism regulating bone resorption, serum calcium, sensitivity to insulin, and energy balance as a whole (Fig. 2A). However, this area is still largely under-investigated and further research is required to understand the evolved mechanisms available to animals required to survive periods of extreme environmental conditions while maintaining the skeleton (Fig. 2B).
Although we propose that the complex pathways of various hormones are important in regulating bone remodeling, their actual biological contribution to the skeleton remains to be investigated in hibernators. Many of the hormones discussed above (e.g., adiponectin) have competing functions when it comes to energy demand versus skeletal remodeling. Regulation of energy requirements may take precedence over maintenance of bone during extreme environmental challenges, such as food shortage and low temperatures. In this case, endocrine and neuroendocrine functions likely override signals of mechanotransduction and skeletal metabolism is greatly reduced. Thus, care must be taken when interpreting the actions of single molecules on bone or interactions among several systems when survival is dependent on energy reserves.
The relationship between adipose tissue and bone, and the interacting neuropeptides and sex hormones involved in skeletal and fat metabolism pathways highlight the adaptation of highly coordinated endocrine functions to environmental challenges in hibernating animals. Indeed, the interconnected signaling between the metabolic systems of bone and fat is still largely being defined in non-hibernators. By understanding skeletal plasticity in mammals exposed to annual extremes in temperature and food shortages, it is possible to identify not only aspects of the evolution of hibernation itself but also the disease processes prevalent in biomedical research today.
Acknowledgments
We thank Sherri Wiseman for data collection and assistance with the manuscript.
Funding
This work was supported by Grant Number AR050420 from The National Institute of Arthritis and Musculoskeletal and Skin Diseases to S.W.D. Funding was also provided to G.L.F. by the Lipid Foundation at CSU (#645848).
References
- Agid R, Ambid L, Sable-Amplis R, Sicart R. Aspects of metabolic and endocrine changes in hibernation. In: Wang LCH, Hudson JW, editors. Strategies in cold: natural torpidity and thermogenesis. New York: Academic Press; 1978. pp. 499–540. [Google Scholar]
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–8. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- Allison SJ, Baldock PA, Herzog H. The control of bone remodeling by neuropeptide Y receptors. Peptides. 2007;28:320–5. doi: 10.1016/j.peptides.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Allison SJ, Herzog H. NPY and bone. Experientia Supplementum. 2006;95:171–82. doi: 10.1007/3-7643-7417-9_13. [DOI] [PubMed] [Google Scholar]
- Anastasilakis AD, Efstathiadou Z, Plevraki E, Koukoulis GN, Slavakis A, Kita M, Avramidis A. Effect of exogenous intermittent recombinant human PTH 1-34 administration and chronic endogenous parathyroid hormone excess on glucose homeostasis and insulin sensitivity. Horm Metab Res. 2008;40:702–7. doi: 10.1055/s-2008-1078729. [DOI] [PubMed] [Google Scholar]
- Azizi F, Mannix JE, Howard D, Nelson RA. Effect of winter sleep on pituitary-thyroid axis in American black bear. Am J Physiol. 1979;237:E227–30. doi: 10.1152/ajpendo.1979.237.3.E227. [DOI] [PubMed] [Google Scholar]
- Baldock PA, Allison SJ, Lundberg P, Lee NJ, Slack K, Lin EJ, Enriquez RF, McDonald MM, Zhang L, During MJ, et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J Biol Chem. 2007;282:19092–102. doi: 10.1074/jbc.M700644200. [DOI] [PubMed] [Google Scholar]
- Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, Lin EJ, Zhang L, Enriquez RF, Wong IP, et al. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One. 2009;4:e8415. doi: 10.1371/journal.pone.0008415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin BH, Tennant BC, Reimers TJ, Cowan RG, Concannon PW. Circannual changes in serum testosterone concentrations of adult and yearling woodchucks (Marmota monax) Biol Reprod. 1985;32:804–12. doi: 10.1095/biolreprod32.4.804. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE. The effects of high fat diets on the blood-brain barrier transport of leptin: failure or adaptation? Physiol Behav. 2006;88:244–8. doi: 10.1016/j.physbeh.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Barnes BM. Annual cycles of gonadotropins and androgens in the hibernating golden-mantled ground squirrel. Gen Comp Endocrinol. 1986;62:13–22. doi: 10.1016/0016-6480(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Barnes BM, Kretzmann M, Zucker I, Licht P. Plasma androgen and gonadotropin levels during hibernation and testicular maturation in golden-mantled ground squirrels. Biol Reprod. 1988;38:616–22. doi: 10.1095/biolreprod38.3.616. [DOI] [PubMed] [Google Scholar]
- Bauman WA. Seasonal changes in pancreatic insulin and glucagon in the little brown bat (Myotis lucifugus) Pancreas. 1990;5:342–6. doi: 10.1097/00006676-199005000-00015. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne RM, Levy MN, Koeppen BM, Stanton BA. The thyroid gland. In: Berne RM, Levy MN, Koeppen BM, Stanton BA, editors. Physiology. 5th ed. St. Louis: Elsevier, Inc; 2004. pp. 860–82. [Google Scholar]
- Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–9. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Silverberg SJ, Shane E, Parisien M, Dempster DW. Characterization and evaluation of asymptomatic primary hyperparathyroidism. J Bone Miner Res. 1991;6(Suppl 2):S85–9. doi: 10.1002/jbmr.5650061419. discussion S121–4. [DOI] [PubMed] [Google Scholar]
- Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260:R321–7. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Serotonin and appetite. Neuropharmacology. 1984;23:1537–51. doi: 10.1016/0028-3908(84)90098-4. [DOI] [PubMed] [Google Scholar]
- Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol. 2013;9:43–55. doi: 10.1038/nrendo.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes. 1997;46:1235–8. doi: 10.2337/diab.46.7.1235. [DOI] [PubMed] [Google Scholar]
- Bradford R, Buckendahl P, Gundberg C, Henriksen K, Vaughan M, Donahue SW. In The American Society for Bone and Mineral Research. Toronto: 2010. Serum osteocalcin is negatively correlated to insulin and adiponectin in hibernating bears. [Google Scholar]
- Bradford RM. Ph.D. Thesis. Biomedial engineering. Houghton: Michigan Technological University; 2010. Exploration of the role of serum factors in maintaining bone mass during hibernation in black bears; p. 189. [Google Scholar]
- Brandebourg TD, Bown JL, Ben-Jonathan N. Prolactin upregulates its receptors and inhibits lipolysis and leptin release in male rat adipose tissue. Biochem Biophys Res Commun. 2007;357:408–13. doi: 10.1016/j.bbrc.2007.03.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barre M, D'Amour P. Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab. 1996;81:3923–9. doi: 10.1210/jcem.81.11.8923839. [DOI] [PubMed] [Google Scholar]
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol-Reg I. 2000;279:R255–62. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep. 2005;3:98–102. doi: 10.1007/s11914-005-0017-7. [DOI] [PubMed] [Google Scholar]
- Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192:292–7. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–81. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–61. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- Castex C, Tahri A, Hoo-Paris R, Sutter BC. Insulin secretion in the hibernating edible dormouse (Glis glis): in vivo and in vitro studies. Comp Biochem Physiol A Comp Physiol. 1984;79:179–83. doi: 10.1016/0300-9629(84)90729-1. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Cheunsuang O, Morris R. Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia. 2005;52:228–33. doi: 10.1002/glia.20239. [DOI] [PubMed] [Google Scholar]
- Chiu KC, Chuang LM, Lee NP, Ryu JM, McGullam JL, Tsai GP, Saad MF. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49:1501–5. doi: 10.1053/meta.2000.17708. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Ki KH, Yang JY, Jang BY, Song JA, Baek WY, Kim JH, An JH, Kim SW, Kim SY, et al. Chronic central administration of Ghrelin increases bone mass through a mechanism independent of appetite regulation. PLoS One. 2013;8:e65505. doi: 10.1371/journal.pone.0065505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–8. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Concannon P, Levac K, Rawson R, Tennant B, Bensadoun A. Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. Am J Physiol Regul Integr Comp Physiol. 2001;281:951–9. doi: 10.1152/ajpregu.2001.281.3.R951. [DOI] [PubMed] [Google Scholar]
- Concannon PW, Castracane VD, Rawson RE, Tennant BC. Circannual changes in free thyroxine, prolactin, testes, and relative food intake in woodchucks, Marmota monax. Am J Physiol. 1999;277:R1401–9. doi: 10.1152/ajpregu.1999.277.5.R1401. [DOI] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr. 2005;25:469–97. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- Dark J, Pelz KM. NPY Y1 receptor antagonist prevents NPY-induced torpor-like hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R236–45. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- Davis D. Hibernation and circannual rhythms of food consumption in marmots and ground squirrels. Q Rev Biol. 1976;51:477–514. doi: 10.1086/409594. [DOI] [PubMed] [Google Scholar]
- De Maddalena C, Vodo S, Petroni A, Aloisi AM. Impact of testosterone on body fat composition. J Cell Physiol. 2012;227:3744–8. doi: 10.1002/jcp.24096. [DOI] [PubMed] [Google Scholar]
- de Paula FJ, Rosen CJ. Back to the future: revisiting parathyroid hormone and calcitonin control of bone remodeling. Horm Metab Res. 2010;42:299–306. doi: 10.1055/s-0030-1248255. [DOI] [PubMed] [Google Scholar]
- Delhanty PJ, van der Eerden BC, van der Velde M, Gauna C, Pols HA, Jahr H, Chiba H, van der Lely AJ, van Leeuwen JP. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol. 2006;188:37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- Delhanty PJ, van der Eerden BC, van Leeuwen JP. Ghrelin and bone. Biofactors. 2014;40:41–8. doi: 10.1002/biof.1120. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Wilson DM, Mann KG, Riggs BL. Effect of renal function on plasma levels of bone Gla-protein. J Clin Endocrinol Metab. 1983;57:1028–30. doi: 10.1210/jcem-57-5-1028. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Moonga BS, Stein LS, Horbert WR, Antakly T. Glucocorticoids inhibit bone resorption by isolated rat osteoclasts by enhancing apoptosis. J Endocrinol. 1997;154:397–406. doi: 10.1677/joe.0.1540397. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, et al. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab. 1999;84:1562–6. doi: 10.1210/jcem.84.5.5652. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17 beta-estradiol. J Clin Invest. 2001;107:803–12. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divieti P, John MR, Juppner H, Bringhurst FR. Human PTH-(7-84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology. 2002;143:171–6. doi: 10.1210/endo.143.1.8575. [DOI] [PubMed] [Google Scholar]
- Doherty AH. Ph.D. Thesis. Cell and molecular biology. Kent: Kent State Univeristy; 2013. The skeletal biology of hibernating woodchucks (Marmota monax) p. 269. [Google Scholar]
- Doherty AH, Frampton JD, Vinyard CJ. Hibernation does not reduce cortical bone density, area or second moments of inertia in woodchucks (Marmota monax) J Morphol. 2012;273:604–17. doi: 10.1002/jmor.20007. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Galley SA, Vaughan MR, Patterson-Buckendahl P, Demers LM, Vance JL, McGee ME. Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J Exp Biol. 2006a;209:1630–8. doi: 10.1242/jeb.02185. [DOI] [PubMed] [Google Scholar]
- Donahue SW, McGee ME, Harvey KB, Vaughan MR, Robbins CT. Hibernating bears as a model for preventing disuse osteoporosis. J Biomech. 2006b;39:1480–8. doi: 10.1016/j.jbiomech.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Vaughan MR, Demers LM, Donahue HJ. Bone formation is not impaired by hibernation (disuse) in black bears Ursus americanus. J Exp Biol. 2003a;206:4233–9. doi: 10.1242/jeb.00671. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Vaughan MR, Demers LM, Donahue HJ. Serum markers of bone metabolism show bone loss in hibernating bears. Clinl Orthop Relat Res. 2003b;408:295–301. doi: 10.1097/00003086-200303000-00040. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- El Ouezzani S, Lafon P, Tramu G, Magoul R. Neuropeptide Y gene expression in the jerboa arcuate nucleus: modulation by food deprivation and relationship with hibernation. Neurosci Lett. 2001;305:127–30. doi: 10.1016/s0304-3940(01)01825-0. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang XL, Liu XY, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier JS, Minaker KL, Landsberg L, Young JB, Pallotta J, Rowe JW. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes. 1982;31:132–5. doi: 10.2337/diab.31.2.132. [DOI] [PubMed] [Google Scholar]
- Florant GL, Bauman WA. Seasonal variations in carbohydrate metabolism in mammalian hibernators: insulin and body weight changes. In: Van Itallie TB, Hirsch J, editors. Recent advances in obesity research. London: John Libbey; 1984. pp. 57–64. [Google Scholar]
- Florant GL, Fenn AM, Healy JE, Wilkerson GK, Handa RJ. To eat or not to eat: the effect of AICAR on food intake regulation in yellow-bellied marmots (Marmota flaviventris) J Exp Biol. 2010;213:2031–7. doi: 10.1242/jeb.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florant GL, Heller HC. CNS regulation of body temperature in euthermic and hibernating marmots (Marmota flaviventris) Am J Physiol. 1977;232:R203–8. doi: 10.1152/ajpregu.1977.232.5.R203. [DOI] [PubMed] [Google Scholar]
- Florant GL, Lawrence AK, Williams K, Bauman WA. Seasonal changes in pancreatic B-cell function in euthermic yellow-bellied marmots. Am J Physiol. 1985;249:R159–65. doi: 10.1152/ajpregu.1985.249.2.R159. [DOI] [PubMed] [Google Scholar]
- Florant GL, Porst H, Peiffer A, Hudachek SF, Pittman C, Summers SA, Rajala MW, Scherer PF. Fat-cell mass, serum leptin and adiponectin changes during weight gain and loss in yellow-bellied marmots (Marmota flaviventris) J Comp Physiol B. 2004;174:633–9. doi: 10.1007/s00360-004-0454-0. [DOI] [PubMed] [Google Scholar]
- Florant GL, Richardson RD, Mahan S, Singer L, Woods SC. Seasonal changes in CSF insulin levels in marmots: insulin may not be a satiety signal for fasting in winter. Am J Physiol. 1991;260:R712–6. doi: 10.1152/ajpregu.1991.260.4.R712. [DOI] [PubMed] [Google Scholar]
- Florant GL, Richter M, Fried SK. The effect of ambient temperature on body mass, torpor, food intake, and leptin levels: implications on the regulation of food intake in mammalian hibernators. In: Ruf T, Bieber C, Arnold A, Miller C, editors. Living in a seasaonal world: thermoregulatory and metabolic adaptations. New York: Springer; 2012. pp. 507–18. [Google Scholar]
- Florant GL, Weitzman ED. Diurnal and episodic pattern of plasma-cortisol during fall and spring in young and old woodchucks (Marmota monax) Comp Biochem Physiol A Comp Physiol. 1980;66:575–81. [Google Scholar]
- Floyd T, Nelson RA, Wynne GF. Calcium and bone metabolic homeostasis in active and denning black bears (Ursus americanus) Clin Orthop Relat Res. 1990;255:301–9. [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, Takeda S, Takeuchi Y, Fukumoto S, Kangawa K, et al. Ghrelin directly regulates bone formation. J Bone Miner Res. 2005;20:790–8. doi: 10.1359/JBMR.041237. [DOI] [PubMed] [Google Scholar]
- Galster W, Morrison PR. Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol. 1975;228:325–30. doi: 10.1152/ajplegacy.1975.228.1.325. [DOI] [PubMed] [Google Scholar]
- Gardi J, Nelson OL, Robbins CT, Szentirmai E, Kapas L, Krueger JM. Energy homeostasis regulatory peptides in hibernating grizzly bears. Gen Comp Endocrinol. 2011;172:181–3. doi: 10.1016/j.ygcen.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–74. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Geiser F. Hibernation. Curr Biol. 2013;23:R188–93. doi: 10.1016/j.cub.2013.01.062. [DOI] [PubMed] [Google Scholar]
- Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–6. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- Gozariu L, Forster K, Faulhaber JD, Minne H, Ziegler R. Parathyroid hormone and calcitonin: influences upon lipolysis of human adipose tissue. Horm Metab Res. 1974;6:243–5. doi: 10.1055/s-0028-1095713. [DOI] [PubMed] [Google Scholar]
- Gustafson AW, Belt WD. The adrenal cortex during activity and hibernation in the male little brown bat, Myotis lucifugus lucifugus: annual rhythm of plasma cortisol levels. Gen Comp Endocrinol. 1981;44:269–78. doi: 10.1016/0016-6480(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HJ, Beck TDI, Walters LM, Greenhouse SS. Seasonal serum glucose, progesterone, and cortisol levels of black bears (Ursus americanus) Can J Zool. 1990;68:183–7. [Google Scholar]
- Harlow HJ, Lohuis T, Beck TD, Iaizzo PA. Muscle strength in overwintering bears. Nature. 2001;409:997. doi: 10.1038/35059165. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13:51–9. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Haymovits A, Gershon MD, Nunez EA. Calcitonin, serotonin and parafollicular cell granules during the hibernation activity cycle in the bat. Proc Soc Exp Biol Med. 1976;153:383–7. doi: 10.3181/00379727-153-39551. [DOI] [PubMed] [Google Scholar]
- He L, Pan Y, He G, Lin B, Liao CC, Zuo X, Yuan L. Structural and functional studies of leptins from hibernating and non-hibernating bats. Gen Comp Endocrinol. 2010;168:29–35. doi: 10.1016/j.ygcen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Healy JE, Bateman JL, Ostrom CE, Florant GL. Peripheral ghrelin stimulates feeding behavior and positive energy balance in a sciurid hibernator. Horm Behav. 2011;59:512–9. doi: 10.1016/j.yhbeh.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy JE, Florant GL. Ghrelin, leptin, and fatty acids in free-living Callospermophilus lateralis (golden-mantled ground squirrels) In: Ruf T, Bieber C, Arnold A, Millesi E, editors. Living in a seasonal world: thermoregulatory and metabolic adaptations. New York: Springer; 2012. pp. 519–30. [Google Scholar]
- Healy JE, Ostrom CE, Wilkerson GK, Florant GL. Plasma ghrelin concentrations change with physiological state in a sciurid hibernator (Spermophilus lateralis) Gen Comp Endocrinol. 2010;166:372–8. doi: 10.1016/j.ygcen.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren EC, Rogers LL, Seal US. Serum chemistry and hematology of black bears—physiological indexes of habitat quality or seasonal patterns. J Mammal. 1993;74:304–15. [Google Scholar]
- Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51:3412–9. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks WA, Flier JS. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–83. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–32. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissa R, Hohtola E, Tuomala T, Laine T, Kallio H. Seasonal changes in fatty acids and leptin contents in the plasma of the European brown bear (Ursus arctos arcctos) Ann Zool Fennici. 1998;35:215–24. [Google Scholar]
- Hoehn KL, Hudachek SF, Summers SA, Florant GL. Seasonal, tissue-specific regulation of Akt/protein kinase B and glycogen synthase in hibernators. Am J Physiol Regul Integr Comp Physiol. 2004;286:R498–504. doi: 10.1152/ajpregu.00509.2003. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382–9. doi: 10.1210/endo.140.10.7034. [DOI] [PubMed] [Google Scholar]
- Hoo-Paris R, Aina E, Castex C, Sutter B. In vitro B cell response to glucose in the hibernating hedgehog: comparison with the homeothermic hedgehog and the rat. Comp Biochem Physiol A Comp Physiol. 1984;78:559–63. doi: 10.1016/0300-9629(84)90596-6. [DOI] [PubMed] [Google Scholar]
- Hoo-Paris R, Castex C, Sutter BC. Plasma glucose and insulin in the hibernating hedgehog. Diabete Metab. 1978;4:13–8. [PubMed] [Google Scholar]
- Howell-Skalla LA, Bunick D, Nelson RA, Bahr JM. Testicular recrudescence in the male black bear (Ursus americanus): changes in testicular luteinizing hormone-, follicle-stimulating hormone-, and prolactin-receptor ribonucleic acid abundance and dependency on prolactin. Biol Reprod. 2000;63:440–7. doi: 10.1095/biolreprod63.2.440. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–54. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Thomas DW, Kramer DL. The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol Biochem Zool. 2003;76:165–79. doi: 10.1086/367950. [DOI] [PubMed] [Google Scholar]
- Hymoller L, Jensen SK. Vitamin D(3) synthesis in the entire skin surface of dairy cows despite hair coverage. J Dairy Sci. 2010;93:2025–9. doi: 10.3168/jds.2009-2991. [DOI] [PubMed] [Google Scholar]
- Igwe JC, Jiang X, Paic F, Ma L, Adams DJ, Baldock PA, Pilbeam CC, Kalajzic I. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J Cell Biochem. 2009;108:621–30. doi: 10.1002/jcb.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui A. Feeding and body-weight regulation by hypothalamic neuropeptides—mediation of the actions of leptin. Trends Neurosci. 1999;22:62–7. doi: 10.1016/s0166-2236(98)01292-2. [DOI] [PubMed] [Google Scholar]
- Ishimura E, Okuno S, Tsuboniwa N, Norimine K, Fukumoto S, Yamakawa K, Yamakawa T, Shoji S, Nishizawa Y, Inaba M. Significant positive association between parathyroid hormone and fat mass and lean mass in chronic hemodialysis patients. J Clin Endocrinol Metab. 2013;98:1264–70. doi: 10.1210/jc.2012-3883. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M. Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess. 2007;11:iii–iv. doi: 10.3310/hta11070. ix–xi, 1–231. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–53. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Kastner PR, Zatzman ML, South FE, Johnson JA. Renin-angiotensin-aldosterone system of the normothermic marmot. Am J Physiol. 1977;233:R37–43. doi: 10.1152/ajpregu.1977.233.1.R37. [DOI] [PubMed] [Google Scholar]
- Kastner PR, Zatzman ML, South FE, Johnson JA. Renin-angiotensin-aldosterone system of the hibernating marmot. Am J Physiol. 1978;234:R178–82. doi: 10.1152/ajpregu.1978.234.5.R178. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;288:R716–22. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- Khor EC, Baldock P. The NPY system and its neural and neuroendocrine regulation of bone. Curr Osteoporos Rep. 2012;10:160–8. doi: 10.1007/s11914-012-0102-7. [DOI] [PubMed] [Google Scholar]
- Kim MS, Namkoong C, Kim HS, Jang PG, Kim Pak YM, Katakami H, Park JY, Lee KU. Chronic central administration of ghrelin reverses the effects of leptin. Int J Obes Relat Metab Disord. 2004;28:1264–71. doi: 10.1038/sj.ijo.0802647. [DOI] [PubMed] [Google Scholar]
- Kim SW, Her SJ, Park SJ, Kim D, Park KS, Lee HK, Han BH, Kim MS, Shin CS, Kim SY. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone. 2005;37:359–69. doi: 10.1016/j.bone.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Koyama H, Nakade O, Takada Y, Kaku T, Lau KH. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J Bone Miner Res. 2002;17:1219–29. doi: 10.1359/jbmr.2002.17.7.1219. [DOI] [PubMed] [Google Scholar]
- Krishna A, Abhilasha S. Proliferative activity of follicles and serum steroid concentration in Scotophilus heathi (vespertilionid bat) during the period of delayed ovulation. Can J Zool. 2000;78:1301–8. [Google Scholar]
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Schor N, Richardson C, Silvia BA, Kunz TH, Widmaier EP. Dissociation of leptin secretion and adiposity during prehibernatory fattening in little brown bats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1277–81. doi: 10.1152/ajpregu.2000.279.4.R1277. [DOI] [PubMed] [Google Scholar]
- Kulkarni NH, Wei T, Kumar A, Dow ER, Stewart TR, Shou J, N'Cho M, Sterchi DL, Gitter BD, Higgs RE, et al. Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J Cell Biochem. 2007;102:1504–18. doi: 10.1002/jcb.21374. [DOI] [PubMed] [Google Scholar]
- Kumar S, Olukoga AO, Gordon C, Mawer EB, France M, Hosker JP, Davies M, Boulton AJ. Impaired glucose tolerance and insulin insensitivity in primary hyperparathyroidism. Clin Endocrinol (Oxf) 1994;40:47–53. doi: 10.1111/j.1365-2265.1994.tb02442.x. [DOI] [PubMed] [Google Scholar]
- Kung AW. Androgen and bone mass in men. Asian J Androl. 2003;5:148–54. [PubMed] [Google Scholar]
- Laemle LK, Cotter JR. Neuropeptide Y-like immunoreactivity in the diencephalon of the little brown bat (Myotis lucifugus): localized variations with physiological state. J Comp Neurol. 1992;316:447–58. doi: 10.1002/cne.903160405. [DOI] [PubMed] [Google Scholar]
- Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH. Administration of PTH-(7-84) antagonizes the effects of PTH-(1-84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135–8. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- Laurila M, Suomalainen P. Studies in the physiology of the hibernating hedgehog. 19. The changes in the insulin level induced by seasons and hibernation cycle. Ann Acad Sci Fenn Biol. 1974;0:1–40. [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19:161–6. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E, Yom-Tov Y, Hefetz A, Kronfeld-Schor N. Changes in diet, body mass and fatty acid composition during pre-hibernation in a subtropical bat in relation to NPY and AgRP expression. J Comp Physiol B. 2012;183:157–66. doi: 10.1007/s00360-012-0689-0. [DOI] [PubMed] [Google Scholar]
- Li CY, Price C, Delisser K, Nasser P, Laudier D, Clement M, Jepsen KJ, Schaffler MB. Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res. 2005;20:117–24. doi: 10.1359/JBMR.041010. [DOI] [PubMed] [Google Scholar]
- Lindgren JU, Johnell O, DeLuca HF. Studies of bone tissue in rats treated by prednisolone and 1,25-(OH)2D3. Clin Orthop Relat Res. 1983:264–8. [PubMed] [Google Scholar]
- Lohuis TD, Harlow HJ, Beck TD, Iaizzo PA. Hibernating bears conserve muscle strength and maintain fatigue resistance. Physiol Biochem Zool. 2007;80:257–69. doi: 10.1086/513190. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Allison SJ, Lee NJ, Baldock PA, Brouard N, Rost S, Enriquez RF, Sainsbury A, Lamghari M, Simmons P, et al. Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J Biol Chem. 2007;282:19082–91. doi: 10.1074/jbc.M609629200. [DOI] [PubMed] [Google Scholar]
- Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman JM. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA. 1995;92:6957–60. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malluche HH, Faugere MC, Rush M, Friedler R. Osteoblastic insufficiency is responsible for maintenance of osteopenia after loss of ovarian function in experimental beagle dogs. Endocrinology. 1986;119:2649–54. doi: 10.1210/endo-119-6-2649. [DOI] [PubMed] [Google Scholar]
- Mathey J, Horcajada-Molteni MN, Chanteranne B, Picherit C, Puel C, Lebecque P, Cubizoles C, Davicco MJ, Coxam V, Barlet JP. Bone mass in obese diabetic Zucker rats: influence of treadmill running. Calcif Tissue Int. 2002;70:305–11. doi: 10.1007/s00223-001-2077-8. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E. Cortisol inhibits the synthesis of insulin-like growth factor-I in skeletal cells. Endocrinology. 1990;126:1569–75. doi: 10.1210/endo-126-3-1569. [DOI] [PubMed] [Google Scholar]
- McGee-Lawrence ME, Wojda SJ, Barlow LN, Drummer TD, Castillo AB, Kennedy O, Condon KW, Auger J, Black HL, Nelson OL, Robbins CT, Donahue SW. Grizzly bears (Ursus arctos horribilis) and black bears (Ursus americanus) prevent trabecular bone loss during disuse (hibernation) Bone. 2009a;45:1186–91. doi: 10.1016/j.bone.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Lawrence ME, Wojda SJ, Barlow LN, Drummer TD, Bunnell K, Auger J, Black HL, Donahue SW. Six months of disuse during hibernation does not increase intracortical porosity or decrease cortical bone geometry, strength, or mineralization in black bear (Ursus americanus) femurs. J Biomech. 2009b;42:1378–83. doi: 10.1016/j.jbiomech.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee ME, Maki AJ, Johnson SE, Nelson OL, Robbins CT, Donahue SW. Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (Ursus arctos horribilis) Bone. 2008;42:396–404. doi: 10.1016/j.bone.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin JM, Seal US, Rogers L, Erickson AW. Annual testosterone rhythm in the black bear (Ursus americanus) Biol Reprod. 1976;15:163–7. doi: 10.1095/biolreprod15.2.163. [DOI] [PubMed] [Google Scholar]
- Melvin RG, Andrews MT. Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol Metab. 2009;20:490–8. doi: 10.1016/j.tem.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millesi E, Strauss A, Burger T, Hoffmann IE, Walzl M. Follicular development in European ground squirrels (Spermophilus citellus) in different phases of the annual cycle. Reproduction. 2008;136:205–10. doi: 10.1530/REP-08-0090. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Boshes M. Meal patterns and food intakes of ground squirrels during circannual cycles. Appetite. 1986;7:163–75. doi: 10.1016/s0195-6663(86)80016-2. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Wahner HW, Jones JD, Ellefson RD, Zollman PE. Metabolism of Bears before, during, and after Winter Sleep. Am J Physiol. 1973;224:491–6. doi: 10.1152/ajplegacy.1973.224.2.491. [DOI] [PubMed] [Google Scholar]
- Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–6. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo PJ, Wellik DL, Bagley NA, Nelson RA. Insulin and glucagon responses in the hibernating black bear. Int Conf Bear Res Manage. 1983;5:291–6. [Google Scholar]
- Pardy CK, Wohl GR, Ukrainetz PJ, Sawers A, Boyd SK, Zernicke RF. Maintenance of bone mass and architecture in denning black bears (Ursus americanus) J Zool Lond. 2004;263:359–64. [Google Scholar]
- Patsch JM, Kiefer FW, Varga P, Pail P, Rauner M, Stupphann D, Resch H, Moser D, Zysset PK, Stulnig TM, et al. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism. 2011;60:243–9. doi: 10.1016/j.metabol.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60:1500–10. doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Powers PS. Osteoporosis and eating disorders. J Pediatr Adolesc Gynecol. 1999;12:51–7. doi: 10.1016/s1083-3188(00)86626-7. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Reid MS, Kilduff TS, Romero LM, Florant GL, Dement WC, Heller HC. Monoaminc and metabolite levels in the cerebrospinal fluid of hibernating and euthermic marmots. J Sleep Res. 1992;1:45–50. doi: 10.1111/j.1365-2869.1992.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Reuss S, Hurlbut EC, Speh JC, Moore RY. Neuropeptide Y localization in telencephalic and diencephalic structures of the ground squirrel brain. Am J Anat. 1990;188:163–74. doi: 10.1002/aja.1001880206. [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Wang FL, Rodriguez-Rojas AM, Wu Z, Trice WJ, Hoffman SJ, Votta B, Stroup GB, Kumar S, Nuttall ME. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39:1361–72. doi: 10.1016/j.bone.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, et al. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–31. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy T, Nanes MS, Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol. 2000;278:C1126–32. doi: 10.1152/ajpcell.2000.278.6.C1126. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy TC, Zhu L, Roy E, Nanes MS, Fan X. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J Biol Chem. 2003;278:34018–25. doi: 10.1074/jbc.M302822200. [DOI] [PubMed] [Google Scholar]
- Saboureau M. Hibernation in the hedgehog: influence of external and internal factors. In: Heller HC, Musacchia XJ, Wang LCH, editors. Living in the cold: physiological and biochemical adaptations. New York: Elsevier Science Publishing Co., Inc; 1986. pp. 253–63. [Google Scholar]
- Sato M, Tsubota T, Komatsu T, Watanabe G, Taya K, Murase T, Kita I, Kudo T. Changes in sex steroids, gonadotropins, prolactin, and inhibin in pregnant and nonpregnant Japanese black bears (Ursus thibetanus japonicus) Biol Reprod. 2001;65:1006–13. doi: 10.1095/biolreprod65.4.1006. [DOI] [PubMed] [Google Scholar]
- Sato M, Tsubota T, Yamamoto K, Komatsu T, Hashimoto Y, Katayama A, Hazumi T, Kita I, Kudo T. Serum progesterone and estradiol-17beta concentrations in captive and free-ranging adult female japanese black bears (Ursus thibetanus japonicus) J Vet Med Sci. 2000;62:415–20. doi: 10.1292/jvms.62.415. [DOI] [PubMed] [Google Scholar]
- Schilling A, Holzmann T, Rueger J, Karsenty G. Leptin regulates bone mass in rats. Bone. 2001 S88:OR74. [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–23. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Seger RL. Ph.D. Thesis. Ecology and environmental sciences. Orono: University of Maine; 2008. Elucidating the mechanism for maintaining eucalcemia despite immobility and anuria in the hibernating black bear (Ursus americanus) p. 114. [Google Scholar]
- Seger RL, Cross RA, Rosen CJ, Causey RC, Gundberg CM, Carpenter TO, Chen TC, Halteman WA, Holick MF, Jakubas WJ, et al. Investigating the mechanism for maintaining eucalcemia despite immobility and anuria in the hibernating American black bear (Ursus americanus) Bone. 2011;49:1205–12. doi: 10.1016/j.bone.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Seriwatanachai D, Krishnamra N, van Leeuwen JP. Evidence for direct effects of prolactin on human osteoblasts: inhibition of cell growth and mineralization. J Cell Biochem. 2009;107:677–85. doi: 10.1002/jcb.22161. [DOI] [PubMed] [Google Scholar]
- Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, Suthiphongchai T, Krishnamra N. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone. 2008;42:535–46. doi: 10.1016/j.bone.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–29. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- Shivatcheva TM, Ankov VK, Hadjioloff AI. Circannual fluctuations of the serum cortisol in the European ground squirrel, Citellus citellus L. Comp Biochem Physiol A Comp Physiol. 1988;90:515–8. doi: 10.1016/0300-9629(88)90229-0. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- Sinha TK, Thajchayapong P, Queener SF, Allen DO, Bell NH. On the lipolytic action of parathyroid hormone in man. Metabolism. 1976;25:251–60. doi: 10.1016/0026-0495(76)90083-4. [DOI] [PubMed] [Google Scholar]
- Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–23. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- Spafford DC, Pengelley ET. The influence of the neurohumor serotonin on hibernation in the golden-mantled ground squirrel, Citellus lateralis. Comp Biochem Physiol A Comp Physiol. 1971;38:239–50. doi: 10.1016/0300-9629(71)90051-x. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Krishna A. Adiposity associated rise in leptin impairs ovarian activity during winter dormancy in Vespertilionid bat, Scotophilus heathi. Reproduction. 2007;133:165–76. doi: 10.1530/rep.1.01019. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Krishna A. Melatonin modulates glucose homeostasis during winter dormancy in a vespertilionid bat, Scotophilus heathi. Comp Biochem Physiol A Mol Integr Physiol. 2010;155:392–400. doi: 10.1016/j.cbpa.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–92. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression: the biochemistry of mammalian hibernation. Adv Clin Chem. 2010;52:77–108. [PubMed] [Google Scholar]
- Swoap SJ. Why one enters torpor: focus on “NPY Y1 receptor antagonist prevents NPY-induced torpor-like hypothermia in cold-acclimated Siberian hamsters”. Am J Physiol Regul Integr Comp Physiol. 2008;294:R234–5. doi: 10.1152/ajpregu.00773.2007. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Tobias J, Chambers TJ. Glucocorticoids impair bone resorptive activity and viability of osteoclasts disaggregated from neonatal rat long bones. Endocrinology. 1989;125:1290–5. doi: 10.1210/endo-125-3-1290. [DOI] [PubMed] [Google Scholar]
- Tøien Ø, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331:906–9. doi: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- Tokuyama K, Galantino HL, Green R, Florant GL. Seasonal glucose uptake in marmots (Marmota flaviventris): the role of pancreatic hormones. Comp Biochem Physiol A Comp Physiol. 1991;100:925–30. doi: 10.1016/0300-9629(91)90316-5. [DOI] [PubMed] [Google Scholar]
- Tomasi TE, Hellgren EC, Tucker TJ. Thyroid hormone concentrations in black bears (Ursus americanus): hibernation and pregnancy effects. Gen Comp Endocrinol. 1998;109:192–9. doi: 10.1006/gcen.1997.7018. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–5. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- Townsend K, Schulz LC, Widmaier EP. Prehibernatory changes in leptin receptor and signaling pathways in bat hypothalamus. J Exp Zool A Comp Exp Biol. 2006;305A:185. [Google Scholar]
- Townsend KL, Kunz TH, Widmaier EP. Changes in body mass, serum leptin, and mRNA levels of leptin receptor isoforms during the premigratory period in Myotis lucifugus. J Comp Physiol B. 2008;178:217–23. doi: 10.1007/s00360-007-0215-y. [DOI] [PubMed] [Google Scholar]
- Tozum TF, Oppenlander ME, Koh-Paige AJ, Robins DM, McCauley LK. Effects of sex steroid receptor specificity in the regulation of skeletal metabolism. Calcif Tissue Int. 2004;75:60–70. doi: 10.1007/s00223-004-0119-8. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tsubota T, Garshelis DL, Nelson RA, Bahr JM. Sex steroid and prolactin profiles in male American black bears (Ursus americanus) during denning. J Vet Med Sci. 1999;61:81–3. doi: 10.1292/jvms.61.81. [DOI] [PubMed] [Google Scholar]
- Tsubota T, Howell-Skalla L, Nitta H, Osawa Y, Mason JI, Meiers PG, Nelson RA, Bahr JM. Seasonal changes in spermatogenesis and testicular steroidogenesis in the male black bear Ursus americanus. J Reprod Fertil. 1997;109:21–7. doi: 10.1530/jrf.0.1090021. [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214:301–5. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–90. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschueren D, Vandenput L. Androgens and osteoporosis. Andrologia. 2000;32:125–30. doi: 10.1046/j.1439-0272.2000.00333.x. [DOI] [PubMed] [Google Scholar]
- Venken K, Boonen S, Bouillon R, Vanderschueren D. Gonadal steroids. In: Rosen CJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 7th ed. Washington (DC): American Society for Bone and Mineral Research; 2008. pp. 117–23. [Google Scholar]
- Vestergaard P, Stoen OG, Swenson JE, Mosekilde L, Heickendorff L, Frobert O. Vitamin D status and bone and connective tissue turnover in brown bears (Ursus arctos) during hibernation and the active state. PLoS One. 2011;6:1–6. doi: 10.1371/journal.pone.0021483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- Ward JM, Armitage KB. Circannual rhythms of food consumption, body mass, and metabolism in yellow-bellied marmots. Comp Biochem Physiol A. 1981;69:621–6. [Google Scholar]
- Ware JV, Nelson OL, Robbins CT, Carter PA, Sarver BA, Jansen HT. Endocrine rhythms in the brown bear (Ursus arctos): evidence supporting selection for decreased pineal gland size. Physiol Rep. 2013;1:e00048. doi: 10.1002/phy2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenberg GM, Holland JC. The circannual variations of thyroid activity in the woodchuck (Marmota monax) Comp Biochem Physiol A Comp Physiol. 1973;44:775–800. doi: 10.1016/0300-9629(73)90141-2. [DOI] [PubMed] [Google Scholar]
- Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. J Biol Chem. 2001;276:28961–8. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- Xing X, Sun MY, Peng X, Song SY, Yang M. . Expression of orexigenic and anorexigenic neuropeptides before and during hibernation in the daurian ground squirrel (Spermophilus dauricus) In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a seasonal world: thermoregulatory and metabolic adaptations. New York: Springer Heidelberg; 2012. pp. 543–57. [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–89. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA. Interrelationships between body weight, food consumption and plasma thyroid hormone concentration cycles in the woodchuck, Marmota monax. Comp Biochem Physiol A Comp Physiol. 1984;77:533–6. doi: 10.1016/0300-9629(84)90223-8. [DOI] [PubMed] [Google Scholar]
- Young RA, Robinson DS, Vagenakis AG, Saavedra JM, Lovenberg W, Krupp PP, Danforth E., Jr Brain TRH, monoamines, tyrosine hydoxylase, and tryptophan hydroxylase in the woodchuck, Maromota monax, during the hibernation season. Comp Biochem Physiol C. 1979;63C:319–23. doi: 10.1016/0306-4492(79)90081-9. [DOI] [PubMed] [Google Scholar]
- Zengin A, Zhang L, Herzog H, Baldock PA, Sainsbury A. Neuropeptide Y and sex hormone interactions in humoral and neuronal regulation of bone and fat. Trends Endocrinol Metab. 2010;21:411–8. doi: 10.1016/j.tem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]