Abstract
Ecoimmunology is an example of how fruitful integrative approaches to biology can be. Since its emergence, ecoimmunology has sparked constructive debate on a wide range of topics, from the molecular mechanics of immune responses to the role of immunity in shaping the evolution of life histories. To complement the symposium Methods and Mechanisms in Ecoimmunology and commemorate the inception of the Division of Ecoimmunology and Disease Ecology within the Society for Integrative and Comparative Biology, we appraise the origins of ecoimmunology, with a focus on its continuing and valuable integration with disease ecology. Arguably, the greatest contribution of ecoimmunology to wider biology has been the establishment of immunity as an integral part of organismal biology, one that may be regulated to maximize fitness in the context of costs, constraints, and complex interactions. We discuss historical impediments and ongoing progress in ecoimmunology, in particular the thorny issue of what ecoimmunologists should, should not, or cannot measure, and what novel contributions ecoimmunologists have made to the understanding of host–parasite interactions. Finally, we highlight some areas to which ecoimmunology is likely to contribute in the near future.
Ecoimmunology before 2014
In 2014, the discipline of ecological immunology (Sheldon and Verhulst 1996), or ecoimmunology, is going strong (Fig. 1). It comprises diverse approaches and has drawn on, and contributed to, the techniques and conceptual foundations of many disciplines. It addresses questions at multiple levels of biological organization, from comparative studies on the evolution of immunity (e.g., Nunn 2002) to investigations of the short-term physiological dynamics of individuals (e.g., Buehler et al. 2011). It is therefore a truly integrative approach to understanding biological pattern and process (Wake 2003).
Fig. 1.
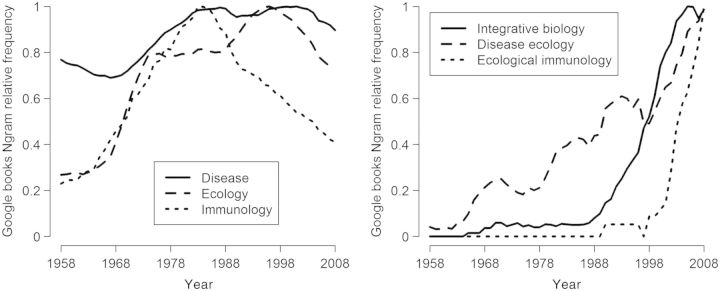
The occurrence of the search terms “disease”, “ecology”, “immunology”, “integrative biology”, “disease ecology”, and “ecological immunology” in Google books Ngram Viewer, a tool for searching the digitized text of ∼4% of all books ever published in English (Michel et al. 2011). Ngram Viewer provides the proportion of words (or phrases composed of the same number of words) in the available corpus of text that match the search term (dubbed “Ngram”). Here, in order to show trends over time independently of absolute frequency, we scaled outputs so that the maximum proportion for each search term between 1958 and 2008 was equal to the maximum for the most commonly occurring search term, which was “disease”. The un-scaled maximum proportions were: “disease” 1.23 × 10−4%, “ecology” 8.71 × 10−6%, “immunology” 1.01 × 10−6%, “integrative biology” 4.53 × 10−9%, “disease ecology” 1.17 × 10−8%, and “ecological immunology” 6.87 × 10−10%.
The current state of ecoimmunology is the result of more than three decades of wide-ranging research, which arguably was initiated by the proposition of the immunocompetence handicap hypothesis (ICHH; Folstad and Karter 1992). The ICHH was a product of behavioral ecology, which proposed an immune-mediated mechanism to explain variation in sexually selected traits of males. It built on the ideas of the handicap principle (Zahavi 1975) and parasite-mediated selection (Hamilton and Zuk 1982) by suggesting that males’ sexual ornaments were handicaps due to the immunosuppressive effects of the testosterone required for their expression. As it turned out, of course, things were not so simple (Roberts et al. 2004), and the major contribution of the ICHH became the appreciation that the relationship between the neuroendocrine and immune systems is more complex than originally thought, but nonetheless important in the evolution of sexual, and other, traits (Westneat and Birkhead 1998; Demas et al. 2011a).
The ICHH is not solely responsible for the rise of ecoimmunology, though. Ideas that would later become central to the field had been discussed years before (Williams 1966; Grossman 1985; Langman and Cohn 1987; Klasing 1988; Behnke et al. 1992), and changes in the way wider biology was being practiced (Wake 2008) facilitated its emergence. Not least of these was an increasing adoption of integrative approaches, such as behavioral and comparative endocrinology. As researchers in traditionally mechanistic disciplines, such as endocrinology and immunology, conceptually worked upward through levels of biological organization, they began to meet ecologists seeking to understand the mechanisms driving higher-order processes, such as mating behavior, aggression, host–parasite interactions, the evolution of virulence, distributions of animals, and many other phenomena.
Behavioral ecologists, in particular, made instrumental contributions to ecoimmunology. They showed, for example, that immunity varies with the environmental characteristics of the habitats occupied by hosts (Nunn et al. 2000, 2003; Semple et al. 2002; Matson 2006), time of year (Nelson 2004; Martin et al. 2008), life-history strategy (Tieleman et al. 2005; Martin et al. 2006a, 2007; Sparkman and Palacios 2009), life-history stage (Love et al. 2008), and a diversity of other factors. Such research, as well as that carried out by comparative and behavioral endocrinologists, continued to emphasize immune interactions with the neuroendocrine system (Demas 2004; Ashley and Wingfield 2012) by focusing on responses to stress (Casto et al. 2001; Berger et al. 2005; Martin 2009), reproductive physiology (Evans et al. 2000; McKean and Nunney 2007; Nunn et al. 2009), and social behavior (Wilson et al. 2002; Archie et al. 2012). This body of work underlies the major interests of the field today. Moreover, the study of various, sometimes exotic, organisms under natural conditions led to a profound and yet (in retrospect) unsurprising discovery: the magnitude of variation in immunity between individuals, groups, and species is far greater than was implicitly predicted by traditional immunology. In spite of strong selection against disease, most organisms maintain a degree of genetic and physiological vulnerability to infection. This key insight would not have been possible using a small number of model species in the controlled laboratory setting of traditional immunology (Pedersen and Babayan 2011).
The cost of immunity: the field’s greatest contribution
The most influential idea to have emerged from the development of ecoimmunology is that immunity can be costly. Taking their cue from behavioral ecology, the first ecoimmunologists applied an optimality approach to immunity, assuming that defenses are regulated to maximize net fitness in the context of costs, ecological influences, and constraints (van Boven and Weissing 2004). The costs of immunity were investigated in different ways, but much work suggested that they could be high, and that they had ecological and evolutionary ramifications. Evolutionary costs of immunity, for example, can arise through pleiotropy (McKean et al. 2008). Experiments and meta-analyses have shown negative genetic covariance between immunity and growth, reproductive success, and other measures of competitive ability (Verhulst et al. 1999; van der Most et al. 2011). Over shorter time-scales, the maintenance (e.g., Valtonen et al. 2009) and deployment (e.g., Derting and Compton 2003) of immune responses can exact physiological costs, both in terms of energy (Lochmiller and Deerenberg 2000; Martin et al. 2003; Ardia et al. 2012) and materials (Gasparini et al. 2009; Cotter et al. 2011). Importantly, such physiological costs were associated with fitness: induction of immune responses could reduce fitness (Mallon et al. 2003; Schmid-Hempel 2003; Garamszegi et al. 2004; Sanz et al. 2004; Eraud et al. 2005, 2009; Jacot et al. 2005; Uller et al. 2006; Bonneaud et al. 2009), and experimental increases in other activities (e.g., rearing, begging, foraging, and sexual behavior) could decrease immune activity (Deerenberg et al. 1997; Hasselquist et al. 2001; McKean and Nunney 2001; Ahtiainen et al. 2005; Verhulst et al. 2005; Moreno-Rueda 2010).
As researchers came to appreciate the ubiquity of immune costs, they began to ask how ecological factors might affect their expression (Sandland 2003; Sadd and Schmid-Hempel 2009). It was found, for instance, that although experimental increases in the expenditure of energy on other activities could increase the metabolic cost of clearing infection, it could do so without affecting the outcome of infection (e.g., Zala et al. 2008). Thus, ecological context has a strong influence on the cost–benefit ratio of most immune responses (Doeschl-Wilson et al. 2009; Lazzaro and Little 2009); availability of food, for example, may only have an effect on immunity in certain age classes (e.g., Birkhead et al. 1999) and may even affect different arms of the immune system in opposite directions (e.g., Gonzalez et al. 1999). Interactions among environmental factors and genotype (e.g., Adamo and Lovett 2011; Triggs and Knell 2011) can further modulate the balance between the costs and benefits of immune investments.
What to measure: the field’s greatest conundrum
An issue that remains central to ecoimmunology is how best to measure immunity (Siva-Jothy 1995). Early on, researchers seeking to test newly formulated hypotheses about immunity in the context of life history sought to measure immunocompetence, the ability of a host to control or avoid infection and other disease-related threats to fitness. However, this was a red herring (Owens and Wilson 1999), and lost favor quickly; “immunocompetence” was intangible and, more importantly, seemed immeasurable (Norris and Evans 2000), so ecoimmunologists began to take a more nuanced approach. Even as practitioners moved away from the simplistic concept of “immunocompetence” (Adamo 2004), though, they remained limited by the small number of measures of variation in immunity that were available. As ecoimmunologists tended to have greater ecological rather than immunological expertise, simple, field-amenable techniques garnered the most use.
Perhaps the measure that has been used most commonly during the history of ecoimmunology is the phytohemagglutinin (PHA) skin-swelling response (Demas et al. 2011b). PHA was (and is) used to stimulate the proliferation of lymphocytes and local inflammation in vivo (and rarely in vitro). When injected into or under the skin, PHA induces the infiltration of leukocytes (Martin et al. 2006c; Turmelle et al. 2010) and hence a swelling, the thickness of which is interpreted as a measure of the strength of the immune response. This response has been measured in pinnipeds (Hall et al. 1999; Brock et al. 2013a), bats (Turmelle et al. 2010), deer (Fernández-de-Mera et al. 2008), toads (Brown et al. 2011), and many other species. It was favored by ecoimmunologists because of evidence linking it to the host’s fitness (Møller and Saino 2004) and to an energetic cost (Martin et al. 2003). PHA and substances like it have the added advantage that they preclude the possibility of manipulative actions on the part of pathogens confounding the interpretation of the variation in immune responses (Graham et al. 2011).
The principal objection to PHA (and to other measures used early on in the history of ecoimmunology) was that hosts’ responses to PHA might not be representative of the same hosts’ responses to pathogens (Kennedy and Nager 2006). PHA directly and indiscriminately activates lymphocytes, antigen presentation, and other parts of cell-mediated cascades, and may incite acute-phase (febrile) responses at high doses. How reasonable was it, therefore, to interpret more swelling as “better”? Did “better” mean with respect to all parasites (i.e., immunocompetence), some parasites (i.e., those regulated by T-cell, basophil, or other PHA-primed mediators), or something else altogether? Several authors offered opinions, including that PHA should be considered an indicator of the inducibility of pro-inflammatory signaling (Vinkler et al. 2010). Still, as with many options for characterizing immunity, the degree to which responses are related to coping with pathogens is likely to vary among types or strains of pathogens, hosts, and stages of infection (Owen and Clayton 2007). However, something surprising happened when this potentially major impediment was formalized; instead of causing the field to collapse, it animated ecoimmunology. The reason for this probably lies in the recurrent and intelligible patterns of covariation between PHA responses (and other immune parameters) and eco-evolutionary factors (Martin et al. 2001; Tella et al. 2002). Even with coarse tools, ecoimmunologists were able to describe and explain variation in immunity across species. It is possible that by measuring multiple distinct immune responses (Martin et al. 2006b; Matson et al. 2006; Millet et al. 2007; Bradley and Jackson 2008; Boughton et al. 2011; Demas et al. 2011b), ecoimmunologists could approach immunocompetence, or at least identify general categories of immune responses (Schmid-Hempel and Ebert 2003; Martin et al. 2008) and describe protective immune phenotypes (Pedersen and Babayan 2011).
Ecoimmunology in 2014
Today, striking a balance between the relevance and feasibility of measures remains a challenge (Graham et al. 2011), and much current effort in ecoimmunology remains invested in the development of tools (Boughton et al. 2011). Conceptually, a defining feature of modern ecoimmunology is that it embraces and attempts to describe natural variation, which contrasts with traditional laboratory-based immunology. It could be argued that a lack of tools has been beneficial to the field in some ways; because ecoimmunology is forced to evaluate immunity above the levels of the molecule and cell, it gleans insight outside the purview of bench immunology. A good example is the concept of tolerance. Tolerance is defined ecologically as the relationship between the burden of infection and the effects of infection on hosts (immunologists use the term for another process). Mathematically, ecological tolerance is often quantified as the slope of the relationship between parasite burden and host fitness. As much has been written recently about the implications of tolerance, we do not restate those here. What is important is that although we have yet to identify many mechanisms of tolerance (Råberg et al. 2009), we now appreciate that hosts might interact with their parasites in a completely new way. It may not be beneficial to clear infections altogether (i.e., achieve sterilizing immunity); instead, hosts could control the number of parasites or offset their effects. This whole-organism phenomenon was underappreciated as part of immunology until a few years ago. Now it is gaining mainstream attention, opening new doors to the treatment of human disease, and contributing to understanding of the spread and emergence of diseases, including zoonoses (Baucom and de Roode 2011).
Ecoimmunology and disease ecology
Ecoimmunology is likely to continue to make contributions to our understanding of host–parasite interactions; and increasing interchange with another burgeoning field, disease ecology, is likely to enhance these contributions. Disease ecology seeks to explain and predict the transmission and emergence of diseases at the population-level and above, and the organismal and sub-organismal traits mediating large-scale biological processes are black-boxed. By contrast, ecoimmunology has—until recently—focused on investigating how the traits of hosts impact variation in immunity, irrespective of infection (as surprising as that may seem). Thus, even though ecoimmunology (unlike traditional immunology) (Ottaviani et al. 2008; Schulenburg et al. 2009) is more amenable to top-down, bottom-up, and even middle-out pathways of inference (Annila and Baverstock 2014), it will benefit from increasing interdisciplinary exchange with disease ecology, as framing studies of variation in immunity in the context in which immunity operates and evolves (Graham et al. 2011), especially the parasites to which immunity is directed (Pedersen and Greives 2008; Hawley and Altizer 2011), is almost guaranteed to generate greater and more rapid progress.
This integration may also have beneficial knock-on effects on related disciplines such as conservation biology, where the combined tools of ecological immunology and disease ecology have the potential to contribute to understanding of the biology of invasions (Kolar and Lodge 2001) and of anthropogenic effects on the health of wildlife populations (French et al. 2010; Brock et al. 2013b). For example, escape from the natural enemies found in a species’ native range could select for lower investment in defense and greater investment in growth and reproduction (Lee and Klasing 2004; White and Perkins 2012). Such a shift in life-history could modify community structure and the rates of contact between hosts, which could result in the spread or emergence of novel diseases. In addition, the application of the combined approaches of ecoimmunology and disease ecology could lead to better understanding of the amplification of infections that cycle through multi-host systems of disease transmission (Previtali et al. 2012).
Future
The future of ecoimmunology (and disease ecology) looks bright. One area that we expect to see grow is the continued exploration of immune costs, trade-offs, and their context-dependencies. The cost of developing an acquired immune system, for example, is presumed to be high given the amount of protein synthesis and gluconeogenesis required to build up repertoires of B-lymphocytes and T-lymphocytes during early ontogeny (Lochmiller and Deerenberg 2000; Martin et al. 2008). This developmental cost may be offset by the metabolic cheapness of the deployment of acquired immune responses relative to innate immune responses later in life (Råberg et al. 2002), a trade-off that is made possible by the down-regulation of innate inflammatory responses by cytokines released during the activation of acquired responses (Lee 2006). Intriguingly, the costs of immunity may be offset across generations, as recent work has shown that the effects of a mother’s experience, nutritional status, and provisioning behavior may all have impacts on the immunity of her offspring, a line of investigation that is yielding insights relevant to behavioral ecology, immunology, and disease ecology (Pihlaja et al. 2006; Addison et al. 2009; Hasselquist and Nilsson 2009; Garnier et al. 2012; Hasselquist et al. 2012).
For many, future immunology is likely to benefit most from a two-pronged approach, and one that overlaps with ecological immunology (e.g., Turner et al. 2011): parts of studies could be carried out in controlled settings whereas others could be carried out in natural contexts (Graham et al. 2011). Overall, there is consensus that as many types of host, parasite, and environment should be studied as possible. Such broad-reaching work is increasingly feasible because techniques developed in the laboratory are being adapted for use in the field, and in different species. In the coming years, the continued integration with genetics, behavior, environmental stochasticity, diversity of pathogens, co-infection dynamics, and community ecology could produce an immunology that makes great leaps forward (Pedersen and Fenton 2007; Tompkins et al. 2010; Babayan et al. 2011; Pedersen and Babayan 2011).
One of the most exciting prospects for ecoimmunology is its value to public (i.e., human) health. Molecular and evolutionary biology were recently described as:
“… separate disciplines and scientific cultures: The former is mechanistic and focused on molecules; the latter is theoretical and focused on populations.” (Rosenberg and Queitsch 2014)
It was also noted that:
“… these domains are beginning to converge in laboratories addressing molecular mechanisms that explain how evolutionary processes work. And bring these processes to bear on medical problems … Each discipline can be viewed as a missing link in the other’s description of biology, and in medicine.”
Much of this applies to the integration of ecology and immunology too. Ecoimmunology has much in common with evolutionary (or Darwinian) medicine, as both aim to understand responses to infection and their consequences in an eco-evolutionary framework (Trotter et al. 2011)—but do so from different, yet complementary, perspectives. Just as the approaches of ecoimmunology are increasingly being applied on the population-level through integration with disease ecology, so the overlap between ecoimmunology and medicine is increasingly relevant to public health.
Consider the following example: it is axiomatic that diet is an important influence on disease processes in humans, which is why, for instance, school feeding programs are considered public health issues (Bundy et al. 2013). However, there has been a recent increase in interest in the details of the interactions between diet and infectious disease: for example, the contributions of malnutrition and malaria to one another’s impacts in Niger (Burki 2013; Médecine Sans Frontières 2013) and elsewhere (Arinaitwe et al. 2012). Traditionally, public health interventions were founded on data collected without reference to the mechanisms underlying the patterns being observed, as is the case, for example, in studies of cohorts, or in clinical trials. An increase in the perceived utility of understanding the mechanisms driving patterns observed in data on public health has precipitated integration with more traditionally mechanistic disciplines (such as immunology). To the benefit of public health, much integration has already occurred within biology—ecoimmunology being an example. Public health researchers can, therefore, adopt emerging integrative paradigms that link population-level processes to molecular mechanisms. For example, research in public health on infectious disease often uses mechanistic models of disease transmission to assess how public health interventions, incorporated into these models, are best able to reduce predicted transmission (e.g., Griffin et al. 2010). In order for interactions between diet (or other aspects of ecology) and the persistence of disease (or other processes influenced by immunity) to be incorporated into such models, they will need to be described mechanistically. The same applies to developments in the study of the health of wild animals and livestock; understanding the ecoimmunological mechanisms regulating tolerance of parasites versus clearance of parasites in a reservoir species, for example, may significantly increase our ability to predict the frequency and location of disease spillover and emergence events (Martin et al. 2010; Martin and Boruta 2013), which could have positive impacts on human and animal health, and on welfare, economic prosperity, and biodiversity.
More generally, as ecoimmunology matures, it will have a chance to contribute to a fuller understanding of many other processes currently of interest to wider biology, such as the role of the microbiome in health and disease (Kau et al. 2011), the distribution and spread of native and introduced species (Lee and Klasing 2004; Martin et al. 2014), and the effects of anthropogenic stressors on the vitality of populations of humans and of wild organisms (Bradley and Altizer 2007; Acevedo-Whitehouse and Duffus 2009; Martin et al. 2010; Brock et al. 2013b).
Conclusion
The origins, development, and current state of ecoimmunology are representative of the integration of approaches to understanding biological phenomena that has occurred during the past three decades (Pennisi 2014). The exploration of the potential of this integration in the case of ecoimmunology has led to insight relevant to many disciplines, and inspired healthy and constructive inter-disciplinary discussion, even when avenues of development have turned out to be narrower and more obstacle-laden than first envisioned. As ecoimmunology moves forward, it will no doubt continue to contribute to the unraveling of the complex workings of the immune system, and to the discussion and understanding of fundamental biological processes.
Funding
The symposium Methods and Mechanisms in Ecoimmunology was supported by the National Science Foundation (IOS-0947177). P.M.B. was funded by the Natural Environment Research Council (www.nerc.ac.uk), the Universities Federation for Animal Welfare (www.ufaw.org.uk) and the Sea Mammal Research Unit (www.smru.st-and.ac.uk). C.C.M. was funded by the National Science Foundation and the National Institutes of Health (NSF-NIH EID, EF-0914384; NIH-R21, AI096036-01).
Acknowledgments
We would like to thank Cynthia Downs, Jim Adelman and Greg Demas for hosting the symposium Methods and Mechanisms in Ecoimmunology; Dan Ardia, Dana Hawley, Lou Burnett, Raoul Boughton and Travis Wilcoxen for facilitating the establishment of the Division of Ecoimmunology and Disease Ecology (DEDE) within the Society for Integrative and Comparative Biology (SICB); and Hal Heatwole for helpful comments on the manuscript.
References
- Acevedo-Whitehouse K, Duffus ALJ. Effects of environmental change on wildlife health. Phil Trans R Soc B Biol Sci. 2009;364:3429–38. doi: 10.1098/rstb.2009.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo SA. How should behavioural ecologists interpret measurements of immunity? Anim Behav. 2004;68:1443–9. [Google Scholar]
- Adamo SA, Lovett M. Some like it hot: the effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. J Exp Biol. 2011;214:1997–2004. doi: 10.1242/jeb.056531. [DOI] [PubMed] [Google Scholar]
- Addison B, Klasing KC, Robinson WD, Austin SH, Ricklefs RE. Ecological and life-history factors influencing the evolution of maternal antibody allocation: a phylogenetic comparison. Proc R Soc Lond B Biol Sci. 2009;276:3979–87. doi: 10.1098/rspb.2009.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahtiainen JJ, Alatalo RV, Kortet R, Rantala MJ. A trade-off between sexual signalling and immune function in a natural population of the drumming wolf spider Hygrolycosa rubrofasciata. J Evol Biol. 2005;18:985–91. doi: 10.1111/j.1420-9101.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- Annila A, Baverstock K. Genes without prominence: a reappraisal of the foundations of biology. J R Soc Interface. 2014;11:20131017. doi: 10.1098/rsif.2013.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proc Natl Acad Sci USA. 2012;109:9017–22. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardia DR, Gantz JE, Schneider BC, Strebel S. Costs of immunity in insects: an induced immune response increases metabolic rate and decreases antimicrobial activity. ) Funct Ecol. 2012;26:732–9. [Google Scholar]
- Arinaitwe E, Gasasira A, Verret W, Homsy J, Wanzira H, Kakuru A, Sandison TG, Young S, Tappero JWT, Kamya MR, Dorsey G. The association between malnutrition and the incidence of malaria among young HIV-infected and -uninfected Ugandan children: a prospective study. Malar J. 2012;11:90. doi: 10.1186/1475-2875-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley NT, Wingfield JC. Sickness behaviour in vertebrates: allostasis, life-history modulation and hormone regulation. In: Demas G, Nelson R, editors. Ecoimmunology. Oxford: Oxford University Press; 2012. pp. 45–91. [Google Scholar]
- Babayan SA, Allen JE, Bradley JE, Geuking MB, Graham AL, Grencis RK, Kaufman J, McCoy KD, Paterson S, Smith KGC, et al. Wild immunology: converging on the real world. Ann NY Acad Sci. 2011;1236:17–29. doi: 10.1111/j.1749-6632.2011.06251.x. [DOI] [PubMed] [Google Scholar]
- Baucom RS, de Roode JC. Ecological immunology and tolerance in plants and animals. Funct Ecol. 2011;25:18–28. [Google Scholar]
- Behnke JM, Barnard CJ, Wakelin D. Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward. Int J Parasitol. 1992;22:861–907. doi: 10.1016/0020-7519(92)90046-n. [DOI] [PubMed] [Google Scholar]
- Berger S, Martin LB, Wikelski M, Romero LM, Kalko EKV, Vitousek MN, Rödl T. Corticosterone suppresses immune activity in territorial Galápagos marine iguanas during reproduction. Horm Behav. 2005;47:419–29. doi: 10.1016/j.yhbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Birkhead TR, Fletcher F, Pellatt EJ. Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc R Soc Lond B Biol Sci. 1999;266:385–90. [Google Scholar]
- Bonneaud C, Sinsheimer JS, Richard M, Chastel O, Sorci G. MHC polymorphisms fail to explain the heritability of phytohaemagglutinin-induced skin swelling in a wild passerine. Biol Lett. 2009;5:784–7. doi: 10.1098/rsbl.2009.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton RK, Joop G, Armitage SAO. Outdoor immunology: methodological considerations for ecologists. Funct Ecol. 2011;25:81–100. [Google Scholar]
- Bradley JE, Jackson JA. Measuring immune system variation to help understand host–pathogen community dynamics. Parasitology. 2008;135:807–23. doi: 10.1017/S0031182008000322. [DOI] [PubMed] [Google Scholar]
- Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K. Applying the tools of ecological immunology to conservation: a test case in the Galapagos sea lion. Anim Conserv. 2013a;16:19–31. [Google Scholar]
- Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K. Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. PLoS One. 2013b;8:e67132. doi: 10.1371/journal.pone.0067132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GP, Shilton CM, Shine R. Measuring amphibian immunocompetence: validation of the phytohemagglutinin skin-swelling assay in the cane toad, Rhinella marina. Methods Ecol Evol. 2011;2:341–8. [Google Scholar]
- Buehler DM, Versteegh MA, Matson KD, Tieleman BI. One problem, many solutions: simple statistical approaches help unravel the complexity of the immune system in an ecological context. PLoS One. 2011;6:e18592. doi: 10.1371/journal.pone.0018592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy AP, Drake LJ, Burbano C. School food, politics and child health. Public Health Nutr. 2013;16:1012–9. doi: 10.1017/S1368980012004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki TK. Malaria and malnutrition: Niger's twin crises. Lancet. 2013;382:587–8. doi: 10.1016/s0140-6736(13)61732-8. [DOI] [PubMed] [Google Scholar]
- Casto JM, Nolan V, Ketterson ED. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) Am Nat. 2001;157:408–20. doi: 10.1086/319318. [DOI] [PubMed] [Google Scholar]
- Cotter SC, Simpson SJ, Raubenheimer D, Wilson K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct Ecol. 2011;25:186–98. [Google Scholar]
- Deerenberg C, Arpanius V, Daan S, Bos N. Reproductive effort decreases antibody responsiveness. Proc R Soc Lond B Biol Sci. 1997;264:1021–9. [Google Scholar]
- Demas GE. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm Behav. 2004;45:173–80. doi: 10.1016/j.yhbeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Demas GE, Adamo SA, French SS. Neuroendocrine-immune crosstalk in vertebrates and invertebrates: implications for host defence. Funct Ecol. 2011a;25:29–39. [Google Scholar]
- Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS. Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. J Anim Ecol. 2011b;80:710–30. doi: 10.1111/j.1365-2656.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- Derting TL, Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus) Physiol Biochem Zool. 2003;76:744–52. doi: 10.1086/375662. [DOI] [PubMed] [Google Scholar]
- Doeschl-Wilson AB, Brindle W, Emmans G, Kyriazakis I. Unravelling the relationship between animal growth and immune response during micro-parasitic infections. PLoS One. 2009;4:e7508. doi: 10.1371/journal.pone.0007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraud C, Duriez O, Chastel O, Faivre B. The energetic cost of humoral immunity in the collared dove, Streptopelia decaocto: is the magnitude sufficient to force energy-based trade-offs? Funct Ecol. 2005;19:110–8. [Google Scholar]
- Eraud C, Jacquet A, Faivre B. Survival cost of an early immune soliciting in nature. Evolution. 2009;63:1036–43. doi: 10.1111/j.1558-5646.2008.00540.x. [DOI] [PubMed] [Google Scholar]
- Evans MR, Goldsmith AR, Norris SRA. The effects of testosterone on antibody production and plumage coloration in male house sparrows (Passer domesticus) Behav Ecol Sociobiol. 2000;47:156–63. [Google Scholar]
- Fernández-de-Mera IG, Vicente J, Höfle U, Rodriguez O, Gaspar-Lopez E, Gortazar C. The effects of sex and age on phytohaemagglutinin skin-testing of deer. N Z Vet J. 2008;56:71–3. doi: 10.1080/00480169.2008.36811. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139:603–22. [Google Scholar]
- French SS, DeNardo DF, Greives TJ, Strand CR, Demas GE. Human disturbance alters endocrine and immune responses in the Galapagos marine iguana (Amblyrhynchus cristatus) Horm Behav. 2010;58:792–9. doi: 10.1016/j.yhbeh.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi LZ, Møller AP, Torok J, Michl G, Peczely P, Richard M. Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav Ecol. 2004;15:148–57. [Google Scholar]
- Garnier R, Ramos R, Staszewski V, Militão T, Lobato E, González-Solís J, Boulinier T. Maternal antibody persistence: a neglected life-history trait with implications from albatross conservation to comparative immunology. Proc R Soc Lond B Biol Sci. 2012;279:2033–41. doi: 10.1098/rspb.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini J, Piault R, Bize P, Roulin A. Synergistic and antagonistic interaction between different branches of the immune system is related to melanin-based coloration in nestling tawny owls. J Evol Biol. 2009;22:2348–53. doi: 10.1111/j.1420-9101.2009.01831.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Sorci G, Møller AP, Ninni P, Haussy C, de Lope F. Immunocompetence and condition-dependent sexual in male house sparrows advertisement (Passer domesticus) J Anim Ecol. 1999;68:1225–34. [Google Scholar]
- Graham AL, Shuker DM, Pollitt LC, Auld SKJR, Wilson J, Little TJ. Fitness consequences of immune responses: strengthening the empirical framework for ecoimmunology. Funct Ecol. 2011;25:5–17. [Google Scholar]
- Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, Bousema T, Drakeley CJ, Ferguson NM, Basáñez M-G, Ghani A. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000324. e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman C. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–61. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Licence ST, Pomeroy PP. The response of grey seal pups to intradermal phytahaemagglutinin injection. Aquat Mammals. 1999;25:25–30. [Google Scholar]
- Hamilton WD, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–7. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Nilsson J. Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Phil Trans R Soc Lond B Biol Sci. 2009;364:51–60. doi: 10.1098/rstb.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselquist D, Tobler M, Nilsson J. Maternal modulation of offspring immune function in vertebrates. In: Demas G, Nelson R, editors. Ecoimmunology. Oxford: Oxford University Press; 2012. pp. 165–224. [Google Scholar]
- Hasselquist D, Wasson M, Winkler D. Humoral immunocompetence correlates with date of egg-laying and reflects work load in female tree swallows. Behav Ecol. 2001;12:93–7. [Google Scholar]
- Hawley DM, Altizer SM. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol. 2011;25:48–60. [Google Scholar]
- Jacot A, Scheuber H, Kurtz J, Brinkhof MWG. Juvenile immune system activation induces a costly upregulation of adult immunity in field crickets Gryllus campestris. Proc R Soc Lond B Biol Sci. 2005;272:63–9. doi: 10.1098/rspb.2004.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MW, Nager RG. The perils and prospects of using phytohaemagglutinin in evolutionary ecology. Trends Ecol Evol. 2006;21:653–5. doi: 10.1016/j.tree.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Klasing KC. Influence of acute feed deprivation or excess feed intake on immunocompetence of broiler chicks. Poult Sci. 1988;67:626–34. doi: 10.3382/ps.0670626. [DOI] [PubMed] [Google Scholar]
- Kolar CS, Lodge DM. Progress in invasion biology: predicting invaders. Trends Ecol Evol. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- Langman R, Cohn M. The E–T (elephant-tadpole) paradox necessitates the concept of a unit of B cell function: the protection. Mol Immunol. 1987;24:675. doi: 10.1016/0161-5890(87)90050-2. [DOI] [PubMed] [Google Scholar]
- Lazzaro BP, Little TJ. Immunity in a variable world. Phil Trans R Soc Lond B Biol Sci. 2009;364:15–26. doi: 10.1098/rstb.2008.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Klasing KC. A role for immunology in invasion biology. Trends Ecol Evol. 2004;19:523–9. doi: 10.1016/j.tree.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–15. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Love OP, Salvante KG, Dale J, Williams TD. Sex-specific variability in the immune system across life-history stages. Am Nat. 2008;172:E99–112. doi: 10.1086/589521. [DOI] [PubMed] [Google Scholar]
- Mallon EB, Brockmann A, Schmid-Hempel P. Immune response inhibits associative learning in insects. Proc R Soc Lond B Biol Sci. 2003;270:2471–3. doi: 10.1098/rspb.2003.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB. Stress and immunity in wild vertebrates: timing is everything. Gen Comp Endocr. 2009;163:70–6. doi: 10.1016/j.ygcen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Martin LB, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond Ser B Biol Sci. 2003;270:153–8. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Hasselquist D, Wikelski M. Investment in immune defense is linked to pace of life in house sparrows. Oecologia. 2006a;147:565–75. doi: 10.1007/s00442-005-0314-y. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Refining approaches and diversifying directions in ecoimmunology. Integr Comp Biol. 2006b;46:1030–9. doi: 10.1093/icb/icl039. [DOI] [PubMed] [Google Scholar]
- Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol. 2006c;20:290–9. [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007;88:2516–28. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil Trans R Soc Lond B Biol Sci. 2008;363:321–39. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. The effects of anthropogenic global changes on immune functions and disease resistance. Ann N Y Acad Sci. 2010;1195:129–48. doi: 10.1111/j.1749-6632.2010.05454.x. [DOI] [PubMed] [Google Scholar]
- Martin LB, Boruta M. The impacts of urbanization on avian disease transmission and emergence. In: Gill D, Brumm H, editors. Avian urban ecology: behavioural and physiological adaptations. Oxford, UK: Oxford University Press; 2013. pp. 116–28. [Google Scholar]
- Martin LB, Coon CAC, Liebl AL, Schrey AW. Surveillance for microbes and range expansion in house sparrows. Proc R Soc B Biol Sci. 2014;281:20132690. doi: 10.1098/rspb.2013.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TE, Møller AP, Merino S, Clobert J. Does clutch size evolve in response to parasites and immunocompetence? Proc Natl Acad Sci USA. 2001;98:2071–6. doi: 10.1073/pnas.98.4.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson KD. Are there differences in immune function between continental and insular birds? Proc R Soc Lond Ser B Biol Sci. 2006;273:2267–74. doi: 10.1098/rspb.2006.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson KD, Cohen AA, Klasing KC, Ricklefs RE, Scheuerlein A. No simple answers for ecological immunology: relationships among immune indices at the individual level break down at the species level in waterfowl. Proc R Soc Lond B Biol Sci. 2006;273:815–22. doi: 10.1098/rspb.2005.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean KA, Yourth CP, Lazzaro BP, Clark AG. The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol. 2008;8:76. doi: 10.1186/1471-2148-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean KA, Nunney L. Sexual selection and immune function in Drosophila melanogaster. Evolution. 2007;62:386–400. doi: 10.1111/j.1558-5646.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- McKean KA, Nunney L. Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98:7904–9. doi: 10.1073/pnas.131216398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Médecine Sans Frontières. Niger 2013: Tackling the deadly combination of malaria and malnutrition. Online report. 2013 ( http://www.msf.org.uk/sites/uk/files/niger_malaria_pd_2013.pdf) [Google Scholar]
- Michel J-B, Shen YK, Aiden AP, Veres A, Gray MK, The Google Books Team. Pickett JP, Hoiberg D, Clancy D, Norvig P, et al. Quantitative analysis of culture using millions of digitized books. Science. 2011;331:176–82. doi: 10.1126/science.1199644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet S, Bennett J, Lee KA, Hau M, Klasing KC. Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol. 2007;31:188–201. doi: 10.1016/j.dci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Moreno-Rueda G. An immunological cost of begging in house sparrow nestlings. Proc R Soc Lond B Biol Sci. 2010;277:2083–8. doi: 10.1098/rspb.2010.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Saino N. Immune response and survival. Oikos. 2004;104:299–304. [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–92. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Norris K, Evans MR. Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol. 2000;11:19–26. [Google Scholar]
- Nunn CL. A comparative study of leukocyte counts and disease risk in primates. Evolution. 2002;56:177–90. doi: 10.1111/j.0014-3820.2002.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Nunn CL, Gittleman JL, Antonovics J. Promiscuity and the primate immune system. Science. 2000;290:1168–70. doi: 10.1126/science.290.5494.1168. [DOI] [PubMed] [Google Scholar]
- Nunn CL, Gittleman JL, Antonovics J. A comparative study of white blood cell counts and disease risk in carnivores. Proc R Soc Lond B Biol Sci. 2003;270:347–56. doi: 10.1098/rspb.2002.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn CL, Lindenfors P, Pursall ER, Rolff J. On sexual dimorphism in immune function. Phil Trans R Soc Lond B Biol Sci. 2009;364:61–9. doi: 10.1098/rstb.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani E, Malagoli D, Capri M, Franceschi C. Ecoimmunology: is there any room for the neuroendocrine system? BioEssays. 2008;30:868–74. doi: 10.1002/bies.20801. [DOI] [PubMed] [Google Scholar]
- Owen JP, Clayton DH. Where are the parasites in the PHA response? Trends Ecol Evol. 2007;22:228–9. doi: 10.1016/j.tree.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Owens IPF, Wilson K. Immunocompetence: a neglected life history trait or conspicuous red herring? Trends Ecol Evol. 1999;14:170–2. [Google Scholar]
- Pedersen AB, Babayan SA. Wild immunology. Mol Ecol. 2011;20:872–80. doi: 10.1111/j.1365-294X.2010.04938.x. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol Evol. 2007;22:133–9. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Greives TJ. The interaction of parasites and resources cause crashes in a wild mouse population. J Anim Ecol. 2008;77:370–7. doi: 10.1111/j.1365-2656.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Once failing biology society thrives as it nurtures new disciplines. Science. 2014;343:129. doi: 10.1126/science.343.6167.129. [DOI] [PubMed] [Google Scholar]
- Pihlaja M, Siitari H, Alatalo RV. Maternal antibodies in a wild altricial bird: effects on offspring immunity, growth and survival. J Anim Ecol. 2006;75:1154–64. doi: 10.1111/j.1365-2656.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Previtali AM, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R, Martin LB. Relationship between pace of life and immune responses in wild rodents. Oikos. 2012;121:1483–92. [Google Scholar]
- Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav. 2004;68:227–39. [Google Scholar]
- Rosenberg SM, Queitsch C. Combating evolution to fight disease. Science. 2014;343:1088–9. doi: 10.1126/science.1247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Phil Trans R Soc Lond B Biol Sci. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Vestberg M, Hasselquist D, Holmdahl R, Svensson E, Nilsson J. Basal metabolic rate and the evolution of the adaptive immune system. Proc R Soc Lond B Biol Sci. 2002;269:817–21. doi: 10.1098/rspb.2001.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd BM, Schmid-Hempel P. Principles of ecological immunology. Evol Appl. 2009;2:113–21. doi: 10.1111/j.1752-4571.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandland G. Costs of immune defense: an enigma wrapped in an environmental cloak? Trends Parasitol. 2003;19:571–4. doi: 10.1016/j.pt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Sanz JJ, Moreno J, Merino S. A trade-off between two resource-demanding functions: post-nuptial moult and immunity during reproduction in male pied flycatchers. J Anim Ecol. 2004;73:441–7. [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc R Soc Lond B Biol Sci. 2003;270:357–66. doi: 10.1098/rspb.2002.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol Evol. 2003;18:27–32. [Google Scholar]
- Schulenburg H, Kurtz J, Moret Y, Siva-Jothy MT. Introduction. Ecological immunology. Phil Trans R Soc Lond B Biol Sci. 2009;364:3–14. doi: 10.1098/rstb.2008.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple S, Cowlishaw G, Bennett PM. Immune system evolution among anthropoid primates: parasites, injuries and predators. Proc R Soc Lond B Biol Sci. 2002;269:1031–7. doi: 10.1098/rspb.2001.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–21. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Siva-Jothy MT. “Immunocompetence”: conspicuous by its absence. Trends Ecol Evol. 1995;10:205–6. doi: 10.1016/s0169-5347(00)89059-x. [DOI] [PubMed] [Google Scholar]
- Sparkman AM, Palacios MG. A test of life-history theories of immune defence in two ecotypes of the garter snake, Thamnophis elegans. J Anim Ecol. 2009;78:1242–8. doi: 10.1111/j.1365-2656.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- Tella JL, Scheuerlein A, Ricklefs RE. Is cell-mediated immunity related to the evolution of life-history strategies in birds? Proc R Soc Lond B. 2002;269:1059–66. doi: 10.1098/rspb.2001.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman BI, Williams JB, Ricklefs RE, Klasing KC. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc R Soc Lond B Biol Sci. 2005;272:1715–20. doi: 10.1098/rspb.2005.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DM, Dunn AM, Smith MJ, Telfer S. Wildlife diseases: from individuals to ecosystems. J Anim Ecol. 2010;4:1–20. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- Triggs A, Knell RJ. Interactions between environmental variables determine immunity in the Indian meal moth Plodia interpunctella. J Anim Ecol. 2011;81:386–94. doi: 10.1111/j.1365-2656.2011.01920.x. [DOI] [PubMed] [Google Scholar]
- Trotter JH, Liebl AL, Weeber EJ, Martin LB. Linking ecological immunology and evolutionary medicine: the case for apolipoprotein E. Funct Ecol. 2011;25:40–7. [Google Scholar]
- Turmelle AS, Ellison JA, Mendonça MT, McCracken GF. Histological assessment of cellular immune response to the phytohemagglutinin skin test in Brazilian free-tailed bats (Tadarida brasiliensis) J Comp Physiol B Biochem Syst Environ Physiol. 2010;180:1155–64. doi: 10.1007/s00360-010-0486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AK, Begon M, Jackson JA, Bradley JE, Paterson S. Genetic diversity in cytokines associated with immune variation and resistance to multiple pathogens in a natural rodent population. PLoS Genet. 2011;7:e1002343. doi: 10.1371/journal.pgen.1002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uller T, Isaksson C, Olsson M. Immune challenge reduces reproductive output and growth in a lizard. Funct Ecol. 2006;20:873–9. [Google Scholar]
- van Boven M, Weissing FJ. The evolutionary economics of immunity. Am Nat. 2004;163:277–94. doi: 10.1086/381407. [DOI] [PubMed] [Google Scholar]
- van der Most PJ, de Jong B, Parmentier HK, Verhulst S. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct Ecol. 2011;25:74–80. [Google Scholar]
- Valtonen TM, Kleino A, Rämet M, Rantala MJ. Starvation reveals maintenance cost of humoral immunity. Evol Biol. 2009;37:49–57. [Google Scholar]
- Verhulst S, Dieleman SJ, Parmentier H. A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proc Natl Acad Sci USA. 1999;96:4478–81. doi: 10.1073/pnas.96.8.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Riedstra B, Wiersma P. Brood size and immunity costs in zebra finches Taeniopygia guttata. J Avian Biol. 2005;36:22–30. [Google Scholar]
- Vinkler M, Bainova H, Albrecht T. Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct Ecol. 2010;24:1081–6. [Google Scholar]
- Wake MH. What is “integrative biology”? Integr Comp Biol. 2003;43:239–41. doi: 10.1093/icb/43.2.239. [DOI] [PubMed] [Google Scholar]
- Wake MH. Integrative biology: science for the 21st century. Bioscience. 2008;58:349–53. [Google Scholar]
- Westneat DF, Birkhead TR. Alternative hypotheses linking the immune system and mate choice for good genes. Proc R Soc Lond B Biol Sci. 1998;265:1065–73. [Google Scholar]
- White TA, Perkins SE. The ecoimmunology of invasive species. Funct Ecol. 2012;26:1313–23. [Google Scholar]
- Williams GC. Adaptation and natural selection: a critique of some current evolutionary thought. Princeton (NJ): Princeton University Press; 1966. pp. 158–92. [Google Scholar]
- Wilson K, Thomas MB, Blanford S, Doggett M, Simpson SJ, Moore SL. Coping with crowds: density-dependent disease resistance in desert locusts. Proc Natl Acad Sci USA. 2002;99:5471–5. doi: 10.1073/pnas.082461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi A. Mate selection—selection for a handicap. J Theor Biol. 1975;53:205–14. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zala SM, Potts WK, Penn DJ. Exposing males to female scent increases the cost of controlling Salmonella infection in wild house mice. Behav Ecol Sociobiol. 2008;62:895–900. [Google Scholar]


