Abstract
Using the mass-measuring capability of scanning transmission electron microscopy, we demonstrate that membrane crystals of the main light-harvesting complex of plants possess the ability to undergo light-induced dark-reversible disassociations, independently of the photochemical apparatus. This is the first direct visualization of light-driven reversible reorganizations in an isolated photosynthetic antenna. These reorganizations, identified earlier by circular dichroism (CD), can be accounted for by a biological thermo-optic transition: structural changes are induced by fast heat transients and thermal instabilities near the dissipation, and self-association of the complexes in the lipid matrix. A comparable process in native membranes is indicated by earlier findings of essentially identical kinetics, and intensity and temperature dependences of the ΔCD in granal thylakoids.
Keywords: Granal thylakoid membranes, LHCII, Light-induced reorganizations, Lipid–protein interactions, Membrane crystal, Thermo-optic effect
Introduction
Energy capture and conversion in the photosynthetic apparatus of plants is regulated by mechanisms that respond to rapid variations in light intensity (Anderson and Andersson 1988). The need to maximize photosynthetic productivity while minimizing the damaging effects of excess photoexcitation is met by structural and functional flexibilities of light-harvesting complex II (LHCII), the principal Chl a/b antenna complex associated with PSII (Horton et al. 1996). LHCII is subject to distinct photochemical feedback mechanisms: it is functionally regulated by protein phosphorylation (Allen 1992), governed by redox signals, and quenching of the singlet excited Chl a, controlled by the build-up of the transthylakoid proton gradient (Horton et al. 2005). When photochemical turnover rates are light saturated, additional regulatory mechanisms must be engaged. Indeed, light-induced reversible reorganizations of LHCII that are largely independent of the photochemical activity of the membranes have been reported with rates approximately proportional to the amount of photoexcitation above that useful for photosynthesis (Barzda et al. 1996, Cseh et al. 2005).
LHCII exists predominantly as trimers both in vivo and in vitro (Kühlbrandt 1994). In thylakoid membranes, trimers occur in supercomplexes with PSII and in semi-crystalline LHCII-only chiral macrodomains, often facing the domains of PSII–LHCII supercomplexes in the opposing, stacked membrane (Boekema et al. 2000, Garab and Mustárdy 2000, Ford et al. 2002). When isolated, LHCII trimers readily form membrane crystals, with hexagonal order, that form tightly stacked lamellar aggregates (Kühlbrandt 1994). This type of microcrystal exhibits a very strong psi- (polymerization- or salt-induced) type circular dichroism (CD), originating from a long-range chiral order of the chromophores (Garab et al. 1988a, Simidjiev et al. 1997, Miloslavina et al. 2012). In the presence of lipids, the complexes also assemble into chirally organized lamellae, but with loose stacking and considerably weaker psi-type CD (Simidjiev et al. 1997). These lipid–LHCII lamellae have been shown to be capable of undergoing light-induced reversible structural changes, on the time scale of minutres, affecting their chiral macro-organization (Simidjiev et al. 1997, Simidjiev et al. 1998), similar to those in granal thylakoid membranes (Barzda et al. 1996, Garab et al. 1988b). These reorganizations, as well as the monomerization of the LHCII trimers with prolonged illumination in vivo and in vitro, have been ascribed to a novel, biological thermo-optic mechanism (Cseh et al. 2000, Garab et al. 2002). Spectroscopic data and thermal analyses have indicated three consecutive phases of the light-induced reorganizations in granal thylakoid membranes: (i) unstacking of membranes; followed by (ii) a lateral disorganization of the chiral macrodomains; and, with prolonged illumination, (iii) monomerization of the LHCII trimers (Dobrikova et al. 2003, for a review, see Garab 2014). Here we present scanning transmission electron microscopy (STEM) analyses on loosely stacked lamellae of LHCII. STEM offers unique capabilities for determination of local mass per unit area (M/A) in unfixed, unstained specimens in the frozen state (Woodcock et al. 1980). We show that light disrupts the lipid–LHCII membrane crystals into disordered aggregates of trimers, from which ordered, stacked arrays regenerate in the dark. With the direct visualization of a light-driven structural change in an isolated photosynthetic antenna, we provide evidence for the inherent structural flexibility of lipid–LHCII macroassemblies that may contribute significantly to the structural flexibility of the thylakoid membranes.
Results
Loosely stacked lamellar aggregates of LHCII were examined in unstained preparations, using STEM. Single sheets possessed regions discontinuously perforated with holes, in hexagonal arrays (Fig. 1). Rarely, seven adjacent holes defined a complete hexagon. Holey regions usually included or adjoined featureless sheets containing occasional perforations. Analysis of 33 spectra from three LHCII preparations on structures similar to Fig. 1 indicated a periodicity of 123.0 ± 2.8 (SD) Å. This agrees with the model of Kühlbrandt et al. (1983), where the cavity is surrounded by six LHCII trimers with alternating up/down orientation relative to the plane of the lamella; the unit cell has the same repeat distance of approximately 125 Å. Using PCMass28 to select areas adjusted to frame six trimers surrounding the central void, the mass of a single trimer was calculated to be 121 ± 8 kDa, which is attributable to the apoprotein and the pigments, with possible minor mass contributions from bound lipids or detergent. Mass measurements for corresponding areas of unperforated sheets of single membranes that would encompass six trimers yielded values of 976 ± 45 kDa. Subtraction of 6 × 121 kDa, for the expected LHCII trimer content, leaves approximately 250 kDa (26%) of mass, which is attributed to bulk lipid (Simidjiev et al. 1997). The patches of holey membranes depict a novel and presumably delicate structure whose visualization is owed to the fact that STEM samples undergo minimal treatment before analysis and, at 10 e−/Å2 per pixel, experience <3% of beam damage.
Fig. 1.
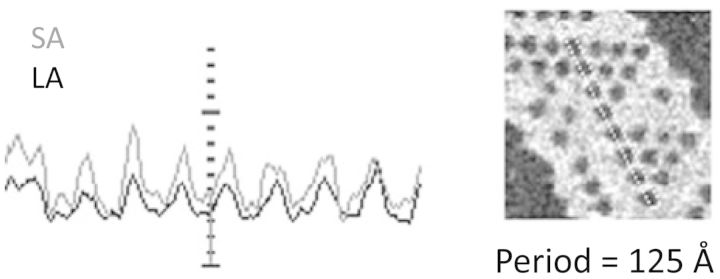
Power spectrum analysis of a holey hexagonal LHCII array by PCMass28 from large angle (LA) and small angle (SA) detectors, showing a repeat distance of approximately 125 Å.
Before illumination, LHCII was largely present as overlapping membrane sheets with M/A in multiples of approximately 31 Da/Å2 (Fig. 2a, left panel, L1–L5, indicating the number of layers), and membrane sheets with holey regions (Fig. 2a, right panel). There were no observations of single lamellae with differing mass density. Illumination of LHCII for 3 min caused a dramatic change in appearance. The few remaining lamellae were no longer in overlapping stacks; they appeared to be sheared, and scarcely holding together (Fig. 2b, left panel). The majority of such images, however, showed only large polymorphic fragments, which frequently were elongated as though comprised of chains of LHCII. Mass analysis was applied to a population of smaller, approximately spherical fragments, released in the light. Over the mass range of 0–600 kDa, a distribution profile was obtained (Fig. 2b, right panel) with a peak around 127 kDa, in good agreement with the LHCII trimer mass determination of approximately 121 kDa discussed above. There was no significant population of LHCII monomers (<50 kDa), suggesting that monomerization occurs only after further disintegration of macroaggregates and trimers at longer or more intense light exposures (Garab et al. 2002).
Fig. 2.
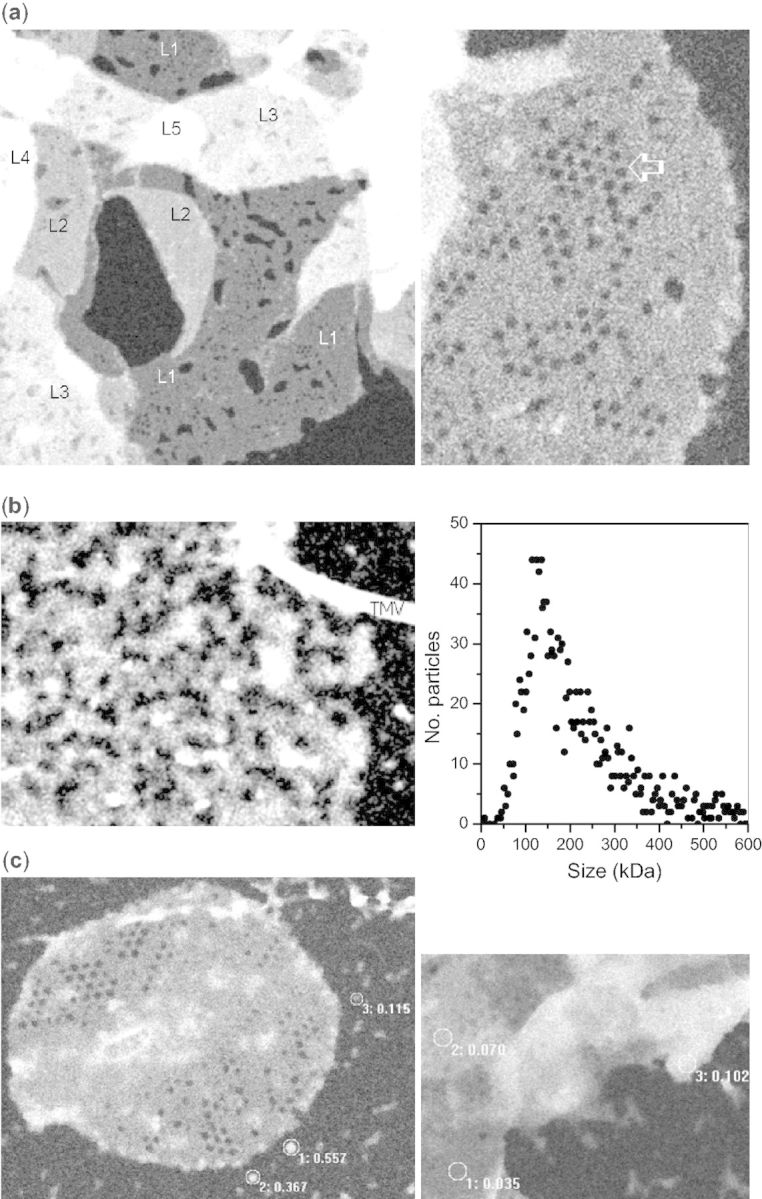
STEM images of lamellar aggregates of LHCII in the dark (a), after 3 min illumination of the sample with heat-filtered red light of 2,200 µmol photons m−2 s−1 (b) and after 3 min light and 10 min dark (c). Scale bar = 500 Å. Tobacco mosaic virus, seen in (b), is added to calibrate density. In (a) and (c), labels indicate the number of superimposed sheets in the circled area and corresponding density (M/A) in kDa/Å2. Shown in (b; right panel) is the mass distribution profile of 1,530 particles from 23 images of the same preparation after 3 min illumination. (“Noise” in these images is due to low-dose imaging conditions needed to preserve structure.)
The structural effects of a 3 min light treatment were substantially reversed in the dark. Within 10 min, lamellae with one level of stacking were already found, and small aggregates of LHCII appeared to be fusing with them (Fig. 2c, right panel); also, large membrane sheets with holey regions appeared (Fig. 2c, left panel). Thirty minutes after illumination, the sheets were multiply stacked and the background was almost clear of small particles and aggregates (not shown). Measurements of M/A showed that the sheets were stacked with densities in multiples of approximately 34 Da/Å2, similar to the state before illumination.
These structural changes detected by STEM can evidently be held responsible for the variations in the chiral macro-organization of the complexes, observed earlier as ΔCD of LHCII lamellae (see the Introduction). The magnitude of the psi-type CD signal has been shown to depend on the size and long-range order of the chirally organized macrodomains both in thylakoid membranes and in LHCII lamellar aggregates (Barzda et al. 1994, Garab and van Amerongen 2009).
As pointed out above (see the Introduction), in thylakoid membranes the light-induced reorganizations have been shown to encompass membrane unstacking, followed by a lateral desorganization of the protein complexes (Dobrikova et al. 2003). It has been shown that these processes are associated with a light-induced reversible release of Mg ions, which occurs both in thylakoid membranes and in lamellar aggregates of LHCII (Garab et al. 1998, Garab et al. 2002). Based on these observations, we propose that the release of Mg ions might be responsible for both the disruption of the multilamellar membrane system of LHCII and the unstacking of granal thylakoid membranes. While it may not be easy to provide direct evidence for this assumption for the case of LHCII lamellae, it is more straightforward to support this statement for thylakoid membranes since stacking of adjacent granum membranes is largely governed by electrostatic forces (Barber and Chow 1979, Barber 1982, Garab et al. 1991, Garab 2014). To substantiate further the proposed role of Mg ions in the light-induced reorganizations of thylakoid membranes, we have investigated the dependence of the magnitude of the light-induced change in psi-type CD (ΔCD) on the concentration of Mg ions. As shown in Fig. 3a, in the absence of cations (in hypotonic medium), no psi-type CD (and thus no ΔCD) could be detected; between 0 and 5 mM, the magnitude of the psi-type CD and ΔCD increased with the concentration of Mg ions, and reached its maximum at approximately 5 mM—in perfect agreement with our earlier data (Garab et al. 1991). Higher concentrations of Mg ions, however, led to a slight decrease in the CD amplitude and significantly inhibited the light-induced CD changes. This latter effect can evidently be attributed to a stronger stacking, and thus the hindrance of unstacking. Similar effects are seen for the monovalent cation K+ (Fig. 3b) and the polycation spermidine (Fig. 3c), which fully arrested ΔCD, while only moderately decreasing the CD amplitude. These data are fully consistent with the role of cations in the stacking interactions and, as reflected by the different ranges of efficient concentrations for mono-, di- and polyvalent cations, with the role of the electrical double layer (cf. Barber and Chow 1979, Barber 1982).
Fig. 3.
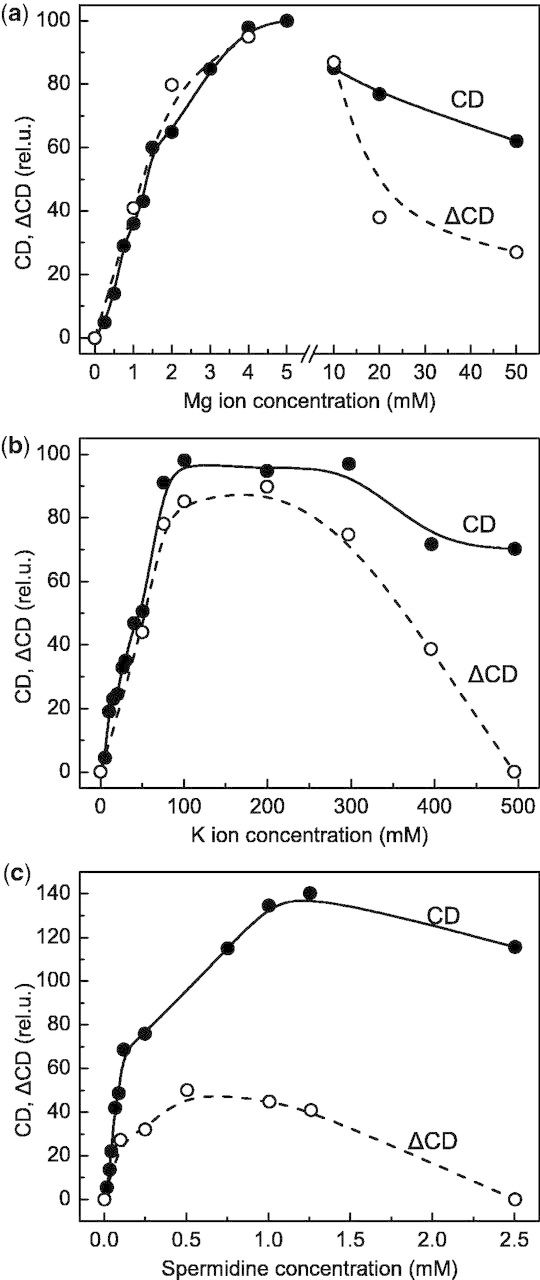
Dependence of the relative amplitudes of the main psi-type circular dichroism bands in the red spectral region (CD) of isolated pea thylakoid membranes, and the relative amplitudes of the light-induced CD changes at (+)690 nm (ΔCD elicited by broad-band blue excitation of 500 W m−2) on the concentration of different cations, Mg2+ (a), K+ (b) and spermidine (c). The maximum amplitudes in (a) were taken as 100%; the same CD and ΔCD amplitudes were used in (b) and (c) as reference values. For further details, see the Materials and Methods.
Discussion
By using STEM, we revealed, albeit with low resolution, the structure of lipid–LHCII membrane crystals, and provided direct evidence that this multilamellar macroassembly with a hexagonal array of the trimeric complexes undergoes light-dependent reversible reorganizations. Upon illumination, the membrane crystals are disrupted, the lamellae are sheared and large aggregates of trimers are formed. These reorganizations most probably can be accounted for by an unstacking of the loosely stacked lamellae, induced by the release of Mg ions, and the consequent destabilization of the long-range order of the complexes in the membrane (lipid matrix), leading to an overall disorganization. The high self-assembly capacity of LHCII readily explains the substantial degree of recovery in the dark, the re-assembly of the multilamellar system containing ordered arrays of trimeric complexes.
In this context, it is also important to point out that thylakoid membranes and LHCII lamellae exhibit very similar self-assembly capacities, which can be monitored by their characteristic psi-type CD signals and similar ΔCD (Garab et al. 1988b, Garab et al. 1991, Barzda et al. 1996), with virtually identical temperature and light intensity dependences (Cseh et al. 2005). Further, both in thylakoid membranes and in LHCII lamellae, the release of Mg ions occurs with rates approximately proportional to the excess light intensity (Garab et al. 1998, Garab 2014). Also, ΔCD in thylakoids has been shown to be largely independent of the photochemical activity of the membranes (Istokovics et al. 1997) and to be approximately linearly proportional to the light intensity above the saturation of photosynthesis (Barzda et al. 1996, Cseh et al. 2005, see also Garab 2014). Thylakoids and lamellar aggregates of LHCII also exhibit very similar transient spectra (Holm et al. 2005). Hence, these data are in line with the notion (Garab and Mustárdy 2000) that a substantial part of the structural flexibility of the thylakoid membranes is ‘borrowed’ from the inherent flexibility of LHCII, and point to the possible role of LHCII-only crystalline-like domains in the grana (Boekema et al. 2000, Holm et al. 2005).
LHCII is known to be inserted unidirectionally with respect to the thylakoid lamellar plane, in contrast to its bifacial arrangement in membrane crystals (Kühlbrandt et al. 1983). The reasonable assumption can be made, however, that regardless of the manner of LHCII packing, illumination can weaken interactions involving the surfaces (stacking and packing) of adjacent trimers in both situations. In the thylakoid membrane, a light-driven, partial disassociation of LHCII-only macrodomains (Boekema et al. 2000, Garab and Mustárdy 2000, Ford et al. 2002) and LHCII–PSII supercomplexes would alleviate excitation pressure on PSII reaction centers and, in conjunction with non-photochemical quenching (NPQ), might protect against photoinhibition and pigment photodestruction (Lambrev et al. 2012). Indeed, fluorescence lifetime experiments have revealed the existence of detached, quenched LHCII in thylakoid membranes in leaves exposed to excess illumination (Holzwarth et al. 2009). The capability of isolated LHCII of exhibiting light-induced reversible quenching with rates proportional to the intensity of the excitation was first shown by Jennings et al. (1991). Thermodynamic analyses of these transients have shown that they are associated with conformational changes in the protein complexes (Barzda et al. 1999). Although the mechanism of NPQ is outside the scope of the present study, we would like to note that, in broad terms, these data are in harmony with the notion that LHCII has an inbuilt capability to undergo transformation into a dissipative state by conformational change (Ruban et al. 2007, Krüger et al. 2011, Krüger et al. 2012). Also, we have shown that the quenched state can be trapped at low temperature despite the rapid decay of the energized state of the membrane (Lambrev et al. 2007). Light-induced structural changes in LHCII are also involved in the regulation of the phosphorylation by light at the substrate level both in isolated LHCII and in the native, thylakoid membrane (Zer et al. 1999, Zer et al. 2003), an additional regulatory mechanism that complements the well-known redox regulation of LHCII phosphorylation (Allen 1992). It is also interesting to note that lipid–protein membranes composed of LHCII isolated from spinach and the thylakoid lipids, monogalcatosyldiacylglycerol and digalactosyldiacylglycerol, assumed strikingly different structures when the proteins were isolated from dark-adapted or high-light- (HL) treated plants. Lipid–LHCII membranes, when isolated from dark-adapted leaves, assembled into planar multibilayers, in contrast to the lipid–LHCII HL-treated membranes, which formed less ordered structures (Janik et al. 2013).
The light-induced disassociation of LHCII from the membrane crystal, reported here, is consistent with the thermo-optic mechanism, according to which fast thermal transients due to dissipated excitation energy can lead to elementary structural transitions in the close vicinity of the site of dissipation (Cseh et al. 2005, Gulbinas et al. 2006, Garab 2014). The elementary structural changes are made possible by the presence of inherent structure instabilities near the site of dissipation, while the reversibility stems from an intrinsic, molecular self-association of the complexes into oligomers and ordered aggregates. Lipid or carotenoid components may play a role in these structural changes. The addition of isolated thylakoid lipids enhances the formation of lamellae (Simidjiev et al. 2000) while lending a structural flexibility for the light-induced reorganizations (Simidjiev et al. 1998). In contrast, when LHCII is delipidated by extraction at higher detergent/Chl ratios, the psi-type CD in the tightly stacked lamellar aggregates increases but LHCII becomes unresponsive to illumination (Simidjiev et al. 1997). Such preparations also exhibit an increased thermal stability, in contrast to lipid–LHCII lamellae, which appear to possess ‘built-in’ thermal instabilities well below the denaturation temperature (Simidjiev et al. 2004). These several effects of lipid content on the stability of LHCII membrane crystals support the notion that the holes in the LHCII arrays, seen in Figs. 1 and 2a and c, arise by loss of interstitial lipid; however, the similar densities of lipid and protein in unstained STEM images does not allow us to exclude the possibility that the holes were formerly occupied by LHCII itself. The photoisomerization or interconversion of carotenoids can also influence the organization of LHCII (Grudzinski et al. 2002). Further work is required to discover the specific site(s) of dissipation and the heat-sensitive structural element(s), to determine the magnitude and ‘radius’ of the heat package and, in general, the exact mechanism of the structural transitions in LHCII (macro)assemblies.
When interpreting the presently available data in terms of the thermo-optic mechanism proposed by Cseh et al. (2000, 2005) (reviewed by Garab 2014), we recall that dissipation of excitation energy causes a thermal transient with a sizeable temperature jump in the near vicinity (∼1 nm) of the dissipation site and with a decay time of about 20–200 ps (Gulbinas et al. 2006). This heat package, with some (low) probability, might induce an elementary structural change in the protein, especially if the temperature jump induces a release of protein-bound cation, as, in fact, has been observed both for LHCII and for isolated thylakoid membranes (Garab et al. 1998, Garab et al. 2002, Garab 2014). It is proposed that the outer loop segment near the N-terminus might readily release a cation upon heat dissipation at the nearby ‘terminal emitter’ Chl a cluster (Chl a610, a611, a612). This region, the outer loop segment near the N-terminus, has been shown, by electron paramagnetic resonance (EPR) labeling, to be highly structurally flexible (Dockter et al. 2012). Cations that are released via a thermo-optic mechanism will not be rebound immediately after the heat jump, but may instead re-bind on a much slower time scale; in fact, the cations (Mg2+) reversibly released from lamellar aggregates of LHCII upon illumination with continuous light can be detected on the time scale of seconds to minutes (Garab et al. 1998). The dissipation-assisted release of Mg ions is probably responsible for the fast (<200 ns, instrument limited) electric signal, most probably from charge displacement, detected upon excitation of oriented LHCII lamellae with a 20 ns laser pulse (Garab et al. 2002). Cations play an essential role in stacking of LHCII-containing membranes (Garab et al. 1991) by screening the negative charges exposed on the LHCII surface (Fig. 4A). Thus, cation release may affect the array of LHCII complexes held together via electrostatic forces in thylakoid membranes. During an illumination period of a few minutes, such or similar events (schematically illustrated in Fig. 4B) might lead to macroscopically observable structural changes detected as changes in the psi-type CD signal of LHCII macroassemblies in vitro or in vivo.
Fig. 4.
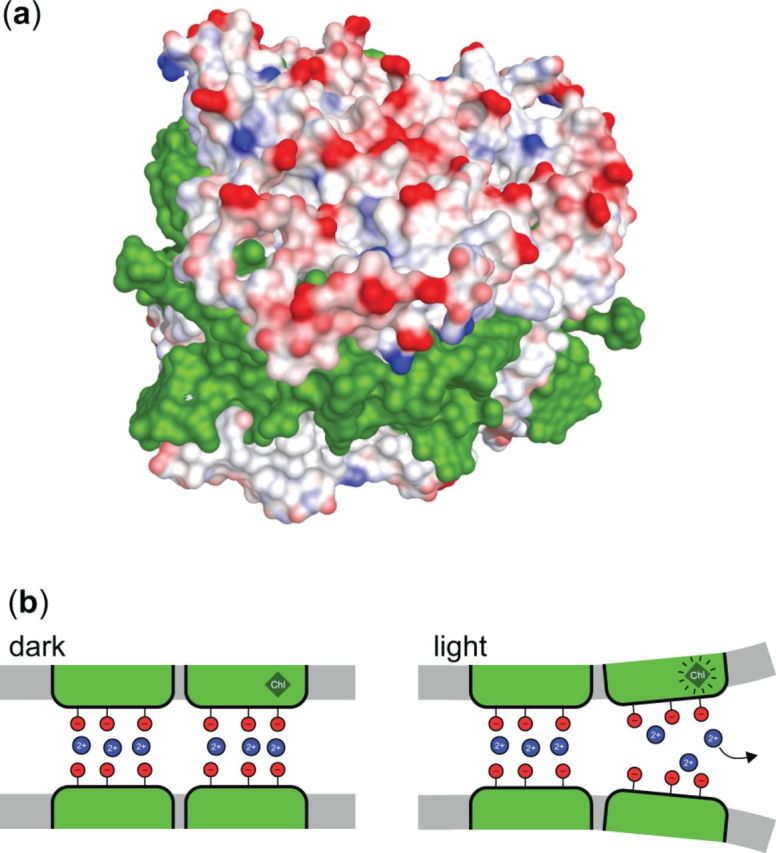
A model illustration of the role of cations in thylakoid membrane stacking. Partial charges on the stromal-exposed surface of the LHCIIb trimer (PDB ID 2BHW) rendered using Accelrys Discovery Studio (a). Negative charges are in red, positive in blue, and cofactors (Chls and xanthophylls) in green. Apart from the positively charged N-terminus (not present in the image), the surface contains solely negatively charged residues; membrane stacking thus requires screening cations (Mg2+) between the layers. Schematic illustration of the cation-mediated stacking in dark conditions and unstacking in light conditions induced by thermal deactivation of Chl excited states, with the heat package causing the release of cations.
In conclusion, the comparable time courses and reversibility of the structural changes observed using STEM and CD measurements—seen in both isolated LHCII and the native thylakoid membrane—support the concept that the LHCII aggregation state in situ is flexibly responsive to illumination, largely independent of ΔpH, photochemical processes and the reaction centers. This ability of LHCII evidently plays an important role in light adaptation and photoprotection of plants.
Materials and Methods
The procedure of Simidjiev et al. (1997), based on the isolation method introduced by Krupa et al. (1987), was used to prepare LHCII. Briefly, thylakoid membranes were obtained from 10-day-old leaves of pea by homogenization in ice-cold sorbitol/Tricine medium lacking Mg ions. Buffered Triton X-100 was added at a controlled detergent/Chl ratio (optimized for the leaf material in preliminary trials) and incubated at 25°C for 45 min. Solubilized LHCII was recovered by centrifugation at 4°C and purified by aggregation with 20 mM MgCl2 plus 100 mM KCl. The aggregate was re-extracted with Triton X-100 and precipitated again with MgCl2/KCl. The pellets were washed in 20 mM Tricine/KOH, pH 7.8 and stored on ice in the same buffer containing 0.1 M sorbitol and 1 mM azide, in which they were stable for at least 15 d. Dilutions in Tricine/KOH buffer, to 20 or 50 µg Chl ml−1, were illuminated at 20°C for the indicated times in 2 mmol m−2 s−1 of heat-filtered red light defined by a Kodak Wratten 25 filter and 2 cm of water. Equivalent samples were maintained in the dark.
Samples for STEM analysis were withdrawn and applied directly to carbon-coated grids, which were briefly washed in 20 mM ammonium acetate then manually frozen within 5 s in liquid nitrogen and dehydrated in vacuo at −100°C. Images were analyzed using PCMass28 (available at ftp.stem.bnl.gov). Mean M/A of lamellae was obtained within a circular field of at least 800 Å diameter. Mass values for particles were obtained using the automated mass determination feature of PCMass28. Preliminary manual counting into 10 kDa bins was used to establish density, background and shape parameters, and only particles completely fitting within a circular mask of radius 80 Å were accepted. PCMass28 was customized accordingly to perform automated screening. Mass values were referenced to the M/A for Tobacco mosaic virus deposited on the same grid.
Thylakoid membranes were isolated from 2-week-old pea leaves, and light-induced CD changes were recorded as described earlier (Istokovics et al. 1997). CD spectroscopy and ΔCD measurements were performed at room temperature, using a Jobin-Yvon CD6 spectropolarimeter, on thylakoid membrane suspended in 30 mM Tricine supplemented with different amounts of cations, as indicated in Fig. 3 [Chl (a + b) content, 20 µg ml−1; optical pathlength, 1 cm; slit width, 4 nm]. The amplitude of the psi-type CD signal was determined as the difference between the major positive and negative psi-type bands at around 690 and 670 nm. The amplitude of the light-induced CD changes (ΔCD) was measured at 690 nm, using a broad-band excitation in the blue of about 500 W m−2 intensity.
Funding
This work was supported by the Hungarian National Innovation Office; A*STAR Singapore [NIH-A*STAR TET_10-1-2011-0279]; the Hungarian Scientific Research Fund/National Office for Research and Technology [No. 80345 TÁMOP-4.2.2.A-11/1/KONV-2012-0060 to G.G.]; the Hungarian Scientific Research Fund, OTKA [PD-014530 to P.H.L.]. STEM experiments were performed at the BNL STEM facility with support from the U.S.D.O.E. Office of Energy Research, Office of Basic Energy Sciences, Division of Energy Biosciences, to G.H. The STEM Resource Center was supported by NIH P41-RR01777.
Acknowledgments
We thank B. Lin and M. Simon for sample preparation and microscopy, H. Li and Z. Chen for comments on the manuscript, and L. Kovács for providing some of the preparations.
Glossary
Abbreviations
- CD
circular dichroism
- ΔCD
light-induced reversible changes in the CD
- LHCII
light-harvesting complex II
- M/A
mass per unit area
- NPQ
non-photochemical quenching of the singlet excited state of Chl A
- psi
polymerization- or salt-induced
- STEM
scanning transmission electron microscopy
Disclosures
The authors have no conflicts of interest to declare.
References
- Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Andersson B. The dynamic photosynthetic membrane and regulation of solar-energy conversion. Trends Biochem. Sci. 1988;13:351–355. doi: 10.1016/0968-0004(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Barber J. Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 1982;33:261–295. [Google Scholar]
- Barber J, Chow WS. A mechanism for controlling the stacking and unstacking of chloroplast thylakoid membranes. FEBS Lett. 1979;105:5–10. [Google Scholar]
- Barzda V, Istokovics A, Simidjiev I, Garab G. Structural flexibility of chiral macroaggregates of light-harvesting chlorophyll a/b pigment–protein complexes. Light-induced reversible structural changes associated with energy dissipation. Biochemistry. 1996;35:8981–8985. doi: 10.1021/bi960114g. [DOI] [PubMed] [Google Scholar]
- Barzda V, Jennings RC, Zucchelli G, Garab G. Kinetic analysis of the light-induced fluorescence quenching in light-harvesting chlorophyll a/b pigmentprotein complex of photosystem II. Photochem. Photobiol. 1999;70:751–759. [Google Scholar]
- Barzda V, Mustárdy L, Garab G. Size dependency of circular dichroism in macroaggregates of photosynthetic pigment–protein complexes. Biochemistry. 1994;33:10837–10841. doi: 10.1021/bi00201a034. [DOI] [PubMed] [Google Scholar]
- Boekema EJ, van Breemen JFL, van Roon H, Dekker JP. Arrangement of photosystem-II supercomplexes in crystalline macrodomains within the thylakoid membrane of green plant chloroplasts. J. Mol. Biol. 2000;301:1123–1133. doi: 10.1006/jmbi.2000.4037. [DOI] [PubMed] [Google Scholar]
- Cseh Z, Rajagopal S, Tsonev T, Busheva M, Papp E, Garab G. Thermooptic effect in chloroplast thylakoid membranes. Thermal and light stability of pigment arrays with different levels of structural complexity. Biochemistry. 2000;39:15250–15257. doi: 10.1021/bi001600d. [DOI] [PubMed] [Google Scholar]
- Cseh Z, Vianelli A, Rajagopal S, Krumova S, Kovács L, Papp E, et al. Thermo-optically induced reorganizations in the main light-harvesting antenna of plants. I. Non-Arrhenius type of temperature dependence and linear light-intensity dependencies. Photosynth. Res. 2005;86:263–273. doi: 10.1007/s11120-005-5104-1. [DOI] [PubMed] [Google Scholar]
- Dobrikova AG, Várkonyi Zs, Krumova SB, Kovács L, Kostov GK, Todinova SJ, et al. Structural rearrangements in chloroplast thylakoid membranes revealed by differential scanning calorimetry and circular dichroism spectroscopy. Thermo-optic effect. Biochemistry. 2003;42:11272–11280. doi: 10.1021/bi034899j. [DOI] [PubMed] [Google Scholar]
- Dockter C, Müller AH, Dietz C, Volkov A, Polyhach Y, Jeschke G, et al. Rigid core and flexible terminus: structure of solubilized light-harvesting chlorophyll a/b complex (LHCII) measured by EPR. J. Biol. Chem. 2012;287:2915–2925. doi: 10.1074/jbc.M111.307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford RC, Stoylova SS, Holzenburg A. An alternative model for photosystem II/light harvesting complex II in grana membranes based on cryo-electron microscopy studies. Eur. J. Biochem. 2002;269:326–336. doi: 10.1046/j.0014-2956.2001.02652.x. [DOI] [PubMed] [Google Scholar]
- Garab G. Hierarchical organization and structural flexibility of thylakoid membranes. Biochim. Biophys. Acta. 2014;1837:481–494. doi: 10.1016/j.bbabio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Garab G, Cseh Z, Kovács L, Rajagopal S, Várkonyi Zs, Wentworth M, et al. Light-induced trimer to monomer transition in the main light-harvesting antenna complex of plants: thermo-optic mechanism. Biochemistry. 2002;41:15121–15129. doi: 10.1021/bi026157g. [DOI] [PubMed] [Google Scholar]
- Garab G, Faludi-Dániel A, Shutherland JC, Hind G. Macroorganization of chlorophyll a/b light-harvesting complex in thylakoids and aggregates: information from circular differential scattering. Biochemistry. 1988a;27:2425–2432. [Google Scholar]
- Garab G, Istokovics A, Butiuc A, Simidjiev I, Dér A. Light-induced ion movements in thylakoid membranes and isolated LHC II. In: Garab G, editor. Photosynthesis: Mechanisms and Effects. Dordrecht, The Netherlands: Springer; 1998. pp. 341–344. [Google Scholar]
- Garab G, Kieleczawa J, Sutherland JC, Bustamante C, Hind G. Organization of pigment–protein complexes into macrodomains in the thylakoid membranes of wild-type and chlorophyll b-less mutant of barley as revealed by circular dichroism. Photochem. Photobiol. 1991;54:273–281. [Google Scholar]
- Garab G, Leegood RC, Walker DA, Sutherland JC, Hind G. Reversible changes in macroorganization of the light-harvesting chlorophyll a/b pigment protein complex detected by circular dichroism. Biochemistry. 1988b;27:2430–2434. [Google Scholar]
- Garab G, Mustárdy L. Role of LHCII-containing macrodomains in the structure, function and dynamics of grana. Aust. J. Plant Physiol. 2000;27:279–285. [Google Scholar]
- Garab G, van Amerongen H. Linear dichroism and circular dichroism in photosynthesis research. Photosynth. Res. 2009;101:135–146. doi: 10.1007/s11120-009-9424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzinski W, Krupa Z, Garstka M, Maksymiec W, Swartz TE, Gruszecki WI. Conformational rearrangements in light-harvesting complex II accompanying light-induced chlorophyll a fluorescence quenching. Biochim. Biophys. Acta. 2002;1554:108–117. doi: 10.1016/s0005-2728(02)00218-9. [DOI] [PubMed] [Google Scholar]
- Gulbinas V, Karpicz R, Garab G, Valkunas L. Nonequilibrium heating in LHCII complexes monitored by ultrafast absorbance transients. Biochemistry. 2006;45:9559–9565. doi: 10.1021/bi060048a. [DOI] [PubMed] [Google Scholar]
- Holm JK, Várkonyi Zs, Kovács L, Posselt D, Garab G. Thermo-optically induced reorganizations in the main light harvesting antenna of plants. II. Indications for the role of LHCII-only macrodomains in thylakoids. Photosyth. Res. 2005;86:275–282. doi: 10.1007/s11120-005-5302-x. [DOI] [PubMed] [Google Scholar]
- Holzwarth AR, Miloslavina Y, Nilkens M, Jahns P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 2009;483:262–267. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Horton P, Wentworth M, Ruban A. Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett. 2005;579:4201–4206. doi: 10.1016/j.febslet.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Istokovics A, Simidjiev I, Lajkó F, Garab G. Characterization of the light-induced reversible changes in the chiral macroorganization of the chromophores in chloroplast thylakoid membranes—temperature-dependence and effect of inhibitors. Photosynth. Res. 1997;54:45–53. [Google Scholar]
- Janik E, Bednarska J, Zubik M, Puzio M, Luchowski R, Grudzinski W, et al. Molecular architecture of plant thylakoids under physiological and light stress conditions: a study of lipid–light-harvesting complex II model membranes. Plant Cell. 2013;25:2155–2170. doi: 10.1105/tpc.113.113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings RC, Garlaschi FM, Zucchelli G. Light-induced fluorescence quenching in the light-harvesting chlorophyll a/b protein complex. Photosynth. Res. 1991;27:57–64. doi: 10.1007/BF00029976. [DOI] [PubMed] [Google Scholar]
- Krüger TPJ, Wientjes E, Croce R, van Grondelle R. Conformational switching explains the intrinsic multifunctionality of plant light-harvesting complexes. Proc. Natl Acad. Sci. USA. 2011;108:13516–13521. doi: 10.1073/pnas.1105411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger TPJ, Ilioaia C, Johnson MP, Ruban AV, Papagiannakis E, Horton P, et al. Controlled disorder in plant light-harvesting complex II explains its photoprotective role. Biophys. J. 2012;102:2669–2676. doi: 10.1016/j.bpj.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa Z, Huner NPA, Williams JP, Maissan E, James DR. Development at cold hardening temperatures—the structure and composition of purified rye LHCII. Plant Physiol. 1987;84:19–24. doi: 10.1104/pp.84.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W. Structure and function of the plant light-harvesting complex, LHC II. Curr. Opin. Struct. Biol. 1994;4:519–528. [Google Scholar]
- Kühlbrandt W, Thaler Th, Wehrli E. The structure of membrane crystals of the light-harvesting chlorophyll a/b protein complex. J. Cell Biol. 1983;96:1414–1424. doi: 10.1083/jcb.96.5.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrev PH, Miloslavina Y, Jahns P, Holzwarth AR. On the relationship between non-photochemical quenching and photoprotection of Photosystem II. Biochim. Biophysic. Acta. 2012;1817:760–769. doi: 10.1016/j.bbabio.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Lambrev PH, Tsonev T, Velikova V, Georgieva K, Lambreva MD, Yordanov I, et al. Trapping of the quenched conformation associated with non-photochemical quenching of chlorophyll fluorescence at low temperature. Photosynth. Res. 2007;94:321– 332. doi: 10.1007/s11120-007-9216-7. [DOI] [PubMed] [Google Scholar]
- Miloslavina Y, Lambrev PH, Jávorfi T, Várkonyi Z, Karlický V, Wall JS, Hind G, Garab G. Anisotropic circular dichroism signatures of oriented thylakoid membranes and lamellar aggregates of LHCII. Photosynth Res. 2012;111:29–39. doi: 10.1007/s11120-011-9664-y. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–578. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- Simidjiev I, Barzda V, Mustárdy L, Garab G. Isolation of lamellar aggregates of the light-harvesting chlorophyll a/b protein complex of photosystem II with long-range chiral order and structural flexibility. Anal. Biochem. 1997;250:169–175. doi: 10.1006/abio.1997.2204. [DOI] [PubMed] [Google Scholar]
- Simidjiev I, Barzda V, Mustárdy L, Garab G. Role of thylakoid lipids in the structural flexibility of lamellar aggregates of the isolated light-harvesting chlorophyll a/b complex of photosystem II. Biochemistry. 1998;37:4169–4173. doi: 10.1021/bi972726m. [DOI] [PubMed] [Google Scholar]
- Simidjiev I, Stoylova S, Amenitsch H, Jávorfi T, Mustárdy L, Laggner P, et al. Self-assembly of large, ordered lamellae from nonbilayer lipids and integral membrane-proteins in vitro. Proc. Natl Acad. Sci. USA. 2000;97:1473–1476. doi: 10.1073/pnas.97.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simidjiev I, Várkonyi Zs, Garab G. Isolation and characterization of lamellar aggregates of LHCII and lipid–LHCII macroassemblies with light-inducible structural transitions. In: Carpentier R, editor. Photosynthesis Research Protocols. Totowa, NJ: Humana Press; 2004. pp. 105–114. [Google Scholar]
- Woodcock CLF, Frado L-LY, Wall JS. Composition of native and reconstituted chromatin particles: direct mass determination by scanning transmission electron microscopy. Proc. Natl Acad. Sci. USA. 1980;77:4818–4822. doi: 10.1073/pnas.77.8.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zer H, Vink M, Keren N, Dilly-Hatrwig H, Paulsen H, Herrmann RG, et al. Regulation of thylakoid protein phosphorylation at the substrate level: reversible light-induced conformational changes expose the phosphorylation site of the light-harvesting complex II. Proc. Natl Acad. Sci. USA. 1999;96:8277–8282. doi: 10.1073/pnas.96.14.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zer H, Vink M, Shochat S, Herrmann RG, Andersson B, Ohad I. Light affects the accessibility of the thylakoid light harvesting complex II (LHCII) phosphorylation site to the membrane protein kinase(s) Biochemistry. 2003;42:728–738. doi: 10.1021/bi020451r. [DOI] [PubMed] [Google Scholar]


