Abstract
Background
The need to develop new, improved treatments for tuberculosis (TB) remains urgent, and the repurposing of existing drugs represents a possible shortcut to market. Recently, there has been significant interest in host-directed adjuvant therapy to enhance bacillary killing. HMG-CoA reductase inhibitors (statins), which are among the most commonly prescribed drugs, have immunomodulatory properties and improve the clinical outcomes of bacterial infections.
Methods
We studied the tuberculocidal activity of simvastatin alone and in combination with first-line anti-TB drugs in J774 macrophages and during chronic TB infection.
Results
Exposure to 5 μM simvastatin significantly increased the tuberculocidal activity of isoniazid in J774 macrophages at Day 3 after infection versus isoniazid alone (P = 0.02). Similarly, relative to the standard oral regimen of rifampicin (10 mg/kg), isoniazid (10 mg/kg) and pyrazinamide (150 mg/kg) given five times weekly, the addition of 25 mg/kg simvastatin enhanced bacillary killing, reducing the number of lung cfu by an additional 1 log10 at Day 28 (P < 0.01) and by a further 1.25 log10 at Day 56 (P < 0.01).
Conclusions
The potential additive activity of simvastatin to first-line TB treatment holds promise. However, further studies to identify the optimal statin and dosing are required. In addition the ability of combination treatment with statins to accelerate the time required to achieve a stable cure remains to be explored.
Keywords: TB, statins, lipids, mice
Introduction
The high cost and protracted nature of preclinical tuberculosis (TB) drug screening,1,2 combined with low profitability,3 have hampered the development of new drugs, spurring new efforts to identify and repurpose existing drugs for TB treatment. In particular, host-directed adjunctive therapies have recently raised considerable interest.4–6
Statins lower cholesterol level through an inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. Statins also have broad immunomodulatory and anti-inflammatory properties, reducing mortality in patients with bacteraemia and multiple organ dysfunction.7,8 Atorvastatin and lovastatin reduced the in vitro and in vivo growth of Chlamydia pneumonia and Salmonella enterica.9,10
Following infection by Mycobacterium tuberculosis, macrophages accumulate lipid bodies, acquiring a ‘foamy’ phenotype. Although their precise role remains poorly characterized, these lipid bodies may serve as a food source for intracellular bacilli. Their accumulation has also been associated with M. tuberculosis growth restriction and phenotypic drug tolerance.11 Phagolysosomal maturation is delayed in foamy macrophages due to enhanced IL-10 induction, alternative macrophage polarization and blunting of the innate immune response.12 Therefore foamy macrophages provide a haven for persistent M. tuberculosis in the infected host. Foamy macrophage formation seems to be dependent on the protein called 6 kDa early secretory antigenic target (ESAT-6) and susceptible to pharmacological inhibition.13 A recent study demonstrated an anti-TB activity of statins in macrophages and in the lungs of M. tuberculosis-infected mice.14 In the current study we hypothesized that the addition of simvastatin might increase the killing activity of standard anti-TB drugs in macrophages and in chronically infected mice.
Methods
Macrophage infection and in vitro antibiotic assays
J774 macrophages were seeded at a density of 2 × 105 cells/well in 24-well plates before activation using 50 ng/mL IFN-γ (R&D) overnight, followed by incubation with 200 ng/mL lipopolysaccharide (Sigma-Aldrich) for 3 h. Following activation, cells were infected with M. tuberculosis CDC1551 at a multiplicity of infection of 10 : 1 for 3 h and washed with (×3) PBS and (×1) RPMI (Life Technologies). After the final wash, the cells were incubated with 200 μg/mL amikacin (Sigma-Aldrich) for 1 h to kill extracellular bacteria and washed, as previously, before resuspension in RPMI medium containing simvastatin (5 μM), isoniazid (0.05 μg/mL) or vehicle control (2% DMSO). The cells were washed in PBS before lysis with 1% (v/v) Triton X-100 and plating on 7H11 selective medium (Fisher, Pittsburgh). For determination of the MIC of simvastatin for axenic M. tuberculosis, a total of 105 bacilli (optical density at 600 nm = 0.5) were inoculated into separate tubes containing 1 mL of supplemented Middlebrook 7H9 broth lacking Tween with increasing concentrations of simvastatin (0–320 μM). Tubes were left standing at 37°C for 14 days. The MIC was defined as the lowest concentration failing to produce a visible pellet.
Intracellular lipid analysis
Nile red staining was measured by flow cytometry to assess intracellular lipid content.15 Briefly, cells were harvested from 24-well plates and incubated with Nile red (Invitrogen) (1 mg/mL) for 10 min and washed, and the fluorescence measured at 584 nm by flow cytometry (FACSCalibur, Becton-Dickinson).
Animals
Female, 4–6-week-old BALB/c mice were purchased from Charles River (Wilmington, MA, USA). All veterinary care and procedures were carried out in accordance with the guidelines of the Johns Hopkins Institutional Animal Care and Use Committee.
Animal chemotherapy study design and endpoints
Frozen aliquots of M. tuberculosis CDC1551 were thawed and grown to mid log-phase in Middlebrook 7H9 broth (Fisher). Aerosol infection with 3.7 log10 cfu was achieved using the Inhalation Exposure System (Glas-Col).16 In the first study the animals received 25 mg/kg simvastatin or vehicle (2% DMSO in PBS) daily (5 times/week) by oesophageal cannulation for 6 weeks, beginning 1 day post-infection. In the second study the animals received rifampicin (10 mg/kg), isoniazid (10 mg/kg) and pyrazinamide (150 mg/kg) as standard treatment with or without simvastatin (25 mg/kg) by gavage on 5 days per week for 8 weeks. Rifampicin doses were separated from the accompanying drugs by at least 1 h to minimize drug–drug interactions. The dose of simvastatin was chosen based on previous publications17 and the manufacturer's determination of human-equivalent drug exposures. At each timepoint lungs were aseptically removed, homogenized and plated on Middlebrook 7H11 plates (Becton Dickinson) for enumeration of cfu. Following each timepoint, the lungs were fixed in formalin, processed and sectioned for staining with haematoxylin and eosin.
Results
Simvastatin, alone and in combination with isoniazid, reduces bacterial burden in macrophages
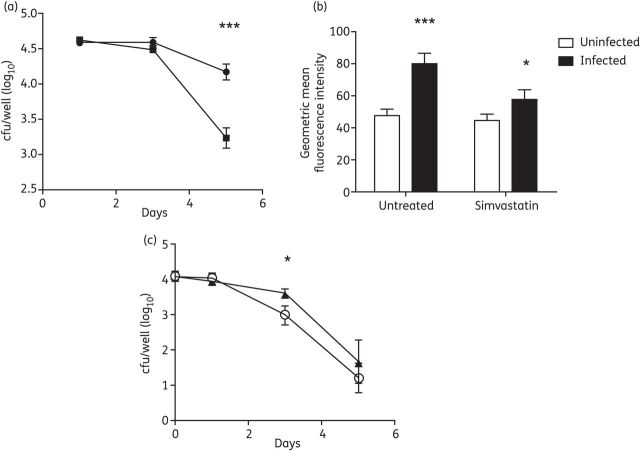
J774 macrophage-like cells were cultured in the absence or presence of simvastatin immediately following infection with M. tuberculosis CDC1551. Cells cultured in the presence of simvastatin had a significantly lower intracellular bacterial burden at Day 5 post-infection (Figure 1a; P < 0.01). Next, cells were harvested and analysed for their lipid content using Nile red-based flow cytometry (Figure 1b). M. tuberculosis infection of the cells resulted in a significant increase in cellular lipid levels (P < 0.01). Infected cells treated with simvastatin had a significantly lower lipid content than those treated with vehicle control 3 days after treatment (P = 0.02). Since lipid body accumulation in macrophages has been associated with a restriction of M. tuberculosis growth and phenotypic drug tolerance,11 we tested the ability of simvastatin to enhance the susceptibility of intracellular bacilli to isoniazid. Exposure of the cells to simvastatin significantly increased the killing activity of isoniazid at Day 3 after infection (P = 0.02); however, this effect was not statistically significant by Day 5 (Figure 1c).
Figure 1.
Simvastatin reduces lipid content and M. tuberculosis survival in macrophages. J774 macrophage-like cells were infected with M. tuberculosis CDC1551 at a multiplicity of infection of 10 : 1. (a) Effect of simvastatin (5 μM) (squares) or vehicle control (2% DMSO) (circles) on intracellular bacillary burden. (b) Effect of simvastatin on macrophage lipid content, as measured by Nile red staining on Day 3 after infection. (c) Effect of combined isoniazid (0.05 μg/mL) and simvastatin (5 μM) (open circles) versus isoniazid alone (0.05 μg/mL) (triangles) on intracellular bacillary burden. Data represent mean number of cfu per well (log10) ± SD (a and c) or geometric mean fluorescence intensity (b). Statistical comparison between groups was performed using a two-tailed Student's t-test. ***P < 0.01; *P = 0.02. All experiments were performed in triplicate.
To determine whether the differences observed in cfu number were due to direct antibacterial effects of the drug, the MIC of simvastatin for wild-type M. tuberculosis in 7H9 broth was assessed. No inhibitory activity was observed at concentrations of simvastatin up to 320 μM (64 times that used in intracellular assays). Furthermore, 320 μM simvastatin failed to lower the observed MIC of isoniazid for M. tuberculosis in nutrient-rich broth (data not shown).
Addition of simvastatin to standard TB treatment enhances bacterial killing
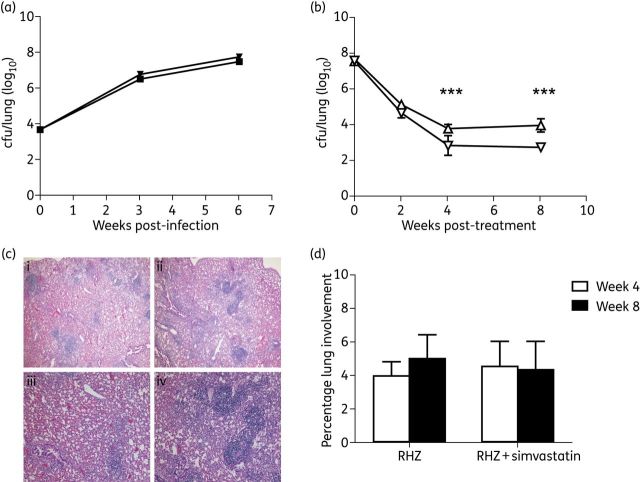
Next we studied the anti-TB activity of a combination regimen containing the first-line drugs and simvastatin against chronic TB infection in BALB/c mice. Mice were treated with either simvastatin or a vehicle control (2% DMSO in PBS) beginning 1 day post-infection for a total of 6 weeks. No difference in cfu was noted after 6 weeks of treatment (P = 0.16), suggesting that simvastatin alone lacks anti-TB activity during the acute stage of infection (Figure 2a).
Figure 2.
Simvastatin enhances the anti-TB activity of the first-line regimen in mice. (a) Simvastatin 25 mg/kg alone (squares) given on the day after aerosol infection does not alter the course of acute infection compared with vehicle control (inverted triangles). (b) In combination with rifampicin/pyrazinamide/isoniazid (RHZ; inverted triangles), simvastatin significantly reduces the lung bacillary burden compared with standard treatment alone (triangles). (c) Histology of animals receiving 8 weeks of RHZ (i and iii) and RHZ + simvastatin (ii and iv). Haematoxylin/eosin stain, ×40 (i and ii) and ×100 (iii and iv) magnification. (d) No significant differences were observed between groups in the percentage of lung with inflammatory involvement. Results are presented as mean cfu per lung (log10) ± SD (a and b) taken from five animals per group, per timepoint, or as the percentage of lung with inflammatory involvement (d). Statistical comparison between groups was performed using a two-tailed Student's t-test. ***P < 0.01. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
In a separate study, infection was allowed to progress for 6 weeks prior to the initiation of treatment with rifampicin/isoniazid/pyrazinamide or rifampicin/isoniazid/pyrazinamide + simvastatin (Figure 2b). After 4 (P = 0.007) and 8 (P < 0.001) weeks of treatment, the combination regimen containing simvastatin showed greater activity against bacilli in the lungs relative to rifampicin/isoniazid/pyrazinamide alone, suggesting that statins may enhance the activity of the first-line regimen. A morphometric analysis of lung histology revealed no discernible differences between the two groups (Figure 2c and d).
Discussion
Despite the availability of potent anti-TB drugs, TB remains one of the world's leading causes of death by infectious diseases. Current research efforts have focused on reducing the duration of curative treatment for TB. The repurposing of currently available drugs with anti-TB activity would accelerate the time to market. Host-directed therapies have recently received significant attention in the context of TB treatment.6,18 Here we show that the use of simvastatin reduces bacterial load in an in vitro macrophage model and enhances the effects of standard treatment in chronically infected BALB/c mice.
The lipid-loaded or foamy macrophage provides M. tuberculosis with a nutrient-rich intracellular environment of reduced bactericidal activity.19 The treatment of activated, infected murine macrophage-like cells with simvastatin significantly reduced bacterial load compared with vehicle control-treated cells (Figure 1a) and enhanced the bactericidal activity of isoniazid (Figure 1c). The addition of simvastatin to the standard regimen significantly lowered the lung bacillary burden of chronically infected mice at weeks 4 and 8 post-infection relative to rifampicin/pyrazinamide/isoniazid alone (Figure 2b). Since simvastatin appears to lack direct tuberculocidal activity, the drug appears to act through a modulation of the intracellular environment within the macrophages. Recent data show that statins prevent the M. tuberculosis-mediated inhibition of autophagy and phagosome maturation.14 In addition, the treatment of M. tuberculosis-infected macrophages with statins reduces the accumulation of lipid droplets, which provide M. tuberculosis with a nutrient-rich intracellular environment of reduced bactericidal activity.14,19 Contrary to the findings by Parihar et al.,14 we did not observe a statistically significant activity of statin monotherapy in M. tuberculosis-infected mice. This discrepancy may be attributable to our study design: we used BALB/c rather than C57BL/6 mice, and M. tuberculosis CDC1551 rather than H37Rv; treatment was initiated 1 day post-infection rather than 6 weeks before infection; and the drug was dosed orally at 25 mg/kg five times weekly rather than intraperitoneally at 20 mg/kg every other day. In particular, the oral administration of simvastatin in our study may partially explain the drug's lack of bacillary killing in the lungs of mice despite its tuberculocidal activity in macrophages, perhaps due to decreased bioavailability in the lung tissues.
Our data demonstrate that the addition of statins to standard anti-TB treatment may potentially improve treatment outcomes. Future studies will focus on identifying the ideal lipid-modulating agent to use as adjunctive therapy and on evaluating its role in shortening the duration of time to achieve a relapse-free state.
Funding
This work was supported by R01 HL106788 to M. L. G. and R01 AI083125 and R01 HL106786 to P. C. K.
Transparency declarations
None to declare.
References
- 1.van den Boogaard J, Kibiki GS, Kisanga ER, et al. New drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother. 2009;53:849–62. doi: 10.1128/AAC.00749-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner CA, Acharya T, Pablos-Méndez A. The global alliance for tuberculosis drug development—accomplishments and future directions. Clin Chest Med. 2005;26:341–7. doi: 10.1016/j.ccm.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien RJ, Nunn PP. The need for new drugs against tuberculosis. Am J Respir Crit Care Med. 2001;163:1055–8. doi: 10.1164/ajrccm.163.5.2007122. [DOI] [PubMed] [Google Scholar]
- 4.Skerry C, Harper J, Klunk M, et al. Adjunctive TNF inhibition with standard treatment enhances bacterial clearance in a murine model of necrotic TB granulomas. PLoS One. 2012;7:e39680. doi: 10.1371/journal.pone.0039680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo MS, Manca C, Yang G, et al. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One. 2011;6:e17091. doi: 10.1371/journal.pone.0017091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Cohen KA, Winglee K, et al. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:574–6. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tleyjeh IM, Kashour T, Hakim FA, et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1658–67. doi: 10.1001/archinternmed.2009.286. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt H, Hennen R, Keller A, et al. Association of statin therapy and increased survival in patients with multiple organ dysfunction syndrome. Intensive Care Med. 2006;32:1248–51. doi: 10.1007/s00134-006-0246-y. [DOI] [PubMed] [Google Scholar]
- 9.Catron DM, Lange Y, Borensztajn J, et al. Salmonella enterica serovar Typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infect Immun. 2004;72:1036–42. doi: 10.1128/IAI.72.2.1036-1042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erkkila L, Jauhiainen M, Laitinen K, et al. Effect of simvastatin, an established lipid-lowering drug, on pulmonary Chlamydia pneumoniae infection in mice. Antimicrob Agents Chemother. 2005;49:3959–62. doi: 10.1128/AAC.49.9.3959-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel J, Maamar H, Deb C, et al. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011;7:e1002093. doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan S, Dkhar HK, Chandra V, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol. 2012;188:5593–603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- 13.Singh V, Jamwal S, Jain R, et al. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe. 2012;12:669–81. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Parihar SP, Guler R, Khutlang R, et al. Statin therapy reduces the Mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209:754–63. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 15.Podechard N, Le Ferrec E, Rebillard A, et al. NPC1 repression contributes to lipid accumulation in human macrophages exposed to environmental aryl hydrocarbons. Cardiovasc Res. 2009;82:361–70. doi: 10.1093/cvr/cvp007. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad Z, Fraig MM, Pinn ML, et al. Effectiveness of tuberculosis chemotherapy correlates with resistance to Mycobacterium tuberculosis infection in animal models. J Antimicrob Chemother. 2011;66:1560–6. doi: 10.1093/jac/dkr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94:2489–94. doi: 10.1210/jc.2008-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbian S, Tsenova L, O'Brien P, et al. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog. 2011;7:e1002262. doi: 10.1371/journal.ppat.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peyron P, Vaubourgeix J, Poquet Y, et al. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]