Abstract
Sarcoidosis is a granulomatous disease, in which liver affection is common, contrary to a primary hepatic lymphoma that is very rarely seen. On MRI both present with almost the same imaging features: hypointense in T1-weighted and hyperintense in T2-weighted sequences. Our patient with a histologically confirmed sarcoidosis in the lungs showed liver lesions that were similar to sarcoidosis manifestations of the liver. Due to size, progression and overlapping features with secondary malignant liver lesions within an interval of 5 months, a biopsy was conducted and confirmed a primary hepatic lymphoma with diffuse large b-cells. Thus, we would recommend performing a biopsy in ambiguous lesions with indistinguishable characteristics and progression within a short follow-up interval.
Keywords: Hepatic MRI, primary hepatic lymphoma, sarcoidosis
Sarcoidosis is a systemic disorder of unknown etiology with non-caseating granulomas that can affect several organs (1). Hepatic manifestations are seen in 18% of the patients, which in most cases are asymptomatic and only manifest with elevated liver enzymes (1). Aside from lymph nodes, spleen, and bone marrow, the liver is in the context of lymphoma the most frequent affected organ indicating disseminated tumor stage. In contrast primary lymphoma of the liver is seen very rarely (2–5).
In imaging, liver sarcoidosis can display with minimal hepatomegaly and the merged granulomas can appear as multiple small nodules or sometimes confluent, rather sharp delineated lesions in the liver (6). These lesions are usually hypoattenuating in CT (6,7). The imaging features of hepatic sarcoidosis in MRI are described as lesions hypointense in T1-weighted (T1W) sequences and hyperintense in T2-weighted (T2W) sequences (6,8,9). However, larger current series do not exist, and there is no knowledge on the appearance of sarcoidosis with more recent magnetic resonance (MR) techniques such as diffusion-weighted imaging.
There are a few case reports available on which hepatic involvement in sarcoidosis has mimicked malignant disease. We present here the opposite case with a histologically confirmed non-Hodgkin’s lymphoma of the liver mimicking hepatic sarcoidosis on state-of-the-art MRI of the liver.
Case report
A 36-year-old man with diagnosed sarcoidosis was referred to our clinic for a reassessment of liver lesions, first seen on MRI 5 months ago. The sarcoidosis had been known for 3 years and the diagnosis was ensured by biopsy of the lung. The treatment with azathioprine and methotrexate had been stopped due to intolerance and switched to steroids, the actual immunosuppressive therapy. Due to idiopathic thrombocytopenic purpura a splenectomy had been performed 10 years ago.
On physical examination the liver was slightly enlarged and apart from that unremarkable. The laboratory show elevated calcium (3.76 mmol/L; normal range, 2.05–2.65 mmol/L), γ-glutamyl transpeptidase (218 U/L; normal range, <55 U/L), alkaline phosphatase (926 U/L; normal range, <135 U/L), LDH (256 U/L; normal range, <250 U/L), and creatinine (1.5 mg/dl; normal range, 0.5–1.2 mg/dl). The blood count showed reactive thrombocytosis (596 G/L; normal range, 150–440 G/L), lymphocytosis (48%; normal range, 25–40%), leukocytosis (17.2 G/L; normal range, 4.0–11.0 G/L), and a slight anemia with low hemoglobin (12.3 g/dl; normal range, 14.0–18.0 g/dl). The angiotensin-converting enzyme concentration was not measured on hospital admission.
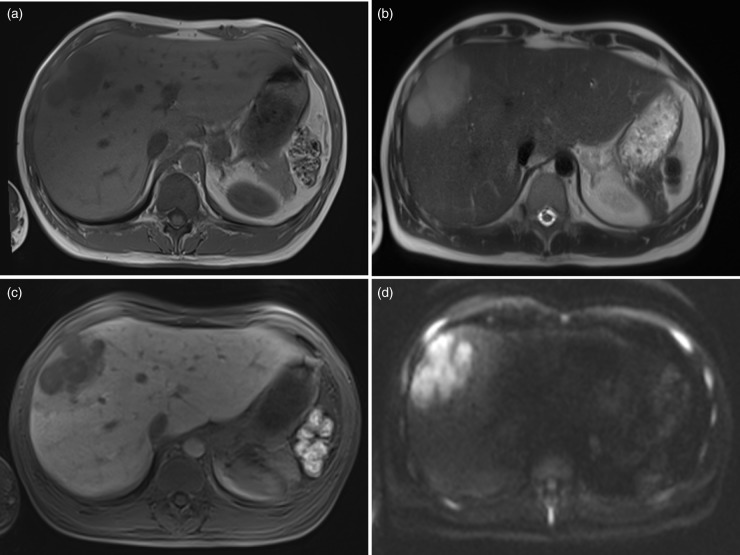
A MRI of the liver was performed on a 1.5 Tesla Magnetom Avanto (Siemens AG, Erlangen, Germany) with the hepatobiliary contrast agent Gd-EOB-DTPA (Primovist, Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) and including diffusion-weighted sequences (Fig. 1). In liver segment 8 two confluent lesions with a geographic pattern were seen with lesion diameters of 2.9 cm and 7.4 cm, respectively, which presented in plain MRI as hypointense in T1W and slightly hyperintense in T2W sequences. After bolus injection of 8 mL Gd-EOB-DTPA the lesions displayed as a hypovascular lesions with only moderate contrast agent uptake in the porto-venous phase. In the hepatocyte phase no liver-specific uptake was seen in the lesion. The lesion showed restricted diffusion in the DWI sequence with an ADC value of 0.85 mm2/s. Compared to the print-outs of the initial MR examination done in another hospital 5 months ago the lesions were growing in size. Pronounced lymph nodes with a maximal size of 1.4 cm in short diameter were detected in the retroperitoneum. The mesenteric root and the liver hilum did not show enlarged lymph nodes. The spleen could not be assessed due to previous splenectomy.
Fig. 1.
MR images showing the confluent lesion in liver segment 8 as a hypointense lesion in a plain T1W 2D GRE sequence (a) with slightly hyperintensity in the T2W single shot sequence (b). In the hepatocyte phase 20 min after injection of Gd-EOB-DTPA, no liver-specific uptake is seen in the lesion (c). In the single-shot EPI sequence for DWI (d) the lesion remained with high signal intensity in the b = 800 s/mm2 images with an ADC value of 0.85 mm2/s, indicating restricted diffusion.
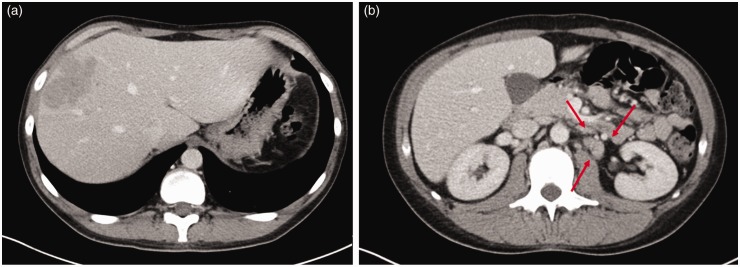
Although the lesions did not present like typical malignant liver masses, mainly because of the shape, the increase in size was suspicious, so that a CT-guided biopsy was performed in due course. For biopsy planning, a liver CT was performed including an arterial and portovenous phase (Fig. 2). The biopsy was made subsequently under CT-fluoroscopy with a 16 G needle and a TruCut biopsy system (Magnum Reusable Core Biopsy System, Bard Biopsy Systems, Tempe, AZ, US) (3). The CT showed similarly to the MRI a hypovascular rather confluent, sharply delineated lesion. The histology examination revealed a diffuse large b-cell non-Hodgkin’s lymphoma. Due to that finding a bone marrow biopsy was added and revealed a bone marrow infiltration by lymphoma. Re-evaluation of the CT and MRI images showed no other manifestation of lymphoma in thorax or abdomen beside the mentioned liver lesions and the enlarged retroperitoneal lymph nodes. The sarcoidosis of the lung appeared well-controlled at that point under long-term steroids. After diagnosis of a primary hepatic diffuse large b-cell lymphoma stage IVA, the patient started immuno-chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and steroids (R-CHOP protocol).
Fig. 2.
CT in portovenous phase (a) showing the same lesion as displayed in Figure 1 with an interval of 4 days. Similarly to the MR findings the lesion presented with hypovascularization. Adjacent to the aorta the CT scan shows enlarged lymph nodes (indicated by arrows) up to a short diameter of 1.4 cm (b).
Discussion
The term sarcoidosis-lymphoma syndrome was first coined by Brincker (10). This has been controversially discussed. Brinker suggested that sarcoidosis is preceding malignant lympho-proliferative diseases. In contrast to that, several cases on patients with a newly diagnosed sarcoidosis following lymphoma have been reported (11–13). Diffuse large b-cell lymphoma represents the most common aggressive lymphoma and makes up 80% of all aggressive lymphomas and 25 % of all non-Hodgkin’s lymphomas. Lymph nodes, spleen, bone marrow, and extranodal manifestations including the liver are the most common manifestations. In contrast the liver as the first manifestation of lymphoma, known as primary hepatic lymphoma, is very rare and makes up only 0.016% of all non-Hodgkin’s lymphoma (14). Only a few hundred cases are reported worldwide. There are currently no data about the frequency of lymphoma entities in primary hepatic lymphoma.
In patients with hepatic sarcoidosis very often malignancy is suspected and liver biopsy serves to confirm the diagnosis of sarcoidosis (8). In our case the situation was rather the opposite. The presented imaging features of a confluent lesion being hypointense in T1W and hyperintense in T2W sequences with hypovascularization are well in line with hepatic sarcoidosis. Liver metastases from various primary tumor sites usually present as round focal liver lesions with an unsharp margin and rim like enhancement, and primary liver tumors very often present with hypervascularization in the arterial phase. These features were not seen in our patient. Reports about imaging of hepatic sarcoidosis with DWI or liver-specific contrast agents are not available, but it seemed reasonable that also sarcoidosis would not show uptake of a hepatobiliary contrast agent and that also sarcoidosis might show a restricted diffusion. So the evaluation of image data in our patient without knowledge of previous imaging might have resulted in the wrong assumption of hepatic sarcoidosis. However, size progression and the in general overlapping features to secondary malignant liver lesions (restricted diffusion, no hepato-biliary uptake, hypovascular appearance) prompted us to go for liver biopsy of one of the lesions, which then turned out to be a lymphoma. Hepatic non-Hodgkin’s lymphoma often appears with multiple lesions hypointense on T1W images and hyperintense on T2W images (15). Spleen involvement in sarcoidosis is seen more often than liver involvement and occurs in 50–60% with lager nodules. Retroperitoneal lymphadenopathy in sarcoidosis is rather rare – as opposed to lymphoma (6).
The patient presented with stage IV diffuse large b-cell lymphoma (multiple liver lesions, bone marrow infiltration, and retroperitoneal lymphadenopathy) and an age-adjusted international prognostic index (aaIPI) of 2 (high–intermediate risk). Long-term immunosuppression including steroids due to sarcoidosis therapy might have promoted the development of lymphoma. The patient received immuno-chemotherapy after the R-CHOP protocol (repeated every 15 days) under support with hematopoetic growth factor. According to the aaIPI the 5-year relapse-free survival is 53% with an overall survival rate of 46% (16).
The diagnosis of lymphoma was postponed in our patient due to the initial decision to watch and wait which seemed to be justified at that time. But with the general strategy to validate hepatic sarcoidosis with biopsy the diagnosis of lymphoma could have been made already 5 months earlier. Our decision to biopsy was influenced by the increase in size, the signal intensities in MRI, the overall extent and the numbers of enlarged lymph nodes. Nevertheless, we did not expect an infiltration but rather supposed a progression of hepatic sarcoidosis with a high disease activity, so that the final biopsy result was a surprise.
In conclusion primary hepatic lymphoma can have imaging features that resemble sarcoidosis of the liver. We would recommend doing a biopsy of liver lesions with unclear features and/or rapid size progression.
References
- 1.Dempsey OJ, Paterson EW, Kerr KM, et al. Sarcoidosis. BMJ 2009; 339: b3206–b3206 [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Dorfman RF, Rosenberg SA. Pathology of malignant lymphomas in the liver: application in staging. Prog Liver Dis 1976; 5: 683–698 [PubMed] [Google Scholar]
- 3.Chabner BA, Johnson RE, Chretien PB, et al. Percutaneous liver biopsy, peritoneoscopy and laparotomy: an assessment of relative merits in the lymphomata. Br J Cancer 1975; 2(Suppl): 242–247 [PMC free article] [PubMed] [Google Scholar]
- 4.Schweiger F, Shinder R, Rubin S. Primary lymphoma of the liver: a case report and review. Can J Gastroenterol 2000; 14: 955–957 [DOI] [PubMed] [Google Scholar]
- 5.Jaffe ES. Malignant lymphomas: pathology of hepatic involvement. Semin Liver Dis 1987; 7: 257–268 [DOI] [PubMed] [Google Scholar]
- 6.Koyama T, Ueda H, Togashi K, et al. Radiologic manifestations of sarcoidosis in various organs. Radiographics 2004; 24: 87–104 [DOI] [PubMed] [Google Scholar]
- 7.Warshauer DM, Molina PL, Hamman SM, et al. Nodular sarcoidosis of the liver and spleen: analysis of 32 cases. Radiology 1995; 195: 757–762 [DOI] [PubMed] [Google Scholar]
- 8.Jung G, Brill N, Poll LW, et al. MRI of hepatic sarcoidosis: large confluent lesions mimicking malignancy. AJR Am J Roentgenol 2004; 183: 171–173 [DOI] [PubMed] [Google Scholar]
- 9.Warshauer DM, Semelka RC, Ascher SM. Nodular sarcoidosis of the liver and spleen: appearance on MR images. J Magn Reson Imaging 1994; 4: 553–557 [DOI] [PubMed] [Google Scholar]
- 10.Brincker H. The sarcoidosis-lymphoma syndrome. Br J Cancer 1986; 54: 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami T, Siddique S, Cohen P, et al. The sarcoid-lymphoma syndrome. Clin Lymphoma Myeloma Leuk 2010; 10: 241–247 [DOI] [PubMed] [Google Scholar]
- 12.Kornacker M, Kraemer A, Leo E, et al. Occurrence of sarcoidosis subsequent to chemotherapy for non-Hodgkin's lymphoma: report of two cases. Ann Hematol 2002; 81: 103–105 [DOI] [PubMed] [Google Scholar]
- 13.Suen JS, Forse MS, Hyland RH, et al. The malignancy-sarcoidosis syndrome. Chest 1990; 98: 1300–1302 [DOI] [PubMed] [Google Scholar]
- 14.Noronha V, Shafi NQ, Obando JA, et al. Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol Hematol 2005; 53: 199–207 [DOI] [PubMed] [Google Scholar]
- 15.Rizzi EB, Schinina V, Cristofaro M, et al. Non-hodgkin's lymphoma of the liver in patients with AIDS: sonographic, CT, and MRI findings. J Clin Ultrasound 2001; 29: 125–129 [DOI] [PubMed] [Google Scholar]
- 16. Sweetenham JW. Diffuse large B-cell lymphoma: risk stratification and management of relapsed disease. Hematology Am Soc Hematol Educ Program 2005:252–259. [DOI] [PubMed]