Abstract
We experienced a case with a gastric varix that did not have a catheterizable main drainage vein and had multiple afferent veins. For this case we successfully performed percutaneous transhepatic sclerotherapy using the following procedure. After the drainage vein was embolized by metallic coils and n-butyl cyanoacrylate from a microcatheter that was advanced through the gastric varix, 5% ethanolamine oleate-iopamidol was infused into the gastric varix from one main afferent vein under balloon occlusion.
Keywords: Gastric varix, percutaneous transhepatic sclerotherapy, percutaneous transhepatic obliteration
Introduction
Balloon-occluded retrograde transvenous obliteration (B-RTO) has been widely accepted as a treatment of gastric varices (1) since its safety and effectiveness were reported by Kanagawa et al. (2). However, occasionally we encounter a gastric varix without a catheterizable main drainage vein (3), making B-RTO impossible. Percutaneous transhepatic sclerotherapy (PTS) has been recommended as a good option in such cases (4,5). PTS is usually performed by placing metallic coils in the afferent veins to reduce blood flow into the gastric varix, after which a sclerosing agent is injected in the antegrade direction into the gastric varix (4,5). When there are multiple afferent veins, this procedure sometimes must be performed for all such veins.
Recently, we experienced a case of a gastric varix without a catheterizable main drainage vein and with multiple afferent veins. For this case we successfully performed PTS with obliteration of the drainage vein through the gastric varix.
Case report
The institutional review board at our institution does not require approval for this type of case report.
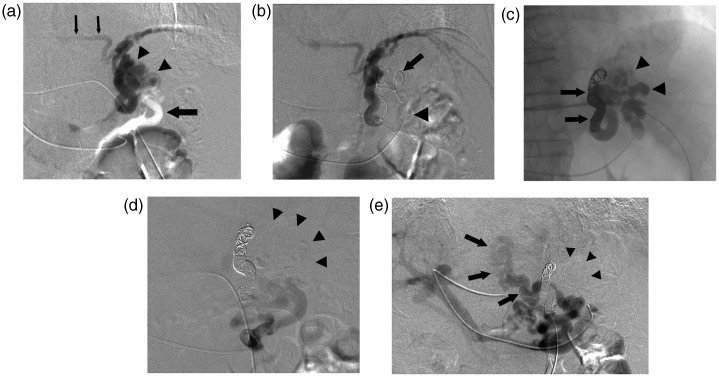
A 76-year-old man with Child’s class A liver cirrhosis caused by hepatitis C virus infection was admitted for treatment of a large gastric varix for prevention of rupture. Endoscopic examination revealed a bead-shaped moderate varix without the red color sign located at the gastric cardia and fornix. Contrast-enhanced multi-detector row computed tomography (MDCT) confirmed that the gastric varix was located in the cardia and fornix of the stomach; however, neither a gastrorenal shunt nor another drainage vein could be demonstrated. Then, computed tomography during arterial portography (CTAP) via the splenic artery was performed. The CTAP revealed that the gastric varix was mainly fed from the posterior gastric vein and partially fed from the short gastric vein and drained into the left inferior phrenic vein. Moreover, a large and tortuous paraesophageal vein, without connection with the gastric varix that was fed from the other posterior gastric vein and the left gastric vein draining into the azygos venous system, was revealed. Initially we planned a B-RTO approach from the left inferior phrenic vein. However, the segment of the left inferior phrenic vein entering into the inferior vena cava was too narrow to be catheterized so we decided to undertake PTS. After written informed consent was obtained from the patient, PTS was performed. Using the percutaneous transhepatic approach, a 5-Fr catheter was inserted into the portal vein system. Splenic venography and selective posterior gastric venography revealed that the gastric varix was fed from one posterior gastric vein and drained into the left inferior phrenic vein (Fig. 1a) and that the paraesophageal vein was fed from the other posterior gastric vein and the left gastric vein. The short gastric vein feeding into the gastric varix was not detected on venography. As the initial step of the procedure, a 5-Fr balloon catheter with a diameter of 11 mm (Moiyan, Miyano Medical Instruments, Hyogo, Japan) was inserted into the posterior gastric vein and advanced to a position near the gastric varix. Balloon occluded venography of the posterior gastric vein revealed the entire gastric varix and drainage vein with relatively good stagnation of the contrast material. Next, 7 mL of 5% ethanolamine oleate-iopamidol (EOI) (mixture of equal amounts of 10% EO and 370 mgI/mL iopamidol) was continuously administered from the balloon catheter. However, the 5% EOI was not distributed in the entire gastric varix and did not sufficiently stagnate. Then a microcatheter (Renegade, Boston Scientific, Natick, MA, USA) was advanced over a cobra-shaped micro-guide wire (GT, Termo, Tokyo, Japan) through the gastric varix into the drainage vein via the balloon catheter positioned in the posterior gastric vein (Fig. 1b). The drainage vein was embolized completely using four detachable coils (GDC; Boston Scientific), nine microcoils, and 0.75 mL of n-butyl cyanoacrylate (NBCA) (Histoacryl-Blue; Braun, Melsungen, Germany) mixed with Lipiodol (Laboratoire Guerbet, Roissy, France). The NBCA-to-Lipiodol ratio was 1:1.5. Balloon occluded venography of the posterior gastric vein after embolization of the drainage vein showed the entire gastric varix with good stagnation of the contrast material. Then 5% EOI was delivered to the gastric varix from the balloon catheter in the posterior gastric vein (Fig. 1c). Over a 50-min period, 23 mL of 5% EOI was administered by four injections. Cone beam CT image obtained after the injections revealed that the entire gastric varix was filled with the sclerosing agent. Fluoroscopy 70 min after the injections showed that the 5% EOI remained in the gastric varix. Finally, the balloon catheter was withdrawn. During these procedures, 4000 units of human haptoglobin were administered intravenously to prevent renal failure owing to hemolysis. Splenic venography and selective posterior gastric venography after PTS revealed that the gastric varix had disappeared and only the flow into the paraesophageal vein remained (Fig. 1d and e). The 5-Fr sheath and 5-Fr catheter were withdrawn after positioning a coil in the needle tract in the liver parenchyma.
Fig. 1.
A 76-year-old man with a gastric varix locating at the gastric cardia and fornix. (a) Posterior gastric venography revealed that the gastric varix (arrowheads) was fed from one posterior gastric vein (large arrow) and drained into the left inferior phrenic vein (small arrows). (b) The microcatheter was advanced into the drainage vein (arrow) through the gastric varix via the balloon catheter (arrowhead) in the posterior gastric vein. (c) Roentgenogram obtained after embolization of the drainage vein with coils and NBCA (arrows) and injection of 23 mL of 5% EOI showed complete filling of sclerotic agents in the gastric varix (arrowheads). (d) Posterior gastic venography after PTS revealed disappearance of the gastric varix (arrowheads). (e) Splenic venography after PTS revealed that the gastric varix disappeared (arrowheads) and only the flow into the paraesophageal vein without connection with the gastric varix, which was fed from the other posterior gastric vein and the left gastric vein and drained into the azygos venous system (arrows), was evident.
Complete disappearance of enhancement in the gastric varix was confirmed on the contrast-enhanced MDCT scan obtained 1 week after the procedure, and disappearance of the varix was confirmed on endoscopic examination performed 4 months later. No complications were encountered during the procedure. Hepatic function, renal function, and blood cell counts were not significantly changed after the procedure.
Discussion
Percutaneous transhepatic obliteration (PTO) has been used for treatment of ruptured varices. In classic PTO, embolic materials are introduced into the afferent gastric vein. Although classic PTO may result in initial hemostasis, a high rate of recurrence or rebleeding occurs (6). More recently, the effectiveness of PTS, which is a modification of the classic PTO, to treat gastric varices without a catheterizable main drainage vein has been reported (4,5,7–10). PTS, in which metallic coils are placed in the afferent veins after which a sclerosing agent is infused from the afferent veins, can embolize gastric varices more selectively than classic PTO. However, Kameda et al. reported that the rate of early recurrence within 6 months after PTS was higher than after B-RTO (40.0% vs. 16.7%, respectively) (5). They speculated that this might be caused by insufficient injection of the sclerosing agent into the gastric varix and drainage vein. Usually, in the PTS procedure, metallic coils are used to reduce blood flow into the gastric varix. On the other hand, Hirota et al. reported that instead of using coils in PTS, a balloon catheter could be placed into the afferent vein via the percutaneous transhepatic route and the sclerosing agent (5% EOI) infused under balloon occlusion (10). Other reports of PTS described coil placement in the drainage vein through the gastric varix as well as the afferent veins to reduce blood flow before injection of the sclerosing agent (8,9).
In our case, the gastric varix was fed by two afferent veins; however, one was too small to be identified during the PTS procedure. Although the sclerosing agent injected from the main afferent vein under balloon occlusion distributed in the gastric varix, it did not stagnate sufficiently to obliterate the varix. We suspected that inflow from the other afferent vein might have diluted the sclerosing agent. Blood flow was almost completely stopped due to complete occlusion of the drainage vein close to the varix with coils and NBCA and complete occlusion of the main afferent vein with the balloon catheter. This allowed the sclerosing agent to be distributed into the entire varix and to stagnate sufficiently.
In conclusion, we consider that PTS with embolization of the drainage vein could be effective if the catheter could be advanced into the drainage vein through the gastric varix, especially for cases in which some of the afferent veins cannot be catheterized.
Conflict of interest
None declared.
References
- 1.Hirota S, Matsumoto S, Tomita M, et al. Retrograde transvenous obliteration of gastric varices. Radiology 1999; 211: 349–356. [DOI] [PubMed] [Google Scholar]
- 2.Kanagawa H, Mima S, Kouyama H, et al. Treatment of gastric fundal varices by Balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 1996; 11: 51–58. [DOI] [PubMed] [Google Scholar]
- 3.Maeda H, Hirota S, Yamamoto S, et al. Radiologic variation in gastrorenal shunts and collateral veins from gastric varices in images obtained before Balloon-occluded retrograde transvenous obliteration. Cardiovasc Intervent Radiol 2007; 30: 410–414. [DOI] [PubMed] [Google Scholar]
- 4.Ninoi T, Nakamura K, Kaminou T, et al. TIPS versus transcatheter sclerotherapy for gastric varices. Am J Roentgenol 2004; 183: 369–376. [DOI] [PubMed] [Google Scholar]
- 5.Kameda N, Higuchi K, Shiba M, et al. Management of gastric fundal varices without gastro-renal shunt in 15 patients. World J Gastroenterol 2008; 14: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith-Laing G, Scott J, Long RG, et al. Role of percutaneous transhepatic obliteration of varices in the management of hemorrhage from gastroesophageal varices. Gastroenterology 1981; 80: 1031–1036. [PubMed] [Google Scholar]
- 7.Chikamori F, Kuniyoshi N, Kagiyama S, et al. Role of percutaneous transhepatic obliteration for special types of varices with portal hypertension. Abdom Imaging 2007; 32: 92–95. [DOI] [PubMed] [Google Scholar]
- 8.Kiyosue H, Matsumoto S, Yamada Y, et al. Transportal intravariceal sclerotherapy with N-butyl-2-cyanoacrylate for gastric varices. J Vasc Interv Radiol 2004; 15: 505–509. [DOI] [PubMed] [Google Scholar]
- 9.Kwak HS, Han YM. Percutaneous transportal sclerotherapy with N-butyl-2-cyanoacrylate for gastric varices: technique and clinical efficacy. Korean J Radiol 2008; 9: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirota S, Ichikawa S, Matsumoto S, et al. Interventional radiologic treatment for idiopatic portal hypertension. Cardiovasc Intervent Radiol 1999; 22: 311–314. [DOI] [PubMed] [Google Scholar]