We studied a cohort of children initiated on treatment doses of combination antiretroviral therapy within 72 hours of birth. In children who achieved sustained virologic suppression, measures of HIV-1 reservoir size in peripheral blood were very low.
Keywords: child, combination antiretroviral therapy, eradication, HIV, proviral DNA
Abstract
Background. A human immunodeficiency virus type 1 (HIV-1)–infected infant started on combination antiretroviral therapy (cART) at 30 hours of life was recently reported to have no detectable plasma viremia after discontinuing cART. The current study investigated the impact of early cART initiation on measures of HIV-1 reservoir size in HIV-1–infected children with sustained virologic suppression.
Methods. Children born to HIV-1–infected mothers and started on cART within 72 hours of birth at 3 Canadian centers were assessed. HIV serology, HIV-1–specific cell-mediated immune responses, plasma viremia, cell-associated HIV-1 DNA and RNA, presence of replication-competent HIV-1, and HLA genotype were determined for HIV-1–infected children with sustained virologic suppression.
Results. Of 136 cART-treated children, 12 were vertically infected (8.8%). In the 4 who achieved sustained virologic suppression, HIV serology, HIV-1–specific cell-mediated immune responses (Gag, Nef), and ultrasensitive viral load were negative. HIV-1 DNA was not detected in enriched CD4+ T cells of the 4 children (<2.6 copies/106 CD4+ T cells), whereas HIV-1 RNA was detected (19.5–130 copies/1.5 µg RNA). No virion-associated HIV-1 RNA was detected following mitogenic stimulation of peripheral blood CD4+ T cells (5.4–8.0 million CD4+ T cells) in these 4 children, but replication competent virus was detected by quantitative co-culture involving a higher number of cells in 1 of 2 children tested (0.1 infectious units/106 CD4+ T cells).
Conclusions. In perinatally HIV-1–infected newborns, initiation of cART within 72 hours of birth may significantly reduce the size of the HIV-1 reservoirs. Cessation of cART may be necessary to determine whether functional HIV cure can be achieved in such children.
A human immunodeficiency virus type 1 (HIV-1)–infected infant (the “Mississippi baby”) with no detectable HIV-1 viremia after treatment cessation and in whom triple combination antiretroviral therapy (cART) had been started at 30 hours of life was recently reported by Persaud et al [1]. Although long-term follow-up will be needed to ascertain whether HIV-1 has been eradicated in this child, this case raises the possibility that very early initiation of cART could prevent establishment of HIV-1 reservoirs or limit reservoir size sufficiently to allow long-term virologic suppression after cessation of therapy. Long-term virologic suppression after interruption of cART has previously been observed in a subpopulation of adults who commenced treatment early during primary HIV-1 infection [2]. In addition to early treatment, host factors, such as human leukocyte antigen (HLA) genotype, may also impact reservoir establishment and disease progression [3].
In our 3 pediatric tertiary care institutions, cART at treatment doses has been used routinely as perinatal HIV-1 postexposure prophylaxis for many years in high-risk situations. This approach is used for infants born to HIV-1–infected mothers with incomplete virologic suppression at delivery or, in the absence of viral load results, if their mothers were nonadherent to antiretroviral medications. Neonatal therapy is initiated as soon as possible after birth and no later than 72 hours of life. Children proven to be HIV-1 infected using standard molecular diagnostic methods [4] are then continued on cART. In this report we describe a cohort of HIV-1–exposed infants who were initiated on cART within 72 hours of life, the vertical transmission rate in this context, and virologic and immunologic findings in a subgroup of infants who achieved sustained virologic suppression.
METHODS
HIV-1–exposed children were eligible for this study if they were started on treatment doses of cART within 72 hours of birth because of incomplete maternal virologic suppression at delivery or, in the absence of maternal viral load results, a maternal history of incomplete adherence or nonadherence to antiretroviral therapy. Subjects were identified from the clinical databases of the 3 participating pediatric HIV care institutions: The Hospital for Sick Children, Toronto; Children's Hospital of Eastern Ontario, Ottawa; and Centre Hospitalier Universitaire Sainte-Justine, Montreal. The study was approved by the research ethics board at each institution. Clinical and demographic data on the mothers and infants were collected retrospectively. Children confirmed to be infected with HIV-1 according to standard criteria [4] and who achieved sustained virologic suppression with cART were approached for study participation; after informed consent was obtained, prospective testing for evidence of residual HIV-1 was undertaken. Sustained virologic suppression was defined by the absence of any detectable virus in standard viral load assays subsequent to achieving an undetectable viral load (<50 copies/mL) for the first time.
HIV serologic testing was performed by standard enzyme-linked immunosorbent assay (ELISA) and Western blot assays. Plasma viremia was determined using the VERSANT HIV-1 3.0 assay (branched DNA) (Bayer Corporation, Berkeley, California; detection limit 50 copies/mL) prior to October 2010 and afterward using the Abbott RealTime HIV-1 assay (Abbott Laboratories, Abbott Park, Illinois; detection limit 40 copies/mL). Residual plasma viremia (<50 copies/mL) was determined by a modified Cobas AmpliPrep/Cobas TaqMan HIV-1 assay as previously described [5]. In brief, to determine the limit of detection of the assay, HIV-1 RNA from HIV-1 Virology Quality Assurance Laboratory RNA Quantification Standard was serially diluted in plasma from HIV-seronegative individuals. These samples were subjected to COBAS AmpliPrep/COBAS TaqMan HIV-1 Test Version 2.0 (Roche Diagnostics) reactions in 18 replicates. The limit of detection was 1.5 copies of HIV-1 RNA per milliliter of plasma. Clinical specimens were subjected to COBAS AmpliPrep/COBAS TaqMan HIV-1 Test version 2.0 (Roche Diagnostics) in quadruplicate (4 × 1 mL plasma).
The level of cell-associated HIV-1 DNA in CD4+ T cells was determined by real-time polymerase chain reaction (PCR) as previously described [5]. To determine the level of cell-associated HIV-1 RNA, total RNA was isolated from highly enriched CD4+ T cells using RNeasy Mini Kit (Qiagen) followed by DNase treatment (Qiagen). Then, 1.5 µg of total RNA was subjected to the Cobas AmpliPrep/Cobas TaqMan HIV-1 assay in duplicate. The assay system contains an internal control (HIV-1 Quantitation Standard Armored RNA), which was added to every specimen to compensate for effects of inhibition and to control the efficiency of target detection.
To assess for the presence of cells carrying replication-competent virus, purified CD4+ T cells were stimulated by mitogen (prostratin analogue) for 3 days and the level of virion-associated HIV-1 RNA in the culture supernatant was quantitated [6]. The supernatant was harvested 3 days following mitogenic stimulation to test for virion-associated HIV-1 RNA due to the timing of maximum CD4+ T-cell stimulation and cytopathic effects and the associated impact of dying cells. In the 2 older children, the presence of replication-competent virus was also tested by standard quantitative co-culture of peripheral blood CD4+ T cells as previously described [7]. To date, quantitiative co-culture has been deferred for the 2 younger children due to blood volume constraints for research.
HIV-specific T-cell responses to Gag and Nef, measured by enzyme-linked immunospot (ELISpot) assay, were performed according to the manufacturer's protocol (Mabtech AB, Sweden) as previously described [8]. Then, 1 µg/mL of each peptide antigen (clade B consensus HIV-1 Gag and/or Nef or CMV-pp65 [National Institutes of Health AIDS reagent program]) was added to appropriate wells. One microgram per milliliter of staphylococcal enterotoxin B was used as a positive control, whereas 0.8% dimethyl sulfoxide (DMSO), the highest DMSO concentration used in peptide stimulations, served as a negative control.
CCR5-delta 32 status and HLA genotypes were determined using PCR and DNA sequencing as previously described [9, 10]. The inferred amino acid sequence of the HLA-B region was examined, and substitutions associated with control of virologic set-point were identified [11].
RESULTS
Over a 10- to 15-year period (varied by institution), 136 newborn infants of HIV-1–infected mothers who had detectable viral load and/or poor adherence to therapy prior to delivery received cART beginning within 72 hours of birth. Twelve of 136 (8.8%) were HIV-1 infected based on detection of HIV-1 DNA or RNA in 2 or more separately timed blood samples. Six of the 12 (50%) had HIV-1 detected by PCR within 48 hours of birth, suggesting in utero infection according to diagnostic standards. For the remaining 6, the timing of infection could not be ascertained because initial testing took place after 48 hours of life. Of the 12 infected children, 4 achieved sustained virologic suppression. Of the remaining 8 children, 6 never achieved consistent virologic suppression due to poor adherence from the time of cART initiation. Two achieved and maintained an undetectable viral load for the first 2–3 years of life, but subsequently experienced virologic rebound as a result of poor adherence.
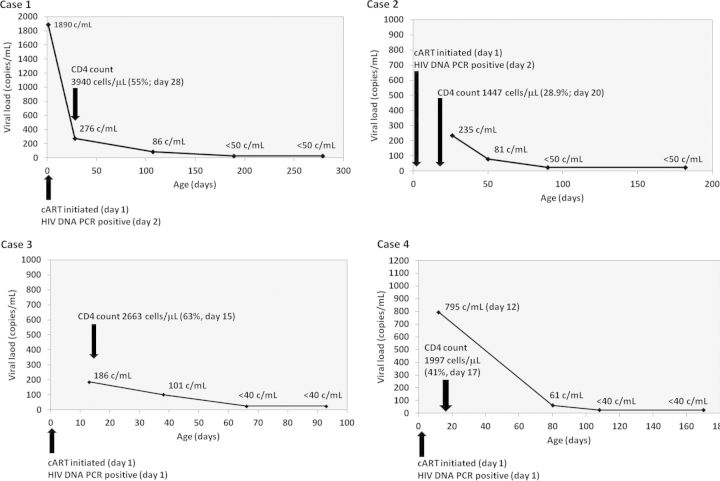
Maternal and infant clinical data as well as HIV-1 diagnostic testing results for the 4 children with sustained virologic suppression are shown in Table 1. All 4 were started on cART consisting of zidovudine, lamivudine, and nevirapine at treatment doses during the first 24 hours of life. HIV-1 PCR was positive within 48 hours of birth in all 4. Only 1 infant (case 1) had viral load testing performed on the first day of life; the remaining 3 had viral load testing after confirmation of a positive birth HIV-1 PCR result. Virologic suppression was first documented between 66 and 189 days of life (median, 99 days; Figure 1). All 4 infants have remained on the same cART regimen, are clinically well, and have normal CD4+ T-cell counts.
Table 1.
Maternal Characteristics and Confirmatory HIV-1 Testing in 4 Infants
| Characteristic | Case 1 | Case 2 | Case 3a | Case 4a |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age | 30 y | 32 y | 29 y | 29 y |
| Viral load prior to deliveryb | 97 701 c/mL | Unknownc | 6326 c/mL | 6326 c/mL |
| CD4+ T-cell count prior to deliveryb | 190 cells/µL | 10 cells/µL | 61 cells/µL | 61 cells/µL |
| Clade | G (CRF 6) | Unknown | C | C |
| Infant characteristics | ||||
| Mode of delivery | Emergency cesarean | Spontaneous | Spontaneous | Spontaneous |
| Gestational age at birth | 34 wk plus 4 d | 27 wk | 36 wk | 36 wk |
| Birth weight | 2980 g | 1070 g | 2270 g | 1640 g |
| HIV PCR (age) | Positive (1 d) | Positive (2 d) | Positive (1 d) | Positive (1 d) |
| CD4+ T cell count at 4 wk | 3940 cells/µL (55.2%) | 1447 cells/µL (28.9%) | 2227 cells/µL (50.9%) | 1690 cells/µL (48.7%) |
| HIV viral load (age) | 1890 c/mL (1 d), 276 c/mL (28 d), 86 c/mL (107 d) |
235 c/mL (26 d), 81 c/mL (50 d) |
186 c/mL (13 d), 101 c/mL (38 d) |
795 c/mL (12 d), 61 c/mL (80 d) |
| Age at which sustained VL <50 c/mL was achieved | 189 d | 90 d | 66 d | 108 d |
Abbreviations: c/mL, copies/mL; CRF, circulating recombinant form; HIV, human immunodeficiency virus; PCR, polymerase chain reaction; VL, viral load.
a Nonidentical twins.
b Timing in relation to delivery (weeks prior): case 1, 3 weeks prior; case 2, onset of labor; case 3, within 24 hours of delivery; case 4, within 24 hours of delivery.
c Presented with catastrophic cerebral event (suspected cerebral toxoplasmosis); died soon after delivery.
Figure 1.
Viral load kinetics for the 4 children with sustained virologic suppression. All 4 children had positive human immunodeficiency virus (HIV) polymerase chain reaction (PCR) within 48 hours of birth and a minimum of 2 subsequent detectable viral load assay results. Only case 1 had a viral load test performed on day 1 of life; the other 3 had viral load testing performed only after receiving the birth HIV PCR result. A biphasic viral load decline is clearly evident in case 1. In the other 3 cases, results are consistent with the second-phase decline of viral load. Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
Additional evaluation of the 4 children was performed at 2.5–7.5 years of age (Table 2). In all 4 cases, HIV serology by both ELISA and Western blot was negative. All 4 had undetectable plasma viremia by ultrasensitive assay (limit of detection, 1.5 copies/mL). No detectable cell-associated HIV-1 DNA (<2.6 copies/106 CD4+ T cells) was demonstrated in the peripheral blood of any of the children, whereas low levels of cell-associated HIV-1 RNA (19.5–130 copies/1.5 µg RNA) were detected in all 4. No virion-associated HIV-1 RNA was detected in the children's CD4+ T cells following mitogenic stimulation (5.4–8.0 × 106 CD4+ T cells). Two of the children had quantitative co-culture performed with a slightly higher number of CD4+ T cells compared with the number of cells used for the detection of virion-associated HIV RNA (10 × 106 for both vs 5.4 × 106 and 7.2 × 106 for cases 1 and 2, respectively). In 1 of these 2 children (case 2), the presence of replication-competent virus was demonstrated by quantitative co-culture at a level of 0.1 infectious units per 106 CD4+ T cells. The negative quantitative co-culture in the second child indicates that if infectious virus was present, it was at a level of <0.1 infectious units per 106 CD4+ T cells.
Table 2.
Genetic Typing and Tests of HIV-1 Persistence
| Case 1 | Case 2 | Case 3a | Case 4a | |
|---|---|---|---|---|
| Age/Sex | 7 y/F | 7 y/F | 2.5 y/F | 2.5 y/M |
| HLA typing | A*01 – A*02; B*27 – B*58; C*02 – C*03 |
A*30 – A*66; B*44 – B*45; C*03 – C*04 |
A*01 – A*66; B*55 – B*58; C*03 – C*03 |
A*01 – A*66; B*55 – B*58; C*03 – C*03 |
| HLA-B variationb | 67CM; 70 K/S; 97R/T | 67S; 70N; 97R | 67Y/M; 70Q/S; 97R/T | 67Y/M; 70Q/S; 97R/T |
| CCR-5 Δ32 status | Wild type | Wild type | Wild type | Wild type |
| Mean CD4+ T-cell count (% past year) | 1432 cells/µL (51.1%) | 1371 cells/µL (38.8%) | 1268 cells/µL (46.3%) | 1263 cells/µL (44.5%) |
| Serology | ||||
| ELISA | Negative | Negative | Negative | Negative |
| Western blot | Negative | Negative | Negative | Negative |
| T-cell responses | ||||
| HIV-1 Gag | Undetectable | Undetectable | Undetectable | Undetectable |
| HIV-1 Nef | Undetectable | Undetectable | Undetectable | Undetectable |
| Plasma viremiac | <1.5 copies/mL | <1.5 copies/mL | <1.5 copies/mL | <1.5 copies/mL |
| Cell-associated DNA | <2.6 copies/106 CD4+ T cells | <2.6 copies/106 CD4+ T cells | <2.6 copies/106 CD4+ T cells | <2.6 copies/106 CD4+ T cells |
| Cell-associated RNAd | 24.9 copies/1.5 µg RNA | 20.0 copies/1.5 µg RNA | 19.5 copies/1.5 µg RNA | 130 copies/1.5 µg RNA |
| RNA in stimulated CD4+ T-cell culturee | Not detected (5.4 M) | Not detected (7.2 M) | Not detected (8.0 M) | Not detected (8.0 M) |
| Quantitative CD4+ T-cell co-culturef | Not detected | 0.1 IU/106 CD4+ T cells | Not done | Not done |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HIV-1, human immunodeficiency virus type 1; HLA, human leukocyte antigen; IU, infectious units; M, million.
a Nonidentical twins; contamination from one twin to the other was not possible as their blood draws were performed on different days and HLA typing was performed on different days with dozens of other individuals samples tested in between.
b HLA-B variation at positions associated with better virologic control (lower set-point); specific amino acid substitutions are indicated in bold.
c Limit of detection: 1.5 copies/mL; each assay performed on 6 mL of whole blood.
d Limit of detection: 1.5 µg RNA; performed in duplicate.
e Limit of detection: 20 copies/mL; M denotes millions of cells.
f Measured as infectious unit per million CD4+ T cells; performed in replicates of 10.
Interferon-γ ELISpot testing revealed no detectable cell-mediated immune responses to HIV-1 Gag or Nef peptide panels (Figure 2). Numerically significant IFN-γ responses directed against cytomegalovirus (CMV) pp65 protein were readily measured in all 4 cases (all 4 were seropositive to CMV).
Figure 2.
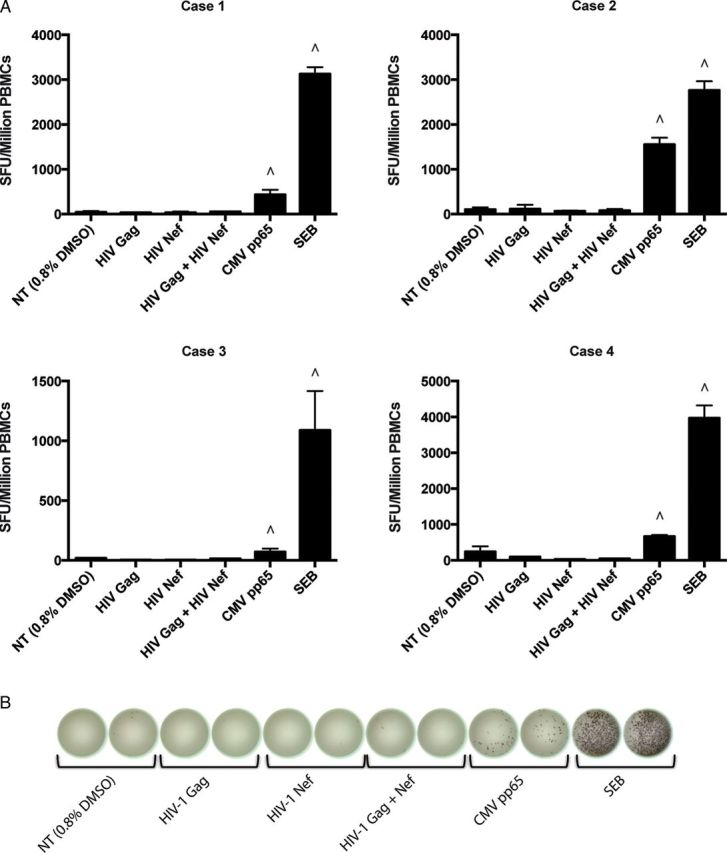
Interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISpot) assay for the 4 children. The 4 cases analyzed did not have detectable human immunodeficiency virus type 1 (HIV-1) Gag or Nef protein-specific T cells in their peripheral blood mononuclear cells (PBMCs). A, HIV-1 Gag and Nef responses were screened using an IFN-γ ELISpot assay. Spot-forming units per million PBMCs are reported after stimulation with each corresponding antigen's peptide pool tested at 1 µg/mL/peptide. ∧ Represents responses characterized by a >2.5-fold increase over the no-treatment (DMSO) condition. B, Representative ELISpot wells for case 1 are shown. Each experiment was performed in duplicate. Abbreviations: CMV, cytomegalovirus; DMSO, dimethyl sulfoxide; HIV-1, human immunodeficiency virus type 1; NT, no treatment; PBMCs, peripheral blood mononuclear cells; SEB, staphylococcal enterotoxin B; SFU, spot-forming units.
All 4 children with sustained virologic suppression were homozygous for wild-type CCR5. Three of the 4 children had HLA B*58, and HLA-B sequence variations previously associated with better HIV control (Table 2) [11]. The 2 early-treated children who experienced virologic rebound due to poor adherence after several years of effective therapy and consistent (though not sustained) virologic suppression were homozygous for wild-type CCR5; neither had HLA B*58 (B*08-B*27, B*07-B*14), but both had potentially beneficial mutations (bold) at positions 67 (C/F and C/Y) and 97 (N/S and S/W) of HLA-B (respectively) [11].
DISCUSSION
The clinical context of our 4 patients with sustained virologic suppression was similar to the child reported by Persaud et al [1], with the caveat that our patients have to date remained on cART. All 4 met the standard diagnostic criteria for HIV-1 infection in infants, consisting of detection of HIV-1 nucleic acid in ≥2 separately timed blood samples. Sustained virologic suppression was achieved by 6 months of age, and subsequent testing demonstrated negative HIV serology by both ELISA and Western blot as well as absence of HIV-1–specific cell-mediated immune responses against Gag and Nef. The detection of HIV-1 RNA in early sequential viral load monitoring samples through days 38–107 of life, while on cART, confirms that all 4 children indeed had HIV-1 infection with actively replicating virus. A biphasic viral load decline was evident in case 1 (for whom results were available from the first day of life before cART was commenced), and the viral load decay patterns of the remaining 3 cases (for whom early results were not available) were likewise consistent with the expected second-phase decline seen with successful cART.
The absence of HIV-1–specific humoral and cell-mediated immune responses in our 4 children is likely a consequence of rapid virologic control by cART and lack of development of a mature B- and T-cell response to HIV-1. It has previously been shown that the majority of children initiating cART within 3 months of birth become seronegative by approximately 16 months of age and that the kinetics of antibody titer decline in these children is similar to that observed with passive maternal antibody in HIV-1–exposed uninfected infants [12, 13]. Similarly, HIV-1–specific CD8+ T-cell responses remain negative or revert to negative in children started on effective cART during the first 6 months of life [14, 15]. HIV-1–specific immune responses can remain negative despite ongoing viral replication as evidenced by detectable low-level viremia (≤5 copies/mL) during cART [13]. However, replication-competent virus can be recovered from the peripheral blood of most children with sustained virologic suppression who were started on cART in early infancy [16, 17].
In our 4 patients with sustained virologic suppression, the positive HIV-1 PCR results within 48 hours of birth combined with the detectable but low viral loads during the first 2–3 weeks of life suggest they were infected in utero close to the time of delivery. In this context, the size of the HIV-1 reservoir at birth, if present, is likely to be extremely small. The finding of a low level (0.1/106 CD4+ T cells) of replication-competent HIV-1 in CD4+ T cells in 1 of our 4 children is consistent with this hypothesis and in line with previous studies demonstrating that the frequency of cells harboring replication-competent virus is significantly lower in infants started on cART within 6 weeks of birth compared with those started at a later age [17–19]. In HIV-1–infected adults, the frequency of CD4+ T cells carrying infectious virus in peripheral blood is also markedly lower in those started on cART within 6 months of primary infection compared to those started in the chronic phase of infection [20]. In experimentally infected macaques, early initiation of cART prior to peak virus replication has been shown to limit systemic dissemination and seeding of the reservoir in peripheral and extralymphoid mucosal compartments [21]. Recovery of infectious virus at extremely low level in peripheral blood (eg, 1/1.7 × 109 CD4+ T cells) and virologic rebound following discontinuation of cART have been noted in early-treated adults with prolonged virologic suppression [20]. Rapid virologic rebound was also reported in a child who initiated cART within a week of birth despite sustained virologic suppression from 1 month of age over a 2-year period and absence of detectable HIV-1 proviral DNA in tonsillar tissue [22]. These findings have important implications for HIV cure research in perinatally infected children, as it may not be feasible to obtain sufficient numbers of peripheral blood mononuclear cells to reasonably exclude the presence of replication-competent virus.
The absence of detectable HIV-1 DNA in the peripheral blood CD4+ T cells of our 4 subjects may also have been related to the number of cells tested. Thus, it is possible that HIV-1 DNA may have been detected had a larger number of replicates been tested. In addition, it is theoretically possible that our PCR primers and probe may not be capable of amplifying certain strains of HIV-1 despite the fact that they were derived from a highly conserved region of the virus.
The significance of detectable levels of cell-associated HIV-1 RNA in the peripheral blood, concurrent with absence of detectable virion-associated HIV-1 RNA in the children's CD4+ T cells following mitogenic stimulation in the limited number of CD4+ T cells tested, is uncertain. The former may reflect transcription of defective HIV-1 genome, a theory put forward to explain similar findings in the “Berlin patient” and the “Mississippi baby” [1, 23]. Although we demonstrated a low level of replication-competent virus (detected in quantitative co-culture) in only 1 child, it is also possible that replication-competent virus was present in the peripheral blood samples of the remaining 3 patients but not detected due to the fact that we could not obtain sufficiently large numbers of cells (for example, >100 million PBMCs). Another potential explanation is failed induction of provirus replication in vitro. In a recent study, it was shown that 11.7% of noninduced virions had intact genomes and normal long terminal repeat function [24].
Multiple HLA class 1 alleles have been linked to the rate of HIV-1 disease progression [3], and individual amino acids within the HLA-B have been associated with influencing the HIV-1 “viral set-point” [11]. Of the 4 children in our cohort with sustained virologic suppression, 3 had protective markers including HLA B*58 and HLA-B sequence variations at positions 67, 70, and 97 [3, 11]. One of these 3 children also had the protective allele HLA B*2701. The child in whom replication-competent virus was recovered by quantitative co-culture lacked these protective genetic markers (case 2). The 2 children who had virologic rebound due to poor adherence after several years of effective therapy and consistent (though not sustained) virologic suppression did not have HLA B*58, but one had HLA B*27, and both had potentially beneficial HLA-B mutations at positions 67 and 97 [11]. Our findings suggest a potential contribution of the infant's (or possibly the mother's) immune system toward virologic control in early-treated perinatally infected children.
Among 136 high-risk HIV-exposed infants who received cART within 72 hours of birth because of lack of or inadequate maternal antiretroviral treatment 12 were infected, a vertical transmission rate of 8.8%. This contrasts with the 0.4% overall transmission rate observed in Canada when maternal cART is initiated >4 weeks before delivery [25]. Furthermore, in the 6 children (50%) tested within 48 hours of birth, positive HIV-1 PCR results suggest that in utero transmission had occurred. These data reinforce the importance of timely maternal diagnosis and provision of appropriate antenatal cART which, if applied universally, would reduce the risk of vertical transmission to <1% and obviate the need for aggressive prophylaxis measures such as treating the neonate with cART.
Based on the results of a randomized clinical trial [26], the US Department of Health and Human Services currently recommends oral zidovudine for the first 6 weeks of life in combination with 3 doses of oral nevirapine in the first week for infants of mothers with incomplete virologic suppression [27]. The potential disadvantages of this strategy are 2-fold. If the child is infected, nevirapine resistance may develop, as has been observed in 12% of children following single-dose peripartum nevirapine [28]. In addition, if infected, this prophylactic rather than treatment regimen could lead to delayed virologic suppression. Combination ART at treatment doses could potentially negate these risks and modulate the establishment and persistence of HIV-1 reservoirs [17, 18]. We therefore advocate that cART at treatment doses be recommended for newborn infants of mothers with inadequately controlled HIV-1 replication at the time of delivery. Formal studies evaluating the pharmacokinetics of antiretroviral agents and the safety of cART in newborn infants are ongoing and of paramount importance in this regard.
In conclusion, the findings in the 4 children with sustained virologic suppression show that early initiation of cART in infants can dramatically reduce the level of proviral HIV-1 DNA and replication-competent virus in peripheral blood CD4+ T cells. Given that it is not possible to examine every cell in each infant, a structured treatment interruption may be the only practical way to determine if HIV-1 eradication or functional cure can be achieved in such children. However, treatment interruption is not without risk, as incomplete virologic control following reinitiation of therapy and development of antiretroviral medication resistance has been observed in adults [29–31]. In addition, if virologic rebound were to occur following cART discontinuation, it would likely be accompanied by expansion of the HIV-1 reservoir, potentially making future HIV-1 reservoir eradication more difficult. Therefore, a thorough discussion of the risks and benefits of stopping cART with patients and caregivers is imperative [32] and, if undertaken, will require intensive and long-term follow-up.
Notes
Acknowledgments. We thank Dr Paul Wender for providing prostratin analogue, and the study volunteers and their caregivers for their participation in this study.
Financial support. H. S. was supported by an infrastructure grant from Réseau SIDA et MI, Fonds de la recherche du Québec-santé.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulder PJ, Walker BD. HIV and HLA class I: an evolving relationship. Immunity. 2012;37:426–40. doi: 10.1016/j.immuni.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. Accessed 15 May 2014.
- 5.Chun TW, Murray D, Justement JS, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–8. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blazkova J, Chun TW, Belay BW, et al. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2012;206:765–9. doi: 10.1093/infdis/jis412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 8.Meiklejohn DA, Karlsson RK, Karlsson AC, Chapman JM, Nixon DF, Schweighardt B. ELISPOT cell rescue. J Immunol Methods. 2004;288:135–47. doi: 10.1016/j.jim.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Brumme ZL, Chan KJ, Dong W, et al. CCR5Delta32 and promoter polymorphisms are not correlated with initial virological or immunological treatment response. AIDS. 2001;15:2259–66. doi: 10.1097/00002030-200111230-00007. [DOI] [PubMed] [Google Scholar]
- 10.Brumme ZL, Brumme CJ, Chui C, et al. Effects of human leukocyte antigen class I genetic parameters on clinical outcomes and survival after initiation of highly active antiretroviral therapy. J Infect Dis. 2007;195:1694–704. doi: 10.1086/516789. [DOI] [PubMed] [Google Scholar]
- 11.Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–7. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol. 2000;74:6984–91. doi: 10.1128/jvi.74.15.6984-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persaud D, Siberry GK, Ahonkhai A, et al. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J Virol. 2004;78:968–79. doi: 10.1128/JVI.78.2.968-979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott ZA, Chadwick EG, Gibson LL, et al. Infrequent detection of HIV-1-specific, but not cytomegalovirus-specific, CD8(+) T cell responses in young HIV-1-infected infants. J Immunol. 2001;167:7134–40. doi: 10.4049/jimmunol.167.12.7134. [DOI] [PubMed] [Google Scholar]
- 15.Luzuriaga K, Chen YH, Ziemniak C, et al. Absent HIV-specific immune responses and replication-competent HIV reservoirs in perinatally infected youth treated from infancy: towards cure. Annual Conference of Retroviruses and Opportunistic Infections (CROI),; Atlanta, GA. 2013. [Google Scholar]
- 16.Persaud D, Ray SC, Kajdas J, et al. Slow human immunodeficiency virus type 1 evolution in viral reservoirs in infants treated with effective antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:381–90. doi: 10.1089/aid.2006.0175. [DOI] [PubMed] [Google Scholar]
- 17.Persaud D, Palumbo PE, Ziemniak C, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS. 2012;26:1483–90. doi: 10.1097/QAD.0b013e3283553638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananworanich J, Puthanakit T, Suntarattiwong P, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28:1015–20. doi: 10.1097/QAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 19.Luzuriaga K, Tabak B, Garber M, et al. Reduced HIV reservoirs after early treatment HIV-1 proviral reservoirs decay continuously under sustained virologic control in early-treated HIV-1- infected children [published ahead of print] J Infect Dis. 2014 doi: 10.1093/infdis/jiu297. doi 10.1093/fdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chun TW, Justement JS, Murray D, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS. 2010;24:2803–8. doi: 10.1097/QAD.0b013e328340a239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okoye AA, Rohankhedkar M, Reyes M, et al. Early treatment in acute SIV infection limits the size and distribution of the viral reservoir (136LB) Top Antivir Med. 2014;22(e-1):68. [Google Scholar]
- 22.Vigano A, Trabattoni D, Schneider L, et al. Failure to eradicate HIV despite fully successful HAART initiated in the first days of life. J Pediatr. 2006;148:389–91. doi: 10.1016/j.jpeds.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Yukl SA, Boritz E, Busch M, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–51. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes JC, Alimenti AM, Singer J, et al. A national review of vertical HIV transmission. AIDS. 2012;26:757–63. doi: 10.1097/QAD.0b013e328350995c. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen-Saines K, Watts DH, Veloso VG, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368–79. doi: 10.1056/NEJMoa1108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed 15 May 2014.
- 28.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dybul M, Nies-Kraske E, Daucher M, et al. Long-cycle structured intermittent versus continuous highly active antiretroviral therapy for the treatment of chronic infection with human immunodeficiency virus: effects on drug toxicity and on immunologic and virologic parameters. J Infect Dis. 2003;188:388–96. doi: 10.1086/376535. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez R, Portilla J, Gimeno A, et al. Immunovirologic consequences and safety of short, non-structured interruptions of successful antiretroviral treatment. J Infect. 2007;54:159–66. doi: 10.1016/j.jinf.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Yerly S, Fagard C, Gunthard HF, Hirschel B, Perrin L. Drug resistance mutations during structured treatment interruptions. Antivir Ther. 2003;8:411–5. [PubMed] [Google Scholar]
- 32.Lo B, Grady C. Ethical considerations in HIV cure research: points to consider. Curr Opin HIV AIDS. 2013;8:243–9. doi: 10.1097/COH.0b013e32835ea1c5. [DOI] [PMC free article] [PubMed] [Google Scholar]