Abstract
Purpose.
Cell surface mucins are a group of highly O-glycosylated transmembrane glycoproteins responsible for the protection of epithelial cells on mucosal surfaces. The aim of this study was to investigate the localization and regulation of mucin 20 (MUC20) at the ocular surface.
Methods.
Localization of MUC20 in human corneal and conjunctival epithelia was evaluated by immunofluorescence microscopy. Immortalized corneal (HCLE) and conjunctival (HCjE) cell lines were grown at different stages of differentiation and subjected to quantitative PCR and Western blot analyses. Cell surface proteins on apical cell membranes were biotinylated and isolated by neutravidin chromatography.
Results.
The MUC20 was detected throughout the entire human ocular surface epithelia, predominantly in cell membranes within intermediate cell layers. In conjunctiva, MUC20 also was observed in the cytoplasm of apical cells within the stratified squamous epithelium, but not in goblet cells. Quantitative PCR and immunoblotting demonstrated expression of MUC20 in HCLE and HCjE cells. Induction of differentiation with serum-containing medium resulted in upregulation of MUC20 mRNA and protein. Biotin labeling of the surface of stratified cultures revealed low levels of MUC20 protein on apical glycocalyces. Further, MUC20 was not detected in the cell culture media or in human tears, suggesting that the extracellular domain of MUC20 is not released from the ocular surface as described previously for other cell surface mucins.
Conclusions.
Our results indicate that MUC20 is a novel transmembrane mucin expressed by the human corneal and conjunctival epithelia, and suggest that differential expression of MUC20 during differentiation has a role in maintaining ocular surface homeostasis.
Keywords: MUC20, transmembrane mucin, cornea, conjunctiva, epithelial cell
Elucidation of the mucin repertoire produced by the ocular surface epithelia is critical to understand how the eye is protected against environmental insult and infection. Here, we report on the expression, distribution, and regulation of a novel transmembrane mucin, MUC20, at the ocular surface.
Introduction
Mucins are high molecular weight glycoproteins produced primarily by wet surfaced epithelia of the respiratory, gastrointestinal, and reproductive tracts, as well as the ocular surface. These molecules are characterized by the presence of central tandem repeats of amino acids rich in serine and threonine, which confer sites for O-linked glycosylation.1 The identification of common structural motifs among mucin gene products has led to their classification as secreted or transmembrane.2 Secreted mucins can be further subclassified as gel-forming or soluble, based on their ability to form polymers. Traditionally, mucins on epithelial cell surfaces have been ascribed hydration and lubrication functions due to their heavily O-glycosylated regions.3 However, recent evidence demonstrates additional roles in barrier function, cell growth and differentiation, cell-cell and cell-matrix interactions, and signal transduction.4
The human ocular surface produces two secreted mucins, the gel-forming MUC5AC and the soluble MUC7. The MUC5AC is produced by the goblet cells of the conjunctiva, whereas MUC7 is synthesized by the lacrimal gland and by the stratified epithelium of the conjunctiva.5,6 At least three transmembrane mucins, MUC1, MUC4, and MUC16, have been described at the ocular surface. They localize to the most apical side of the stratified squamous epithelium of the cornea and conjunctiva.7 Less characterized mucin transcripts detected on the human conjunctiva also include the transmembrane mucins MUC13, MUC15, and MUC17.8 Interestingly, a relatively new member of the transmembrane mucin family, MUC20, has been described as one of the most highly expressed glycogenes in human conjunctiva using a microarray approach.9 However, to our knowledge no attempts have been made to further characterize the expression and distribution of MUC20 at the ocular surface.
The MUC20 gene was originally identified by differential display technology in renal tissues of patients with immunoglobulin A nephropathy.10 Further characterization revealed that the MUC20 gene is localized close to MUC4 on chromosome 3q29 and encodes a moderately small mucin with a polymorphic mucin tandem repeat domain of 19 amino acids.11 Studies using the MDCK and HEK293 kidney cell lines indicate that MUC20 is a membrane protein that localizes on the plasma membrane.11 In addition to kidney, MUC20 mRNA also has been found so far in colon, endometrium, liver, lung, middle ear, placenta, and prostate.11–16 It is overexpressed in colorectal and endometrial cancers, where it recently has been shown to predict recurrence and poor outcome.12,16 Here, we report on the expression, distribution, and regulation of MUC20 in normal human ocular surface epithelia.
Materials and Methods
Human Samples
Conjunctival impression cytology samples and tear washes were obtained as discarded samples from an ongoing study in compliance with Good Clinical Practices, Institutional Review Board (IRB) regulations, informed consent regulations, and the provisions of the Declaration of Helsinki. The subjects completed an IRB approved questionnaire regarding history of ocular allergies; disease; surgery; contact lens wear; current medications; the presence, type, and frequency of symptoms of dry eye and dry mouth; and the use of dry eye therapy. Only samples from normal subjects (defined as those with no allergies, eye diseases, surgery, contact lens wear, or dry eye symptoms) were used in this study. These subjects had normal Schirmer I test (≥10 mm wetting at 5 minutes), no diagnostic dye staining, and normal tear breakup time (TBUT; ≥15 seconds). The conjunctival impression cytology samples (n = 3) and tear washes (n = 3) used in this study were collected as described previously.17 Human corneal and conjunctival tissues stored in optimal cutting temperature compound were obtained as archived material from previously published studies.18,19
Cell Culture
Telomerase-immortalized human corneal-limbal (HCLE) and conjunctival (HCjE) epithelial cells were grown as reported previously.20 Briefly, cells were grown as monolayers in keratinocyte serum-free medium (KSFM; Life Technologies, Carlsbad, CA, USA) to achieve confluence. Cells then were incubated in Dulbecco's modified Eagles's medium (DMEM)/F-12 (Sigma-Aldrich Corp., St. Louis, MO, USA) supplemented with 10% newborn calf serum (Thermo Scientific, Rockford, IL, USA) and 10 ng/mL EGF (Life Technologies) for 7 days to promote stratification and differentiation.
RNA Isolation and cDNA Synthesis
Total RNA was extracted from cell cultures and impression cytology samples using an extraction reagent (TRIzol; Life Technologies) according to the manufacturer's protocol. Residual genomic DNA in the RNA preparation was eliminated by digestion with amplification-grade DNase I (Life Technologies). Reverse transcription of 1 μg of total RNA was performed with random hexamer primers and reverse transcriptase (iScript; Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's protocol.
Quantitative PCR (qPCR)
Detection of MUC20 gene expression was performed by qPCR using PrimePCR MUC20 primers (Unique Assay ID: qHsaCEP0025090; Bio-Rad Laboratories, Inc.). The qPCR reactions were done in a 20 μL reaction volume using 1 μL of cDNA, 1 μL of MUC20 primers and the SYBR Fast master mix (KAPA Biosystems, Wilmington, MA, USA) in a Mastercycler ep realplex thermal cycler (Eppendorf, Hauppauge, NY, USA). The following parameters were used: 2 minutes at 95°C, followed by 40 cycles of 5 seconds at 95°C and 30 seconds at 60°C. All samples were normalized using glyceraldehyde-3-phoshate dehydrogenase (GAPDH) housekeeping gene expression (PrimePCR GAPDH primers; Bio-Rad Laboratories, Inc.). The comparative CT method was used for relative quantitation of the number of MUC20 mucin transcripts, selecting the relative mucin mRNA level in monolayer HCLE cell cultures as the calibrator.
Transient Transfection of siRNAs
Depletion of MUC20 in stratified HCLE cells was achieved using the Silencer Select Pre-designed small interfering RNA ([siRNA], s47303; Life Technologies) targeting a sequence of human MUC20 mRNA (5′-CACUCACAAUGGACAUAUUtt-3′). A nonspecific scrambled siRNA (4390843; Life Technologies) served as the negative control. For knockdown, HCLE cells in 12-well plates were transfected twice (at confluence and 3 days after confluence) by 6-hour incubation with 10 μL siRNA in Lipofectamine 2000 (2 μL; Life Technologies) dissolved in 400 μL Opti-MEM plus GlutaMax reduced-serum medium (Life Technologies). Cells were allowed to recover for 72 hours after each transfection in supplemented DMEM/F-12 medium.
Biotinylation of Cell Surface Proteins
To determine the presence of MUC20 on apical cell membranes of stratified HCLE and HCjE cells, cultures in 6-well plates were serum-starved for 2 hours before biotinylation and isolation of cell surface proteins by chromatography on a neutravidin-agarose affinity column using the Pinpoint Cell Surface Protein Isolation kit (Thermo Scientific) according to the manufacturer's instructions. Biotinylated protein was analyzed by Western blot to determine the amount of MUC20 and MUC16 at the cell surface relative to the flow through.
Electrophoresis and Western Blot
Protein from cell cultures was extracted using radioimmunoprecipitation (RIPA) buffer (150 μM NaCl, 50 μM Tris, pH 8.0, 1% NP 40, 0.5% deoxycholate, 0.1% SDS) supplemented with Complete Protease Inhibitor Cocktail (Roche Biochemical, Indianapolis, IN, USA). After homogenization with a pellet pestle, the cell lysates were centrifuged at 13,500g for 30 minutes, and the protein concentration of the supernatant determined using the Pierce BCA Protein Assay Kit (Thermo Scientific). For analyses of cell culture medium, cells were washed with PBS, pH 7.5, followed by incubation with serum-free DMEM/F12 for 24 hours at 37°C. Medium was collected and centrifuged at 6000g for 4 minutes to remove cellular debris, and concentrated as described.21
For analysis of MUC20 and GAPDH, proteins were resolved in 10% SDS-PAGE, and electroblotted onto nitrocellulose membranes (Bio-Rad Laboratories, Inc.). For analysis of MUC16, proteins were resolved by agarose gel electrophoresis (1%, wt/vol) and transferred to nitrocellulose membranes by vacuum. Nonspecific binding was blocked by incubation with 5% nonfat milk in 0.1% Tween 20 in Tris buffered saline (TTBS) for 1 hour at room temperature. Membranes then were incubated with rabbit anti-MUC20 C-terminus (1:3,000; clone RB13033; Abgent, San Diego, CA, USA), rabbit anti-MUC20 N-terminus (1:3,000; clone RB12372; Abgent), or mouse anti-MUC16 (1:3,000; clone M11; Neomarkers, Fremont, CA, USA) antibodies overnight at 4°C. We used GAPDH as an internal control (1:3,000; FL-335; Santa Cruz Biotechnology, Dallas, TX, USA). Following incubation with the corresponding peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:5,000; Santa Cruz Biotechnology) for 1 hour at room temperature, positive binding was visualized with chemiluminescence (SuperSignal West Pico substrate; Thermo Scientific). Densitometry was performed using ImageJ software (available in the public domain at http://rsb.info.nih.gov/nih-image; National Institutes of Health [NIH], Bethesda, MD, USA).
Immunofluorescence
We localized MUC20 in 7-μm frozen sections of human corneal and conjunctival epithelia using standard protocols. Methanol-fixed slides were blocked with 3% bovine serum albumin (Sigma-Aldrich Corp.) in PBS before incubation with the rabbit anti-MUC20 C-terminus antibody (1:150) overnight at 4°C. The corresponding fluorescein isothiocyanate–conjugated anti-rabbit secondary antibody was applied (1:300) for 1 hour at room temperature. After washing with PBS, specimens were cover-slipped using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Incubation with primary antibody was routinely omitted in control experiments. The slides were viewed with an inverted fluorescence microscope (Axio ObserverZ1; Carl Zeiss Microscopy, Thornwood, NY, USA).
To determine whether MUC20 colocalized with MUC5AC in conjunctival tissue, double-labeling studies were performed. For this purpose, a mixture of rabbit anti-MUC20 C-terminus (1:50) and mouse anti-MUC5AC (1:50; clone CLH2; Santa Cruz Biotechnology) antibodies was applied to tissue sections followed by a mixture of fluorescein isothiocyanate–conjugated anti-rabbit (1:300) and Texas red isothiocyanate–conjugated anti-mouse (1:300) antibodies.
Statistical Analyses
Data are represented as mean ± SD. Statistical analyses were performed with Student's t-test using Excel (Microsoft, Redmond, WA, USA).
Results
Mucin Expression in Human Conjunctival Epithelium
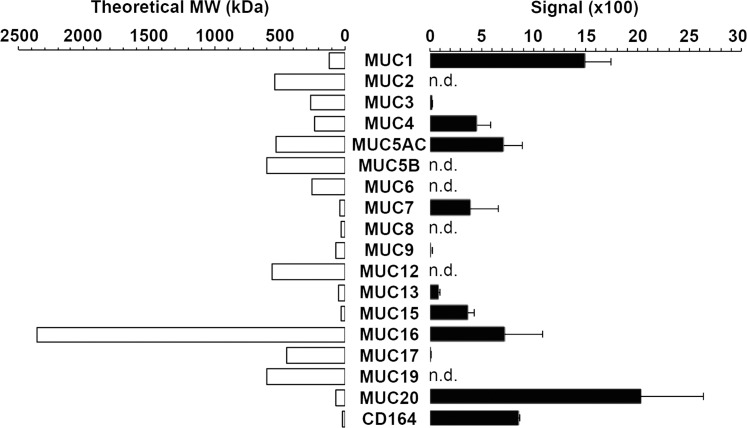
Microarray databases are a valuable tool to evaluate the expression profiles of genes responsible for the glycosylation of proteins, lipids, and proteoglycans in human tissues.22 Analysis of a public microarray depository (available in the public domain at http://www.functionalglycomics.org) revealed that MUC20 is the most highly expressed mucin gene in the human conjunctival epithelium (Fig. 1). Other transcripts detected included MUC1, MUC4, MUC5AC, MUC7, MUC13, MUC15, MUC16, and MUC17, consistent with previous reports showing the presence of these mucins at the ocular surface.7,8 The MUC2 transcripts, known to be expressed at levels 5900-fold lower than MUC5AC,23 were not detected. Moreover, the microarray data also demonstrated significant signal intensity for CD164, also known as MUC24, a transmembrane mucin not described in ocular surface epithelia.
Figure 1.
Mucin gene expression in human conjunctival epithelium. Microarray analysis of impression cytology samples indicates that MUC20 is the most highly expressed mucin gene in human conjunctiva. Detailed data on glycogene expression can be found in the public domain at http://www.functionalglycomics.org/glycomics/publicdata/microarray.jsp; Accession # MAEXP_272_042605. The theoretical molecular weight (MW) can be found in the public domain at the Universal Protein Resource (UniProt) database (http://www.uniprot.org). n.d., not detected.
Unlike MUC20, the presence of the transmembrane mucins MUC1, MUC4, MUC16 has been characterized extensively in corneal and conjunctival epithelia.3 Based on amino acid sequence, MUC16 is the largest with an expected molecular weight of 2.4 MDa, while MUC20 has an expected molecular weight of 72 kDa, comparable to that of MUC1 (122 kDa, Fig. 1).
MUC20 Localizes Throughout the Stratified Epithelia in Human Corneal and Conjunctival Epithelia
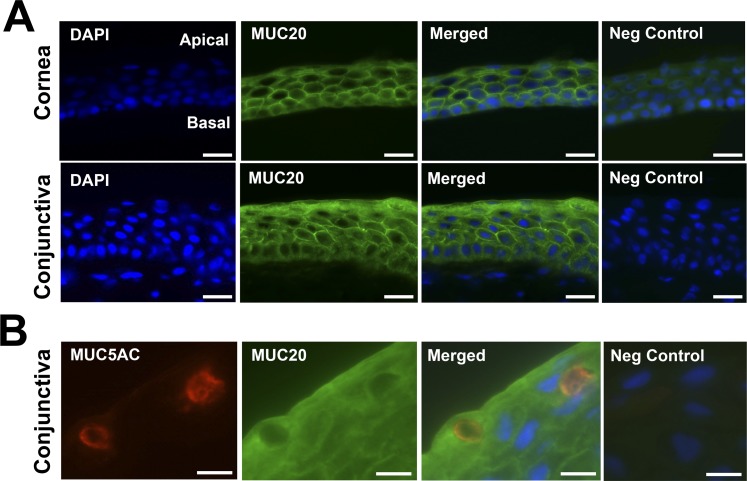
The tissue distribution of MUC20 in human corneal and conjunctival epithelia was evaluated using immunofluorescence microscopy. In cornea, MUC20 was detected primarily along the cell membranes of intermediate cell layers of the stratified epithelium (Fig. 2A). Binding of the MUC20 antibody also was predominant on apical membranes of columnar cells in the basal cell layer, but not on apical membranes of flattened cells in the apical cell layer, in contrast to other transmembrane mucins, such as MUC16.24 A similar distribution pattern of MUC20 along the cell membranes of intermediate cell layers also was observed in conjunctiva. Interestingly, MUC20 had a robust intracellular distribution on superficial cells of the conjunctiva, suggesting a more active trafficking process of the mucin in these cells. The MUC20 was not detected in goblet cells, as demonstrated by lack of colocalization with MUC5AC within secretory packets in the cell (Fig. 2B).
Figure 2.
Immunofluorescence micrographs demonstrating the presence of MUC20 in human corneal and conjunctival epithelia. (A) The MUC20 was primarily observed along the cell membranes of intermediate cell layers of the stratified corneal and conjunctival epithelia. In conjunctiva, MUC20 had an intracellular distribution within superficial cells. Scale bars: 25 μm. (B) The MUC20 did not colocalize with MUC5AC within secretory packets in conjunctival goblet cells. 4′6-Diamidino-2-phenylindole (DAPI) was included in the mounting medium to localize the position of cell nuclei. Scale bars: 10 μm.
Induction of Differentiation In Vitro Promotes MUC20 Gene Expression and Protein Biosynthesis
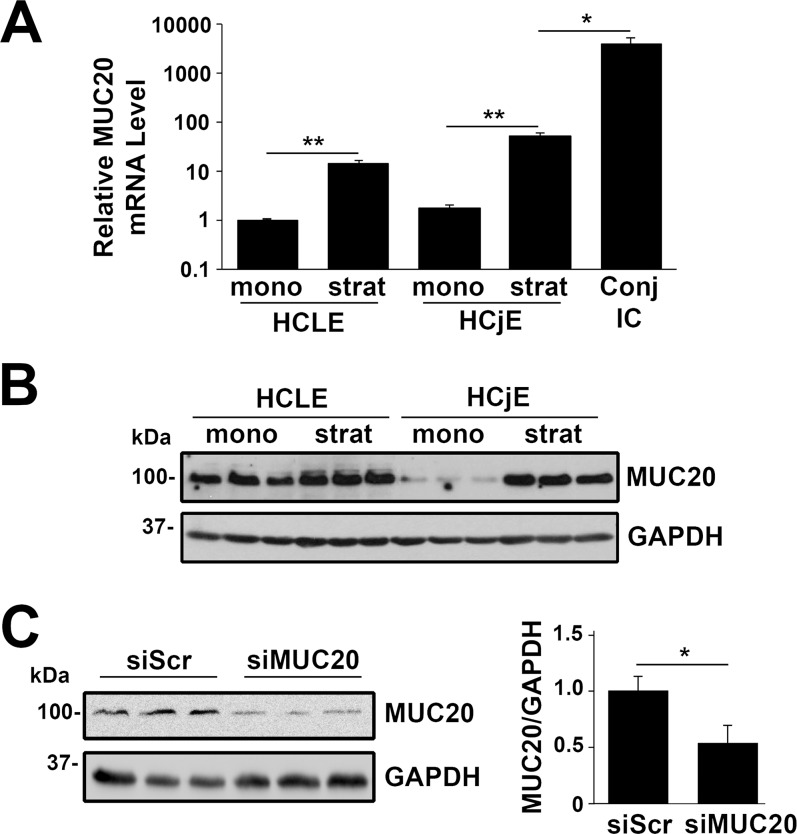
It has been hypothesized that serum derived from vessels in the conjunctiva may have an important role in the regulation of mucins by the ocular surface epithelia.25 Addition of serum to human corneal and conjunctival epithelial cells in vitro is known to induce stratification and cell differentiation.26 Here, we used the HCLE and HCjE cell lines to determine whether induction of differentiation with serum-containing medium regulates MUC20 biosynthesis. By qPCR, we demonstrated that MUC20 transcripts are present in undifferentiated HCLE and HCjE cells grown as monolayers in serum-free medium (Fig. 3A). Upon induction of stratification and differentiation by serum supplementation for 7 days, MUC20 mRNA levels were significantly upregulated in both cell lines. We also found a significantly higher level (72-fold) of MUC20 mRNA in native human conjunctival epithelial cells from impression cytology samples compared to stratified HCjE cultures, indicating that, as previously observed, cells in culture do not achieve the degree of differentiation seen in vivo.27
Figure 3.
Expression of MUC20 is differentially regulated during corneal and conjunctival cell differentiation. (A) The qPCR analysis revealed significant upregulation of MUC20 mRNA after induction of stratification and differentiation with serum-containing medium in HCLE and HCjE cell cultures. The level of MUC20 mRNA was significantly lower in stratified HCjE cells than in native human conjunctival epithelial cells from three impression cytology (IC) samples. Results are expressed on a logarithmic scale. mono, monolayer cultures; strat, stratified cultures. (B) By Western blot, MUC20 protein also was upregulated upon serum addition to HCLE and HCjE cells. (C) The specificity of the antibody for MUC20 was confirmed by knockdown experiments. Transfection of stratified HCLE cell cultures with siRNA specific to MUC20 resulted in a 47% reduction in band intensity compared to scramble control. Experiments were performed independently in triplicate. *P < 0.05, **P < 0.01.
To analyze the expression of MUC20 at the protein level, we performed immunoblot analyses of HCLE and HCjE cells before and after serum supplementation. We found that induction of cell differentiation resulted in upregulation of MUC20 protein in corneal and conjunctival epithelial cells (Fig. 3B), consistent with the induction observed at the mRNA level. The specificity of the antibody for MUC20 was demonstrated in knockdown experiments. Depletion of MUC20 in stratified HCLE cells by siRNA resulted in a 47% reduction in band intensity compared to the scramble control, confirming the identity of the MUC20 band on the gel (Fig. 3C).
MUC20 Shows Limited Release From Ocular Surface Epithelial Cells
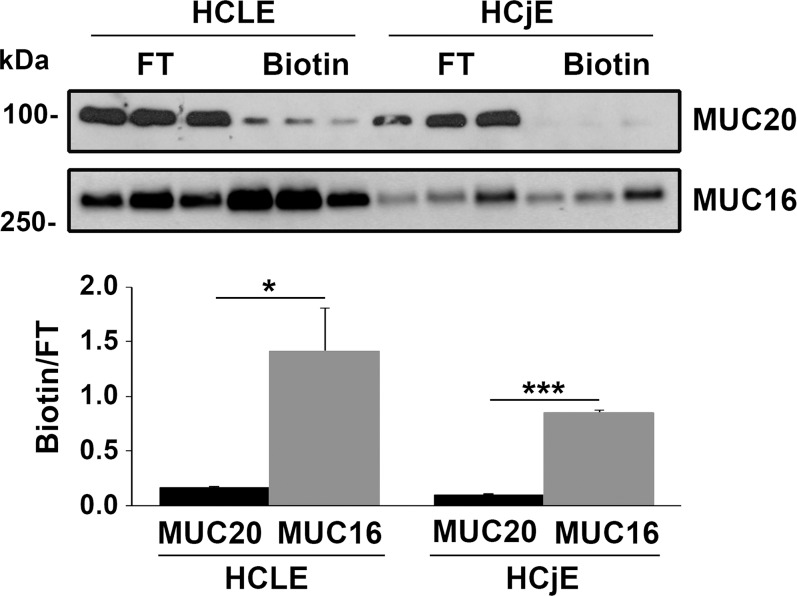
Transmembrane mucins at the ocular surface are known to localize to the apical cell glycocalyx of superficial cells and are especially prevalent on the tips of the microplicae at the tear film interface.28 Surprisingly, our immunolocalization data revealed weak expression of MUC20 on the apical glycocalyx of superficial cells in corneal and conjunctival tissue specimens (Fig. 2A). To determine the extent to which MUC20 is present on the most apical surface in ocular surface epithelial cells, we performed biotinylation experiments in stratified cultures of HCLE and HCjE cells. Consistent with our immunolocalization data, MUC20 was weakly detected on the apical surface of the cells (Fig. 4). This is in contrast with the transmembrane mucin MUC16, which was expressed strongly on the apical surface in our experiments.
Figure 4.
Protein biotinylation demonstrates low levels of MUC20 at the cell surface. Surface-biotinylated proteins were isolated using a neutravidin-agarose affinity column. By Western blot, MUC20 was detected weakly on the apical surface of stratified HCLE and HCjE cells (upper). In contrast, MUC16 was detected robustly on the apical surface, particularly in HCLE cells. Densitometry analysis of the amount of biotinylated protein normalized to the flow through (FT) demonstrates a higher abundance of MUC16 on the cell surface than MUC20 (lower). Experiments were performed independently in triplicate. *P < 0.05, ***P < 0.001.
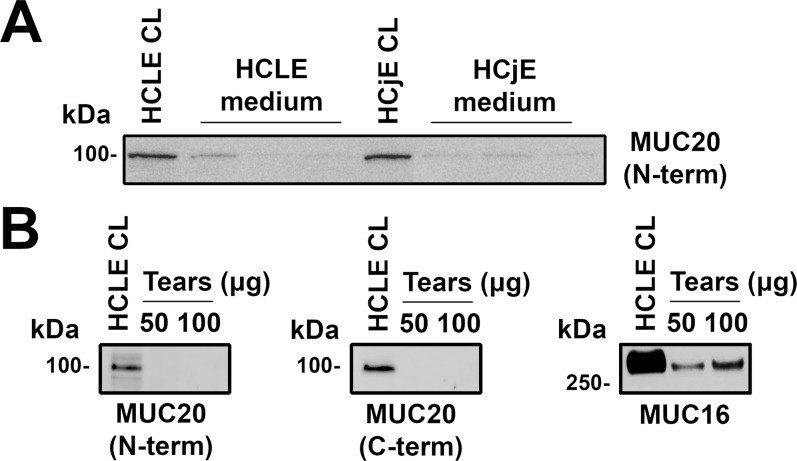
Further, we determined whether MUC20 was released from the ocular surface epithelia. Previous reports have shown that the extracellular domains of MUC1, MUC4, and MUC16 are constitutively shed from the ocular surface into the tear film.21,29 To explore this possibility, we first performed immunoblotting assays with media from stratified HCLE and HCjE cell cultures. For these experiments, we took advantage of an antibody targeting the N-terminal domain of MUC20. As shown in Figure 5A, low levels of MUC20 were detected in the cell culture media. In addition, we performed immunoblotting assays in tear washes collected from normal individuals. Use of antibodies to the N- and C-terminal domains of MUC20 failed to detect the protein in tears (Fig. 5B), suggesting that MUC20 is not released from the ocular surface in vivo.
Figure 5.
Western blot analyses demonstrating limited release of MUC20 by the ocular surface epithelia. (A) Low levels of MUC20 were detected in the cell culture media of stratified HCLE and HCjE cells using an antibody targeting the N-terminal domain of the protein. A total of 40 μg cell lysates (CL) was used as positive control. Experiments were performed independently in triplicate. (B) The MUC20 was not detected in human tear washes pooled from three normal human subjects using antibodies recognizing the N- and C-terminal domains. On the other hand, MUC16 was present in the tear washes, as reported previously.29
Discussion
Elucidation of the mucin repertoire produced by the ocular surface epithelia is critical to understand how the eye is protected against environmental insult and infection. A number of reports have established that the stratified squamous epithelia of the human ocular surface synthesize at least three transmembrane mucins, MUC1, MUC4, and MUC16.7,28 Data obtained in this study provides direct evidence on the expression and regulation of an additional transmembrane mucin, MUC20, at the ocular surface.
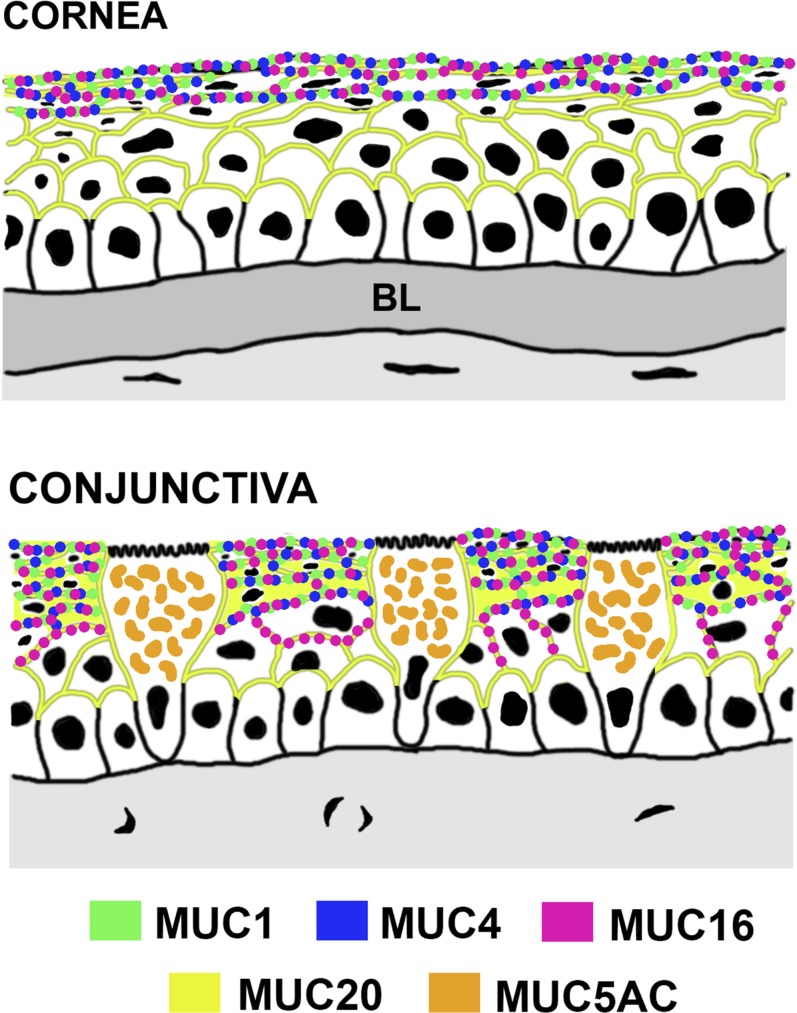
We found that MUC20 has a unique distribution compared to other transmembrane mucins at the ocular surface (Fig. 6). Earlier studies demonstrated that MUC1, MUC4, MUC16 are present along the membranes of the superficial cell layer of the corneal and conjunctival epithelia.28 Indeed, the localization of MUC16 at the tips of the microplicae in the apical glycocalyx of the corneal epithelium has led to studies and demonstration of the role of this mucin in barrier function.24,30,31 Based on the protective role ascribed to transmembrane mucins on the tear film interface, we expected that MUC20 would likewise be present along apical membranes on the apical surface of the stratified epithelia. Surprisingly, immunofluorescence microscopy revealed that MUC20 was predominant along the cell membranes of intermediate cell layers of the stratified epithelium, with limited expression on the apical glycocalyx of superficial cells. Further, MUC20 was weakly detected on biotinylated surface proteins from the HCLE and HCjE cell lines and was absent in human tears. These data suggested that, unlike MUC16, a transmembrane glycoprotein with a molecular weight of more than 2.4 MDa, the small MUC20 may not be primarily involved in glycocalyx barrier function at the ocular surface. Recent evidence indicating that transmembrane mucins do not have a redundant role in the protection of the ocular surface, but may have additional functions, supports this hypothesis.32
Figure 6.
Mucin distribution at the human ocular surface epithelia; MUC1, MUC4, and MUC16 localize primarily within apical cell membranes of the stratified corneal and conjunctival epithelia, whereas the secreted mucin MUC5AC localizes within mucin packets in goblet cells. On the other hand, MUC20 has a unique localization throughout the human corneal and conjunctival epithelia, being predominant in the intermediate cell layers of the stratified epithelia. Modified with permission from Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–388, Copyright 2003, Elsevier; and Gipson IK, Argüeso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49, Copyright 2003, Elsevier.
Although to our knowledge no specific function has yet been identified for MUC20 at the ocular surface, studies derived from the analysis of its C-terminal domain indicate that MUC20 could be involved in the regulation of the Met signaling pathway. Met is a cell-surface receptor for hepatocyte growth factor (HGF) involved in cell motility.33 Using a yeast two-hybrid screen, Higuchi et al.34 showed that the cytoplasmic domain of MUC20 associates with the multifunctional docking site of Met. This interaction prevents the recruitment of the Grb2 adaptor protein to Met; therefore, attenuating the activation of MAPK, inhibiting the expression of matrix metalloproteinases, and preventing the proliferation of kidney epithelial cells induced by HGF. In cornea, HGF is known to facilitate the migration and proliferation of epithelial cells, and to inhibit apoptosis.35 Consistent with the effect of HGF in human corneas, Met expression has been detected throughout the epithelium.36 Moreover, expression of Met mRNA in the corneal epithelium is markedly upregulated following epithelial injury37 but downregulated in human corneas of donors with diabetic retinopathy.38 Although not within the scope of our current study, it is tempting to speculate that MUC20 expression may have a role in the regulation of Met signaling in ocular surface epithelia through binding to its multifunctional docking site.
Serum contains a number of growth factors, vitamins, and anti-inflammatory factors that have been considered important for corneal and conjunctival integrity.39 Indeed, eye drops made from autologous serum are used in clinical practice to treat ocular surface disorders, such as persistent epithelial defects or severe dry eyes intractable to conventional therapy.40 Hori et al.25 proposed that a mechanism for the efficacy of serum may be due in part to its ability to upregulate expression of the transmembrane mucins MUC1, MUC4, and MUC16 in the human ocular surface epithelia, at the mRNA and protein levels. Interestingly, the pattern of regulation of each mucin was different from the others, suggesting that the three transmembrane mucins are independently regulated.25 We found that, similar to MUC1 in corneal and conjunctival epithelial cells,25,26,41 MUC20 mRNA and protein were detectable in cells grown in serum-free conditions. After addition of serum, the expression of MUC20 increased as previously shown with other transmembrane mucins in a process associated with the terminal differentiation of ocular surface epithelia in vitro and in vivo.25,42 The requirement of serum for the induction of MUC20 expression suggests an important role of serum proteins derived from conjunctival vessels in maintaining proper levels of this mucin at the ocular surface.
In summary, our results indicated that MUC20 is a transmembrane mucin strongly expressed at the ocular surface. It has a unique localization throughout the human corneal and conjunctival epithelia, being predominant in the intermediate cell layers of the stratified epithelia. Moreover, we found that MUC20 expression is differentially regulated during cell stratification and differentiation, and that compared to other transmembrane mucins, it is not released into the tear film. Clearly, further studies are needed to provide direct evidence on the biological roles of MUC20 in ocular surface health and disease.
Acknowledgments
The authors thank Ilene Gipson, PhD, at The Schepens Eye Research Institute for providing the human corneal-limbal and conjunctival epithelial cell lines, as well as sections of conjunctival biopsy specimens. The authors also thank Jerome Mauris, PhD, for providing valuable suggestions and discussions.
Supported by National Institutes of Health/National Eye Institute (NIH/NEI; Bethesda, MD, USA) Grant R01 EY014847 (PA).
Disclosure: A. Woodward, None; P. Argüeso, None
References
- 1. Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995; 57: 607–634 [DOI] [PubMed] [Google Scholar]
- 2. Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001; 6: D1192–D1206 [DOI] [PubMed] [Google Scholar]
- 3. Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010; 90: 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004; 4: 45–60 [DOI] [PubMed] [Google Scholar]
- 5. Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003; 22: 41–45 [DOI] [PubMed] [Google Scholar]
- 6. Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996; 37: 1684–1692 [PubMed] [Google Scholar]
- 7. Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008; 8: 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corrales RM, Galarreta D, Herreras JM, et al. Conjunctival mucin mRNA expression in contact lens wear. Optom Vis Sci. 2009; 86: 1051–1058 [DOI] [PubMed] [Google Scholar]
- 9. Mantelli F, Schaffer L, Dana R, Head SR, Argueso P. Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci. 2009; 50: 2666–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waga I, Yamamoto J, Sasai H, et al. Altered mRNA expression in renal biopsy tissue from patients with IgA nephropathy. Kidney Int. 2003; 64: 1253–1264 [DOI] [PubMed] [Google Scholar]
- 11. Higuchi T, Orita T, Nakanishi S, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004; 279: 1968–1979 [DOI] [PubMed] [Google Scholar]
- 12. Chen CH, Wang SW, Chen CW, et al. MUC20 overexpression predicts poor prognosis and enhances EGF-induced malignant phenotypes via activation of the EGFR-STAT3 pathway in endometrial cancer. Gynecol Oncol. 2013; 128: 560–567 [DOI] [PubMed] [Google Scholar]
- 13. Finkbeiner WE, Zlock LT, Morikawa M, Lao AY, Dasari V, Widdicombe JH. Cystic fibrosis and the relationship between mucin and chloride secretion by cultures of human airway gland mucous cells. Am J Physiol Lung Cell Mol Physiol. 2011; 301: L402–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007; 117: 1666–1676 [DOI] [PubMed] [Google Scholar]
- 15. Russo CL, Spurr-Michaud S, Tisdale A, Pudney J, Anderson D, Gipson IK. Mucin gene expression in human male urogenital tract epithelia. Hum Reprod. 2006; 21: 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiao X, Wang L, Wei P, et al. Role of MUC20 overexpression as a predictor of recurrence and poor outcome in colorectal cancer. J Transl Med. 2013; 11: 151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2002; 43: 1004–1011 [PubMed] [Google Scholar]
- 18. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002; 120: 330–337 [DOI] [PubMed] [Google Scholar]
- 19. Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci. 2014; 127: 3141–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Argueso P, Gipson IK. Assessing mucin expression and function in human ocular surface epithelia in vivo and in vitro. Methods Mol Biol. 2012; 842: 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest Ophthalmol Vis Sci. 2008; 49: 1864–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Comelli EM, Head SR, Gilmartin T, et al. A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology. 2006; 16: 117–131 [DOI] [PubMed] [Google Scholar]
- 23. McKenzie RW, Jumblatt JE, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci. 2000; 41: 703–708 [PubMed] [Google Scholar]
- 24. Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003; 44: 2487–2495 [DOI] [PubMed] [Google Scholar]
- 25. Hori Y, Spurr-Michaud S, Russo CL, Argueso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004; 45: 114–122 [DOI] [PubMed] [Google Scholar]
- 26. Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006; 47: 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003; 44: 2496–2506 [DOI] [PubMed] [Google Scholar]
- 28. Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004; 78: 379–388 [DOI] [PubMed] [Google Scholar]
- 29. Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007; 84: 939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blalock TD, Spurr-Michaud SJ, Tisdale AS, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007; 48: 4509–4518 [DOI] [PubMed] [Google Scholar]
- 31. Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009; 284: 23037–23045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB. Comparison of the Transmembrane Mucins MUC1 and MUC16 in Epithelial Barrier Function. PLoS One. 2014; 9: e100393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clague MJ. Met receptor: a moving target. Sci Signal. 2011; 4: pe40 [DOI] [PubMed] [Google Scholar]
- 34. Higuchi T, Orita T, Katsuya K, et al. MUC20 suppresses the hepatocyte growth factor-induced Grb2-Ras pathway by binding to a multifunctional docking site of met. Mol Cell Biol. 2004; 24: 7456–7468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010; 81: 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson SE, Walker JW, Chwang EL, He YG. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993; 34: 2544–2561 [PubMed] [Google Scholar]
- 37. Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999; 68: 377–397 [DOI] [PubMed] [Google Scholar]
- 38. Saghizadeh M, Kramerov AA, Tajbakhsh J, et al. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci. 2005; 46: 3604–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinto GG, Campos M, Behrens A. Autologous serum for ocular surface diseases. Arq Bras Oftalmol. 2008; 71: 47–54 [PubMed] [Google Scholar]
- 40. Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004; 88: 1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiong L, Woodward AM, Argueso P. Notch signaling modulates MUC16 biosynthesis in an in vitro model of human corneal and conjunctival epithelial cell differentiation. Invest Ophthalmol Vis Sci. 2011; 52: 5641–5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones DT, Monroy D, Ji Z, Pflugfelder SC. Alterations of ocular surface gene expression in Sjogren's syndrome. Adv Exp Med Biol. 1998; 438: 533–536 [DOI] [PubMed] [Google Scholar]
- 43. Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003; 231: 1–49 [DOI] [PubMed] [Google Scholar]