Abstract
BACKGROUND
Lowering blood pressure (BP) after stroke remains a challenge, even in the context of clinical trials. The Secondary Prevention of Small Subcortical Strokes (SPS3) BP protocol, BP management during the study, and achieved BPs are described here.
METHODS
Patients with recent symptomatic lacunar stroke were randomized to 1 of 2 levels of systolic BP (SBP) targets: lower: <130mm Hg, or higher: 130–149mm Hg. SBP management over the course of the trial was examined by race/ethnicity and other baseline conditions.
RESULTS
Mean SBP decreased for both groups from baseline to the last follow-up, from 142.4 to 126.7mm Hg for the lower SBP target group and from 143.6 to 137.4mm Hg for the higher SBP target group. At baseline, participants in both groups used an average of 1.7±1.2 antihypertensive medications, which increased to a mean of 2.4±1.4 (lower group) and 1.8±1.4 (higher group) by the end-study visit. It took an average of 6 months for patients to reach their SBP target, sustained to the last follow-up. Black participants had the highest proportion of SBP ≥150mm Hg at both study entry (40%) and end-study visit (17%), as compared with whites (9%) and Hispanics (11%).
CONCLUSIONS
These results show that it is possible to safely lower BP even to a SBP goal <130mm Hg in a variety of patients and settings, including private and academic centers in multiple countries. This provides further support for protocol-driven care in lowering BP and consequently reducing the burden of stroke.
Keywords: achieved blood pressures, blood pressure, blood pressure management, hypertension, ischemic stroke, lacunar stroke, stroke prevention.
Hypertension is the most important risk factor for stroke with data from both randomized trials and meta-analyses supporting the importance of blood pressure (BP) lowering for secondary stroke prevention.1–5 Despite the evidence, studies continue to report inadequate BP control in those with prior stroke.6–9 The challenges of lowering BP are not unique to clinical practice, and even within the context of BP clinical trials, achieving goal systolic BP (SBP) has proven difficult.10–12
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial13 was conducted in 81 sites across 8 countries between May 2003 and April 2012. The primary objective was to identify effective strategies for secondary stroke prevention, including lowering SBP to a target of <130mm Hg. The purpose of this report is to provide detailed information on the implementation of the SPS3 BP protocol, including strategies used to enhance fidelity to the protocol, achieved BPs, and factors associated with failure to achieve SBP goal.
METHODS
The rationale and design of the SPS3 trial are presented in detail elsewhere.13,14 In brief, eligible patients were aged ≥30 years, with a clinical lacunar stroke syndrome15 within 6 months before enrollment with confirmation by magnetic resonance imaging and no surgically amenable ipsilateral carotid artery disease or major-risk cardioembolic sources. They were randomized to 1 of 2 levels of SBP control, a lower target (<130mm Hg) and a higher target (130–149mm Hg), and simultaneously to an antiplatelet regimen. Both normotensive and hypertensive patients were eligible, with status determined by average BP from 2 prerandomization visits. There was no washout period for antihypertensive medications. The SPS3 study was approved by the institutional review boards of all participating centers, and all patients provided written informed consent.
SPS3 BP protocol
Research personnel were trained on a standardized protocol for BP measurement16,17 and provided with a validated automated electronic device.18 Three measurements, separated by 2 minutes, were averaged. Sites were asked to record and use the first 3 measurements to avoid the bias of selecting 3 out of multiple measurements. Printers were installed on a selection of BP machines to ensure that printed values matched values on case report forms and that only the initial 3 measurements were used.
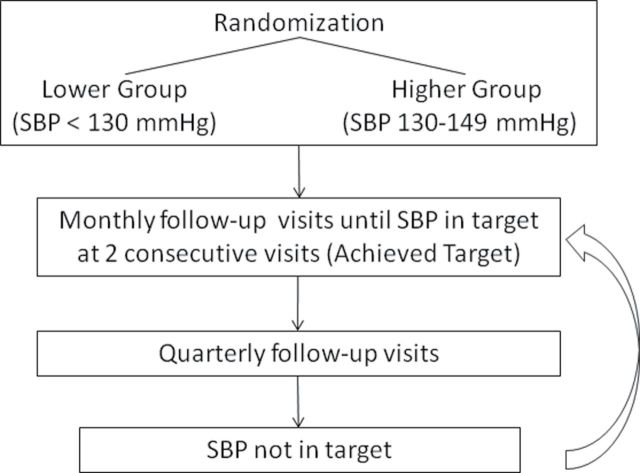
After randomization, all patients were seen monthly for 3 months and thereafter on a quarterly basis for management of SBP to the assigned target. The average of the 3 in-clinic measurements was used to determine whether the patient was within target; those who were not continued to be seen at least monthly for BP management until their SBP was within the assigned target for 2 consecutive visits, the SPS3 definition for achieving SBP target (Figure 1). To assess for orthostatic hypotension, sitting BP was compared with BP after standing for 2 minutes.
Figure 1.
Follow-up algorithm. Abbreviation: SBP, systolic blood pressure.
BP control was overseen at each clinical site by a physician with expertise in BP management. Patients were evaluated for secondary causes of hypertension and treatment tailored to address any secondary causes at baseline and through-out the study. Investigators were encouraged to use the most effective medications to lower BP. An algorithm was developed and distributed to the sites (Supplementary Appendix S1). This nonprotocol-mandated algorithm advocated titration of dose, as well as addition of agents, using a stepwise approach, monitoring carefully for side effects.16 Diastolic BP was considered as needed for safety reasons but not managed per protocol. By protocol, patients assigned to the higher target (130–149 mmHg) with SBP below target and on BP-lowering medications had them discontinued or their dose reduced to bring SBP into target, unless prescribed for reasons other than BP control. Participants with SBP below target in the higher group and on no BP-lowering medications were followed quarterly, and if their SBP increased, they were managed into their assigned target.
Antihypertensive medications were provided free of charge to participants, as deemed appropriate by the local site team. Medications available in the SPS3 formulary included at least 1 drug from most classes (Supplementary Appendix S2) and relied heavily on the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) formulary because of its proven effectiveness.19 Medications were also selected based on their half-life to enhance adherence (daily dosing preferred) and their availability through the Cooperative Studies Program Clinical Research Coordinating Center, Department of Veterans Affairs. The SPS3 formulary was not used in several countries because of the difficulties in shipping medications and the availability of local government-subsidized medication plans. There were several situations where ongoing monitoring of current evidence suggested minor tailoring of the formulary to ensure that best practice was provided to all participants.20,21
Patients were designated “inactive” in the BP protocol if they or their primary care physicians refused to have their BP managed to their assigned target by SPS3 investigators. Patients were designated “failure to achieve assigned target” if for medical reasons their BP could not be managed into the assigned target or for patients who suffered intolerable side-effects, despite trying multiple agents. All participants were followed to a common end-study date, irrespective of active/inactive or failure to achieve assigned target status.
The Steering Committee and the Blood Pressure Steering Sub-Committee provided guidance and oversight to ensure that the BP protocol was implemented in a standardized manner across sites. They were charged with internal monitoring of patient safety and keeping current with publications in the field to ensure that the BP protocol remained ethical and safe.
Adherence to the BP protocol was supported through continuous analysis and feedback to the clinical sites regarding protocol deviations and safety concerns.18 Weekly reports to sites showed the percentage of patients by site within their SBP target, and quarterly reports provided detailed information about BP management, and for safety, participants with very high or low SBP and heart rates. In addition to regular conference calls between the Coordinating Center and the clinical sites, sites with poor performance in managing patients into their assigned targets were identified for additional discussion and recommendations for challenging patient management.
Data analysis
Participant characteristics were compared between the lower and higher SBP target groups using analysis of variance for quantitative measures, χ2 tests of association for nominal categorical measures, and χ2 tests of trend for ordinal categorical measures. Means ± SDs and frequencies (%) are presented. To examine aspects of implementation of the BP protocol by trial personnel, counts and percentages of participants for whom <25%, 25%–49%, 50%–75%, and >75% of all study visits were always active, ever inactive, and failure to achieve assigned target are presented by BP target group and compared with the Cochran–Mantel–Haenszel test. There were a small percentage of normotensive subjects in each treatment arm, and they were included in all analyses. SAS versions 9.2 and 9.3 (SAS Institute, Cary, NC) were used for all statistical analyses.
RESULTS
The average age of the 3,020 participants was 63±11 years, 63% were men, and 51% were white, 30% were Hispanic, and 16% were black. The majority were from the United States (56%), followed by Latin America (23%), Spain (12%), and Canada (9%). Hypertension, hyperlipidemia, and diabetes were the most prevalent vascular risk factors, present in 75%, 49%, and 37% of subjects, respectively. The majority (67%) demonstrated good functional recovery from stroke as evidenced by a modified Rankin Score22 of 0 or 1. Patients were followed for a mean of 3.7±2.0 years.
The mean SBP decreased for both groups from baseline to the last follow-up, from 142.4 to 126.7mm Hg (lower target group) and from 143.6 to 137.4mm Hg (higher target group) (Table 1). Baseline BPs were similar by sex, as were responses to the BP protocol. The percentage of patients with controlled hypertension (SBP < 140mm Hg) was similar for both groups at baseline (approximately 47%); by end-study this increased to 85.1% in the lower SBP target group compared with 57.7% in the higher SBP target group. Approximately 75% of participants were always active in the BP protocol, whereas approximately 22% of participants were designated as inactive at least once and 3%–4% of participants were designated as failure to achieve assigned target, with no differences by SBP target group. The mean number of follow-up and BP visits over the course of the study was the same regardless of SBP target (21.3±11.6 vs. 21.6±12.2, lower and higher groups respectively; P > 0.05), decreasing from an average of 7 visits the first year to approximately 5 visits per year in subsequent years. The majority of participants reported adherence as “excellent” or “good” at >75% of their quarterly visits. There was no difference by treatment group in symptoms possibly related to BP management. Participants who were in target at >75% of study visits were more likely to be always active (87.9% and 83.7% of those always active in lower and higher groups, respectively) compared with being ever inactive (12.1% and 15.4% in lower and higher groups, respectively) or with failure to achieve assigned target (0% and 0.9% in lower and higher groups, respectively; P < 0.0001) (Table 2).
Table 1.
Implementation of blood pressure protocol by treatment group
| Variable | Lower SBP target (<130mm Hg) (n = 1,501) | Higher SBP target (130–149mm Hg) (n = 1,519) |
|---|---|---|
| Mean SBP ± SD at baseline | 142.4±18.5 | 143.6±19.1 |
| Mean SBP ± SD at last follow-up** | 126.7±16.5 | 137.4±16.2 |
| Mean SBP ± SD across quarterly follow-ups (excluding initial 6 months)** | 125.1±14.7 | 137.1±14.4 |
| No. (%) in target at last follow-up | 1,034 (69.6) | 855 (56.9) |
| Mean DBP ± SD at baseline* | 77.6±10.4 | 79.0±10.8 |
| Mean DBP ± SD at last follow-up** | 69.1±10.4 | 74.8±10.9 |
| Mean DBP ± SD across quarterly follow-ups (excluding initial 6 months)** | 68.5±9.9 | 75.0±10.3 |
| SBP <140mm Hg, no. (%) | ||
| Baseline | 722 (48.1) | 699 (46.0) |
| Year 1 | 1,120 (84.1) | 776 (56.9) |
| End of study | 1,100 (85.1) | 754 (57.7) |
| Study Status, no. (%) | ||
| Always active | 1,147 (76.4) | 1,126 (74.1) |
| Ever inactive | 304 (20.3) | 331 (21.8) |
| Ever failure to achieve assigned target | 50 (3.3) | 62 (4.1) |
| Mean number of BP checks ± SD | 21.3±11.6 | 21.6±12.2 |
| Antihypertensive medication adherence excellent or good at >75% of quarterly visits, no. (%) | 1,303 (90.7) | 1,248 (92.5) |
| Side-effects possibly related to blood pressure management, no. (%) | ||
| Unsteadiness when standing | 375 (25) | 355 (24) |
| Blurred vision when standing | 85 (6) | 103 (7) |
| Dizziness when standing | 324 (22) | 304 (20) |
| Light-headedness when standing | 222 (15) | 236 (16) |
| Palpitations when standing | 21 (0.2) | 24 (0.2) |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
* Difference between higher and lower groups is significant at P < 0.001.
** Difference between higher and lower groups is significant at P < 0.0001.
Table 2.
Percentage of quarterly visits with systolic blood pressure (SBP) in target by SBP group and by study status
| Variable | Lower SBP target (<130mm Hg) (n = 1,471)a,* | Higher SBP target (130–149mm Hg) (n = 1,479)a,* | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Always active (n = 1,136) | Ever inactive (n = 286) | Ever FAAT (n = 49) | Overall | Always active (n = 1,114) | Ever inactive (n = 303) | Ever FAAT (n = 62) | |
| ≤25%, no. (%) | 126 (8.6) | 44 (3.9) | 54 (18.9) | 28 (57.1) | 150 (10.1) | 84 (7.5) | 43 (14.2) | 23 (37.1) |
| >25%–50%, no. (%) | 140 (9.5) | 77 (6.8) | 47 (16.4) | 16 (32.7) | 288 (19.5) | 182 (16.3) | 88 (29.0) | 18 (29.0) |
| >50%–75%, no. (%) | 447 (30.4) | 349 (30.7) | 93 (32.5) | 5 (10.2) | 600 (40.6) | 479 (43.0) | 104 (34.3) | 17 (27.4) |
| >75%, no. (%) | 758 (51.5) | 666 (58.6) | 92 (32.2) | 441 (29.8) | 369 (33.1) | 68 (22.4) | 4 (6.5) | |
Abbreviation: FAAT, failure to achieve assigned target.
a There were 37 subjects who had only a baseline visit (22 subjects in the lower SBP group and 15 in the higher SBP group) and 33 participants who did not have valid SBP measurements at their quarterly follow-ups (17 in the higher group and 15 in the lower group).
*P < 0.0001
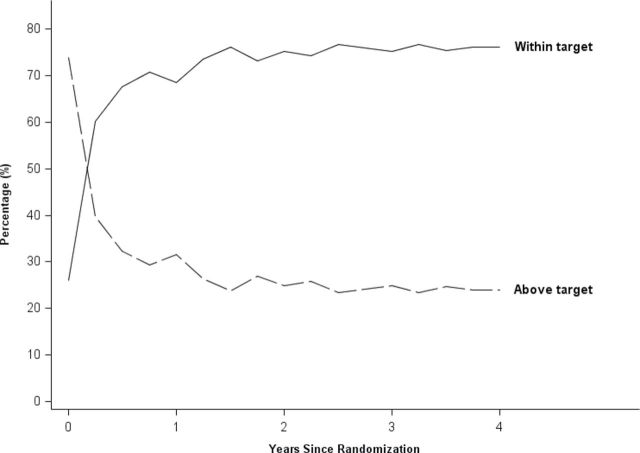
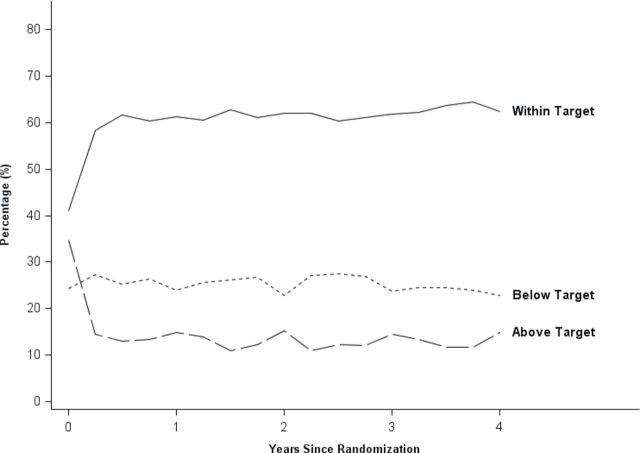
Figures 2 and 3 show the percentage of participants with SBP in target (group percentage and does not necessarily include the same individuals at each point in time) over the course of the study. For the lower SBP target group, the percentage within their assigned target increased during the initial 3–6 months, with continued improvement over the first 2 years of the study. The largest increase in the higher group was in the initial 6 months, and thereafter there was little change in percentage in target.
Figure 2.
Percentage of patients with systolic blood pressure (SBP) within target (<130mm Hg) and above target across the study in the lower SBP target group. Note that the figure includes the 10.3% who were classified as normotensive at baseline.
Figure 3.
Percentage of patients with systolic blood pressure (SBP) within target (130–149mm Hg), above target, and below target across the study in the higher SBP target group. Note that the figure includes the 10.3% who were classified as normotensive at baseline.
Participants in both groups used an average of 1.7±1.2 antihypertensive medications at baseline, which increased to a mean of 2.4±1.4 in the lower SBP target group and 1.8±1.4 in the higher SBP target group by the end-study visit (Table 3). As expected, participants in the lower group used more medications in all classes than the higher group. After year 1, percentages were relatively stable for both groups, with small fluctuations.
Table 3.
Antihypertensive medications across study follow-up
| Variable | Lower SBP target (<130mm Hg) | Higher SBP target (130–149mm Hg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year 1 | Year 2 | Year 3 | End of study | Baseline | Year 1 | Year 2 | Year 3 | End of study | |
| Mean number ± SD of medications | 1.7±1.2 | 2.4±1.3 | 2.5±1.3 | 2.5±1.3 | 2.4±1.4 | 1.7±1.2 | 1.8±1.4 | 1.8±1.4 | 1.8±1.4 | 1.8±1.4 |
| Diuretic, % | 35.7 | 65.6 | 68.7 | 70.8 | 64.3 | 36.8 | 48.1 | 48.7 | 51.2 | 45.9 |
| Beta-blocker, % | 25.6 | 30.7 | 33.5 | 34.9 | 34.9 | 24.1 | 25.0 | 25.4 | 25.8 | 28.3 |
| Calcium-channel blocker, % | 25.8 | 43.1 | 47.5 | 45.5 | 43.2 | 25.6 | 29.8 | 28.8 | 28.0 | 29.3 |
| ACEi, % | 53.3 | 52.0 | 51.3 | 49.6 | 47.5 | 51.2 | 40.8 | 39.7 | 39.7 | 38.7 |
| ARB, % | 15.3 | 30.6 | 32.6 | 35.0 | 33.6 | 16.6 | 23.5 | 23.5 | 23.9 | 23.9 |
| ACEi or ARB/ diuretic, % | 60.8 | 72.9 | 74.4 | 74.1 | 70.7 | 59.5 | 54.5 | 53.7 | 53.6 | 51.8 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; SBP, systolic blood pressure.
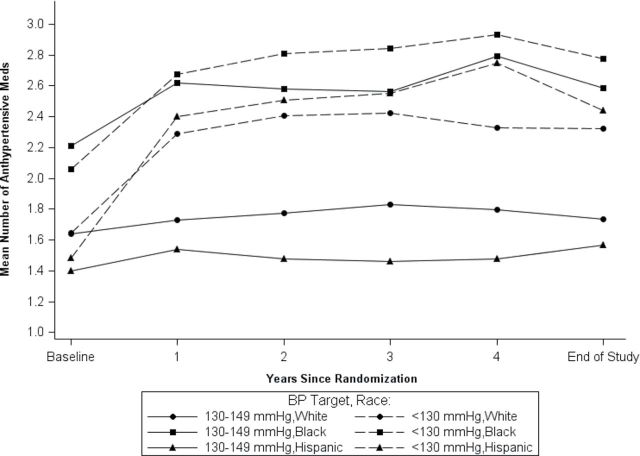
Examination of the achieved SBPs by race/ethnicity (Table 4) shows an increase in the percentage in target from baseline to end-study visit for both groups. This pattern was the same regardless of race/ethnicity. Although the percentage with SBP ≥150mm Hg in the lower group was similar for Hispanics and blacks at baseline (35.9% and 34.9%, respectively), this decreased by end-study visit to 5.3% in Hispanics compared with 13.3% in blacks. Black participants also had the highest proportion of SBP ≥150mm Hg in the higher group at end-study visit (21.1%), as compared with whites (11.8%) and Hispanics (14.8%). The higher SBP readings for black participants were despite a similar mean number of BP visits compared with white and Hispanic participants. Although blacks were prescribed, on average, a higher mean number of medications throughout the study (Figure 4), including higher percentages of diuretics and calcium-channel blockers compared with whites and Hispanics (data not shown), the percentage increase in medications between baseline and the end of the study in the lower SBP target group was lower for black participants compared with white and Hispanic participants.
Table 4.
Baseline and achieved systolic blood pressures by race/ethnicitya
| Time period | Lower SBP target | Higher SBP target | |||||
|---|---|---|---|---|---|---|---|
| <130 | 130–149 | ≥150 | <130 | 130–149 | ≥150 | ||
| Baseline SBP, no. (%) | Overall | 379 (25.8) | 630 (42.9) | 458 (31.2) | 360 (24.3) | 605 (40.9) | 514 (34.8) |
| White | 219 (28.1) | 346 (44.5) | 213 (27.4) | 199 (26.2) | 333 (43.8) | 228 (30.0) | |
| Hispanic | 110 (24.6) | 177 (39.5) | 161 (35.9) | 116 (24.8) | 180 (38.5) | 172 (36.8) | |
| Black | 50 (20.7) | 107 (44.4) | 84 (34.9) | 45 (17.9) | 92 (36.7) | 114 (45.4) | |
| Year 1 SBP, no. (%) | Overall | 892 (68.6) | 326 (25.1) | 83 (6.4) | 319 (23.9) | 816 (61.3) | 197 (14.8) |
| White | 458 (67.2) | 183 (26.8) | 41 (6.0) | 186 (27.2) | 409 (59.7) | 90 (13.1) | |
| Hispanic | 303 (73.2) | 85 (20.5) | 26 (6.3) | 95 (21.7) | 287 (65.5) | 56 (12.8) | |
| Black | 131 (63.9) | 58 (28.3) | 16 (7.8) | 38 (18.2) | 120 (57.4) | 51 (24.4) | |
| End-of-study SBP, no. (%) | Overall | 863 (70.4) | 279 (22.8) | 84 (6.9) | 339 (28.0) | 697 (57.6) | 174 (14.4) |
| White | 438 (69.4) | 156 (24.7) | 37 (5.9) | 177 (29.8) | 346 (58.3) | 70 (11.8) | |
| Hispanic | 312 (78.2) | 66 (16.5) | 21 (5.3) | 114 (27.3) | 242 (57.9) | 62 (14.8) | |
| Black | 113 (57.7) | 57 (29.1) | 26 (13.3) | 48 (24.1) | 109 (54.8) | 42 (21.1) | |
Abbreviation: SBP, systolic blood pressure.
a Because of the small numbers and heterogeneity of the group, the table excludes 74 participants who reported their race as American Indian/Alaskan Native, Asian/Pacific Islander, or Other.
Figure 4.
Mean number of antihypertensive medications over time by race/ethnicity.
DISCUSSION
This article examined SBP management in the 3,020 participants of the SPS3 trial over the course of the study. The main finding is that SBP can be safely lowered and the decrease sustained over time in most patients with established cerebrovascular disease. There were no differences by treatment group in cardiovascular events or adverse events potentially related to lowering SBP.23 These results also suggest the importance of aiming low to achieve good control. Although more than one-third of participants had SBP ≥150mm Hg at study entry, the proportion with SBP ≥150 mmHg was 50% less in the lower SBP target group (7%) compared with the higher SBP target group (15%). Although not a goal for the SPS3 study, the percentage with SBP <140mm Hg was 85% in the lower group and only 58% in the higher group by end-study visit. Although investigators could aim for SBP <140mm Hg in the higher group (130–149 mmHg), it appears that the intensive effort required to reduce SBP to <130mm Hg in the lower SBP target group was important for the higher percentage of control in this group. The implication for practice is that to achieve a high percentage of patients with SBP <140mm Hg, it may be necessary to target SBP management at a lower level, such as 130mm Hg, rather than focusing on the goal of 140mm Hg.
In line with the protocol, BP treatment began with randomization, with patients achieving assigned targets, on average, by the 6-month follow-up. Over the remaining mean 3.7 years of follow-up, the initial gains in the percentage in target were sustained, irrespective of race/ethnicity. An earlier examination of achieved BP control in this cohort with a subset who had completed 1 year of follow-up showed that those patients who were in target at 6 months were 2.4 times more likely to be in target at 1 year compared with the group that had not achieved target.24 The results presented here suggest that this holds true for the duration of the study. Similarly, ALLHAT reported the largest gains in percentage in target occurred early in the trial, with continued improvement over time to 65.6% in target at year 5.25 More recently, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial also showed the biggest gains in achieving target were in the initial 6 months, sustained over the course of the trial.26 Taken together, these results underscore the importance of early and intensive BP management.
Examination of baseline BP in this cohort before randomization and SPS3 study interventions demonstrated that >50% of participants had uncontrolled SBP at approximately 3 months after stroke and almost 20% had baseline SBP values consistent with stage 2 (≥160mm Hg) hypertension despite treatment.9 After 1 year of participation in the trial, however, approximately two-thirds were within their assigned target. Patients, systems including access to care, and providers have been proposed as barriers to BP control. The SPS3 protocol was designed to overcome these barriers in a way that could be reproducible in clinical practice. The SPS3 protocol was a bundle that included a focus on accurate measurement of BP, attention to patient adherence to prescribed treatment at each clinic visit, and timely titration and medication intensification as needed, with an algorithm to guide practice. Furthermore, sites were provided with regular feedback about the proportion of patients in target and comparison with other sites along with conference calls to the sites to discuss difficult cases. A number of studies have recommended a protocol-based strategy along with clinical monitoring and regular clinician feedback for achieving BP control.27–30 In a cluster randomized controlled trial conducted over a 16-week period in general practice, Godwin and colleagues29 reported a significant improvement in BP control at 12 months using a protocol-based strategy for BP control. Similar to SPS3, there was flexibility with regard to the combination of medications used for individual patients, designed to reach their achieved target and to minimize side effects. An adapted version of the SPS3 protocol has already shown effectiveness in reducing SBP in another stroke prevention trial.31
The disparity in prevalence and control of hypertension among black participants reported here is consistent with previous reports of poorer control in this population.32–34 The ALLHAT study showed a lower hypertension control rate in black participants after 4 years of follow-up, with approximately 60% achieving SBP < 140 mmHg in contrast with 68% in white participants.35 This is despite a standardized rigorous protocol that involved treating participants to a target, similar to SPS3. Although black participants were taking a higher mean number of medications than whites or Hispanics at both study entry and across follow-ups, the lower control rates seen here may suggest that a more intensive medication regimen was needed. Medication use in Hispanics increased by 0.9 mean medications from baseline to end-study, and SBP ≥150 mmHg decreased from 36% to 5% in the lower SBP target group. For blacks, the increase was 0.7 mean medications, and SBP ≥150mm Hg decreased from 35% to 13% in the lower SBP target group. Adherence as measured by missing visits and inactive status was not different for blacks.
A challenging aspect of the trial was managing those patients in the higher group (130–149mm Hg) who were below target. The protocol mandated a reduction of dose or number of antihypertensive medications for those patients with SBP <130mm Hg unless there were clinical contraindications such as non-BP management indications. We saw small increases in the proportion with SBP <130mm Hg between baseline and the end-study visit, regardless of race/ethnicity. These findings demonstrate the difficulty of delivering an intervention when there may not be equipoise in the community.
The high rate of controlled hypertension that was achieved is within the context of a clinical trial. Features that are common to clinical trials, such as a high level of commitment of the participants and the close follow-up by research coordinators, may suggest that the control seen here is better than what could be expected in clinical practice. The BP protocol was designed, however, to be generalizable to clinical practice. It was based on published guidelines,16,17 and medications used in the trial are those commonly available. Although medications were made available to participants, the cost to patients would be relatively low because we used mainly generics in our formulary. Furthermore, the study included participants from 8 countries and from 81 sites reflecting a variety of academic and private practices and may be representative of those patients with recent lacunar stroke.
In summary, these results show that it is possible to lower BP in a variety of settings, including an international context. By the end of the study, more than two-thirds of participants were within their assigned target. Importantly there was a significant decrease in the percentage with SBP ≥150mm Hg in both target groups. Without significant changes in primary and secondary prevention practices, the prevalence and costs of stroke are anticipated to increase substantially.36 Protocols such as that used in SPS3 have been shown to be effective in lowering BP, further supporting the evidence for protocol-driven care in lowering BP control28 and, consequently, reducing the burden of stroke.1–5
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENT
The SPS3 work was funded by the National Institute of Neurological Disorders and Stroke (NINDS # 2 U01 NS38529-04A1).
REFERENCES
- 1. PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischemic attack. Lancet 2001; 358:1033–1041 [DOI] [PubMed] [Google Scholar]
- 2. Gueyffier F, Boissel JP, Boutitie F, Pocock S, Coope J, Cutler J, Ekbom T, Fagard R, Friedman L, Kerlikowske K, Perry M, Prineas R, Schron E. Effect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data ANalysis of Antihypertensive intervention trials) Project Collaborators. Stroke 1997; 28:2557–2562 [DOI] [PubMed] [Google Scholar]
- 3. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003; 34:2741–2748 [DOI] [PubMed] [Google Scholar]
- 5. Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet 2011; 377:1681–1692 [DOI] [PubMed] [Google Scholar]
- 6. Brenner DA, Zweifler RM, Gomez CR, Kissela BM, Levine D, Howard G, Coull B, Howard VJ. Awareness, treatment, and control of vascular risk factors among stroke survivors. J Stroke Cerebrovasc Dis 2010; 19:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dawes M. Why is controlling blood pressure after stroke so difficult? CMAJ 2013; 185:11–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roumie CL, Ofner S, Ross JS, Arling G, Williams LS, Ordin DL, Bravata DM. Prevalence of inadequate blood pressure control among veterans after acute ischemic stroke hospitalization: a retrospective cohort. Circ Cardiovasc Qual Outcomes 2011; 4:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White CL, Pergola PE, Szychowski JM, Talbert R, Cervantes-Arriaga A, Clark HD, Del Brutto OH, Godoy IE, Hill MD, Pelegrí A, Sussman CR, Taylor AA, Valdivia J, Anderson DC, Conwit R, Benavente OR. Blood pressure after recent stroke: baseline findings from the Secondary Prevention of Small Subcortical Strokes trial. Am J Hypertens 2013; 26:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003 [DOI] [PubMed] [Google Scholar]
- 11. Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366:895–906 [DOI] [PubMed] [Google Scholar]
- 12. Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004; 363:2022–2031 [DOI] [PubMed] [Google Scholar]
- 13. Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente M-F, Coffey C, McClure LA, Szychowski JM, Conwit R, Heberling PA, Howard G, Bazan C, Vidal-Pergola G, Talbert R, Hart RG. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke 2011; 6:164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White CL, Szychowski JM, Roldan A, Benavente M-F, Pretell EJ, Del Brutto OH, Kase CS, Arauz A, Meyer BC, Meissner I, Demaerschalk BM, McClure LA, Coffey CS, Pearce LA, Conwit R, Irby LH, Peri K, Pergola PE, Hart RG, Benavente OR. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. J Stroke Cerebrovasc Dis 2013; 22:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher CM. Lacunar infarcts—a review. Cerebrovasc Dis 1991; 1:311–320 [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JLJ, Jones DW, Materson BJ, Oparil S, Wright JTJ, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2571 [DOI] [PubMed] [Google Scholar]
- 17. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161 [DOI] [PubMed] [Google Scholar]
- 18. Pergola PE, White CL, Graves JW, Coffey CS, Tonarelli SB, Hart RG, Benavente OR, Reliability and validity of blood pressure measurement in the Secondary Prevention of Small Subcortical Strokes study. Blood Press Monitoring 2007; 12:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The ALLHAT Officers and Coordinators for the ALLHAT Collabortive Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2000; 283:1967–1975 [PubMed] [Google Scholar]
- 20. Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004; 364:1684–1649 [DOI] [PubMed] [Google Scholar]
- 21. Whelton PK, Barzilay J, Cushman WC, Davis BR, Iiamathi E, Kostis JB, Leenen FH, Louis GT, Margolis KL, Mathis DE, Moloo J, Nwachuku C, Panebianco D, Parish DC, Pressel S, Simmons DL, Thadani U. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Int Med 2005; 165:1401–1409 [DOI] [PubMed] [Google Scholar]
- 22. Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 1957; 2:200–215 [DOI] [PubMed] [Google Scholar]
- 23. The SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graves JW, White CL, Szychowski JM, Pergola PE, Benavente OR, Coffey CS, Hornung LN, Hart RG. Predictors of lowering SBP to assigned targets at 12 months in the Secondary Prevention of Small Subcortical Strokes study. J Hypertens 2012; 30:1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT, Jr., Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT). J Clin Hypertens 2002; 4:393–404 [DOI] [PubMed] [Google Scholar]
- 26. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. New Engl J Med 2010; 362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egan BM, Laken MA, Shaun Wagner C, Mack SS, Seymour-Edwards K, Dodson J, Zhao Y, Lackland DT. Impacting population cardiovascular health through a community-based practice network: update on an ASH-supported collaborative. J Clin Hypertens 2011; 13:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frieden TR, King SMC, Wright JS. Protocol-based treatment of hypertension: a critical step on the pathway to progress. JAMA 2014; 311:21–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Godwin M, Birtwhistle R, Seguin R, Lam M, Casson I, Delva D, MacDonald S. Effectiveness of a protocol-based strategy for achieving better blood pressure control in general practice. Fam Pract 2010; 27:55–61 [DOI] [PubMed] [Google Scholar]
- 30. Walsh JME, McDonald KM, Shojania KG, Sundaram V, Nayak S, Lewis R, Owens DK, Goldstein MK. Quality improvement strategies for hypertension management: A systematic review. Med Care 2006; 44:646–657 [DOI] [PubMed] [Google Scholar]
- 31. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, Montgomery J, Nizam A, Lane BF, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride Jr GL, Lynch JR, Zaidat OO, Rumboldt Z, Cloft HJ. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Int Med 2005; 165:2098–2104 [DOI] [PubMed] [Google Scholar]
- 33. Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E, Graham A, Howard V. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke 2006; 37:1171–1178 [DOI] [PubMed] [Google Scholar]
- 34. Safford MM, Halanych JH, Lewis CE, Levine D, Houser S, Howard G. Understanding racial disparities in hypertension control: intensity of hypertension medication treatment in the REGARDS study. Ethn Dis 2007; 17:421–426 [PubMed] [Google Scholar]
- 35. Wright JT, Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, Haywood LJ, Leenen FH, Margolis KL, Papademetriou V, Probstfield JL, Whelton PK, Habib GB. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005; 293:1595–1608 [DOI] [PubMed] [Google Scholar]
- 36. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011; 123:933–944 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.