Abstract
Injury to the ACL is a commonly encountered problem in active individuals. Even partial tears of this intra-articular knee ligament lead to biomechanical deficiencies that impair function and stability. Current options for the treatment of partial ACL tears range from nonoperative, conservative management to multiple surgical options, such as: thermal modification, single-bundle repair, complete reconstruction, and reconstruction of the damaged portion of the native ligament. Few studies, if any, have demonstrated any single method for management to be consistently superior, and in many cases patients continue to demonstrate persistent instability and other comorbidities.
The goal of this study is to identify a potential cell source for utilization in the development of a tissue engineered patch that could be implemented in the repair of a partially torn ACL. A novel protocol was developed for the expansion of cells derived from patients undergoing ACL reconstruction. To isolate the cells, minced hACL tissue obtained during ACL reconstruction was digested in a Collagenase solution. Expansion was performed using DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). The cells were then stored at -80 ºC or in liquid nitrogen in a freezing medium consisting of DMSO, FBS and the expansion medium. After thawing, the hACL derived cells were then seeded onto a tissue engineered scaffold, PLAGA (Poly lactic-co-glycolic acid) and control Tissue culture polystyrene (TCPS). After 7 days, SEM was performed to compare cellular adhesion to the PLAGA versus the control TCPS. Cellular morphology was evaluated using immunofluorescence staining. SEM (Scanning Electron Microscope) micrographs demonstrated that cells grew and adhered on both PLAGA and TCPS surfaces and were confluent over the entire surfaces by day 7. Immunofluorescence staining showed normal, non-stressed morphological patterns on both surfaces. This technique is promising for applications in ACL regeneration and reconstruction.
Keywords: Bioengineering, Issue 86, Anterior Cruciate Ligament, Tissue Engineering, hACL derived cells, PLAGA, in vitro expansion, ACL partial tears
Introduction
The anterior cruciate ligament (ACL) is a commonly injured intra-articular ligament of the knee. Approximately 200,000 (ACL) injuries are reported annually in the United States. Over 75% of patients experiencing ACL injury opt for orthopedic reconstructive surgery 1,2,3,4. Surgical intervention is often indicated due to an inherently poor healing potential3. ACL reconstruction is typically accomplished by means of an autograft or allograft tendon. Autograft and allograft represent the gold standard for reconstruction as they boast high success rates, and primary suture repair, the other treatment option, has shown failure rates of up to 94% 5,6,7.
Partial tears of the ACL represent 10% to 28% of all ACL tears8. In a prospective study, Noyes et al. estimated that 50% of patients with partial tears affecting more than half of the ACL progressed to complete ACL insufficiency after non-operative treatment9. Other studies report persistent instability and decreased function with fewer than 30% of patients able to return to their pre-injury activity level 9,10,11,12,13. Treatment options are limited and include conservative modalities, thermal shrinkage of remaining ACL, or ACL reconstruction. Recently, there has been increased interest in augmented primary repair. These techniques use biologics to enhance primary suture repairs14. Recent research has attempted to harvest mesenchymal-like hACL derived stem cells to circumvent graft limitations,31,32 but the validity and efficacy of these cells is still unknown. The ideal cell source for tissue engineering applications seems to be non-mesechymal hACL derived fibroblast cells.
Current research is focused on identifying a suitable matrix material and cell source for the engineered scaffold. It is standard procedure for a torn ACL to be discarded as surgical waste during reconstruction surgery, however, this damaged ligament may be a quality source for the acquisition of cells needed to develop and enhance an ideal tissue engineered ACL replacement. Our lab has developed a protocol for the in vitro expansion of these harvested hACL derived cells. Using an engineered 2D matrix infused with hACL derived cells, we have designed a patch that could potentially augment partial ACL repair and strengthen torn ligaments.
Protocol
1. Surgical Retrieval
Note: An IRB approval was obtained to collect the ACL stump during ACL reconstruction surgery. IRB exemption was given as the ACL stump used were generally discarded as surgical waste. 20 patients were used to collect the ACL stump.
Administer general anesthesia and pre-operative antibiotics as per institution's protocol to the patient.
Apply tourniquet and inflate 100 mg above systolic blood pressure.
Situate the patient in the supine position. Make a horizontal anterolateral incision with knee in 30-45° flexion for a 5 mm portal and insert the arthroscopic trocar and camera into the suprapatellar pouch at 30°.
Perform a routine diagnostic arthroscopy.
Enter a notch and debride the membranous synovium over the torn ACL.
Use a 4.5 mm shaver and ablator to identify the ACL stump.
Use a duckbill straight bitter to collect multiple specimens from the remaining ACL stump.
Maintain the sample in normal saline in a sterile container.
Send the samples to pathology for decoding and final delivery to the laboratory facility.
Recover patient and perform post-operative care as per institution's protocol.
2. Tissue Digestion and hACL Isolation
Transfer the human ACL tissue received from pathology to a Petri dish with sterile saline/PBS (Phosphate buffered saline).
Mince the tissue using sterile scissors into 1-2 mm3 pieces and wash the minced tissue with saline at least 3 times.
Digest the minced tissue with 0.4% Collagenase in DMEM/F-12 (50/50, 1x) medium supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin for 4-6 hr at 37 °C.
Centrifuge the cells at 1,000 x g for 5 min and then resuspend the cells in the medium.
Wash the cells with the medium for 2-3 times and then seed/culture in T-25 flasks for 2-3 days.
3. Cellular Expansion, Freezing and Thawing
Use a light microscope to visualize the cells cultured for 2-3 days in a T-25 flask.
Maintain the cells at 37 °C in the DMEM/F-12 (50/50, 1x) medium supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin (P/S). Note: Change the media when cells show adherence and standard morphology. Use a light microscope to observe the cells.
Wash the confluent cells with PBS at day 7 (~1.3 X 106 cells/flask). Add Trypsin (0.5 g/L) and incubate the cells at 37 °C for 5 min. Add media to suspended cells to neutralize trypsin. Divide the media-cell mixture between three T-25 flasks.
Wash the confluent cells with PBS, add trypsin (0.5 g/L), and resuspend them in freezing medium containing DMSO, FBS and the complete medium (DMEM/F-12 Medium + 10% FBS + 1% P/S) in the ratio 1:2:7.
Store the cryogenic vials at -80 °C if the cells will be used within a month, and in liquid nitrogen if the cells will be stored for longer durations.
Thaw the vials in a 37 °C water bath for reuse. Note: Typically 90-95% cells survive.
4. Fabrication of Tissue Engineered 2-D Poly Lactic-co-glycolic Acid (PLAGA) Scaffold
Dissolve 1 g of PLAGA in 12 ml dichloromethane in a 20 ml scintillation vial and vortex the solution for 8 hr at a constant speed (800 rpm). Note: Detailed description in Gupta et al.15
Transfer the dissolved solution to a glass Petri dish lined with Fluorinated protection paper.
Place the Petri dish under a vacuum hood for 30 min. Leave the Petri dish at -20 °C overnight and then at room temperature. Place the Petri dishes in lyophilizer to ensure complete evaporation of the solvent to obtain thin films of the polymer.
Place the polymer films in a desiccator for 24 hr.
Cut the polymer films into 12 mm diameter circular disks and store them in a desiccator until needed.
5. Scanning Electron Microscopy (SEM)
Wash the cells (50,000 cells/disk) seeded on the control TCPS and the PLAGA scaffold with PBS 7 days subsequent to seeding.
Use 1.5% Glutaraldehyde in 0.1 M Cacodylate buffer to fix the cells and 2.5% OsO4 in 0.1 M Cacodylate buffer for post-fixation.
Wash the fixed cells with 0.1 M Cacodylate buffer. Dry the fixed cells using serial ethanol dehydration (50%, 70%, 80%, 90% and 100%) for 15 min each, and further dry in Hexamethyldisilazane (HMDS) overnight.
Vent the chamber of SEM sputter coating system with button valve and raise the top plate.
Place the dried samples in chamber. Lower the top plate.
Switch the control knob to pump to start evacuating the chamber.
Open argon leak valve. Wait for the vacuum to drop to 0.05 mbar.
Coat the dried samples with a thin layer (0.13 nm) of Gold/Palladium.
Return the argon leak valve to its closed position.
Switch the control knob to off.
Vent the chamber with button valve and raise the top plate.
Remove the samples and store them in a desiccator for 24 hr.
Observe the samples under an scanning electron microscope.
6. Immunofluorescence Staining
Wash the cells (50,000 cells/disk) seeded on the control TCPS and the PLAGA scaffold with PBS 7 days subsequent to seeding.
Fix the cells using 70% ethanol (cold) for 10 min.
Incubate the fixed cells at room temperature with 1% Bovine Serum Albumin (BSA) in PBS having 0.05% Triton X-100 for 20 min.
Immerse the samples in 1% Tween at room temperature for 20 min.
Add monoclonal mouse Anti-β-actin antibody (1:400) and incubate the cells overnight at 4 °C.
Wash the cells with 0.05% Tween. Add secondary antibody (goat anti-mouse F (ab') 2 fragment of IgG conjugated with a fluorescence probe, 1:400) to the cells and incubate for 1 hr at room temperature.
Wash the cells with the PBS. Stain the cells with nuclear stain and mount using 80% Glycerol.
Observe the cells under a confocal microscope.
Representative Results
The working model for surgical retrieval, tissue digestion and isolation of the human Anterior Cruciate Ligament (hACL) derived cells is shown in Figure 1. The cells migrated from the explants and adhered to the T-25 flasks. These cells were cultured for 3 days and then were visualized under a light microscope (Figure 2). A confluent monolayer was obtained by day 7. The presence of healthy, viable cells indicated the successful retrieval and culture of hACL derived cells.
Cellular attachment to the surface of PLAGA and TCPS was determined qualitatively via SEM. Figure 3 exhibits adherence and proliferation of hACL derived cells on each polymer surface. Cellular confluence was observed for PLAGA and TCPS.
Cellular morphology and adhesion analysis was determined via immunofluorescence staining. β-actin staining demonstrated that hACL derived cells adhered to and proliferated upon the surface of both PLAGA and TCPS. Figure 4 exhibits polymeric adherence and extenuates the normal, non-stressed appearance of the hACL cells.
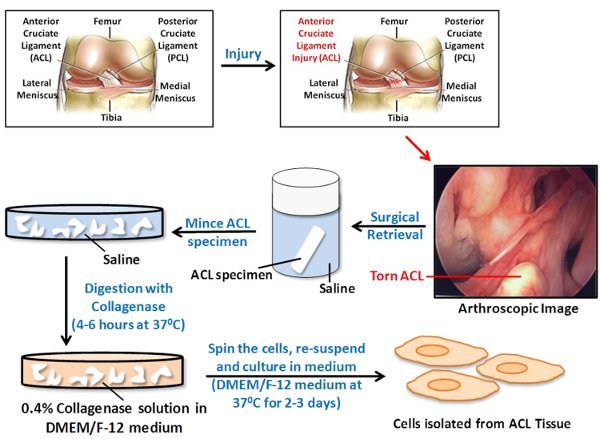 Figure 1. A schematic representation of steps involved in the surgical retrieval, tissue digestion and isolation of cells from injured Anterior Cruciate Ligament (ACL) for tissue engineering applications. During the ACL reconstruction surgery, the remaining (surgical waste) ACL stump is collected and stored in saline. The collected ACL stump is minced into 1-2 mm3 pieces and washed with saline. The minced ACL is digested with 0.4% Collagenase solution in DMEM/F-12 medium. The cells are then centrifuged, resuspended and cultured in DMEM/F-12 medium at 37°C.
Figure 1. A schematic representation of steps involved in the surgical retrieval, tissue digestion and isolation of cells from injured Anterior Cruciate Ligament (ACL) for tissue engineering applications. During the ACL reconstruction surgery, the remaining (surgical waste) ACL stump is collected and stored in saline. The collected ACL stump is minced into 1-2 mm3 pieces and washed with saline. The minced ACL is digested with 0.4% Collagenase solution in DMEM/F-12 medium. The cells are then centrifuged, resuspended and cultured in DMEM/F-12 medium at 37°C.
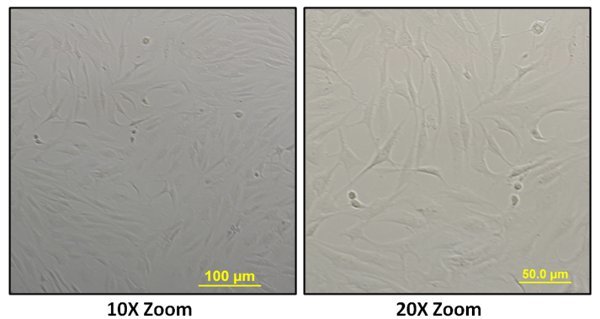 Figure 2. Human Anterior Cruciate Ligament derived cells captured using a light microscope (at 10X and 20X zoom). The spindle or elongated shaped cells were seen growing on the surface of T-25 flasks after 3 days of culture.
Figure 2. Human Anterior Cruciate Ligament derived cells captured using a light microscope (at 10X and 20X zoom). The spindle or elongated shaped cells were seen growing on the surface of T-25 flasks after 3 days of culture.
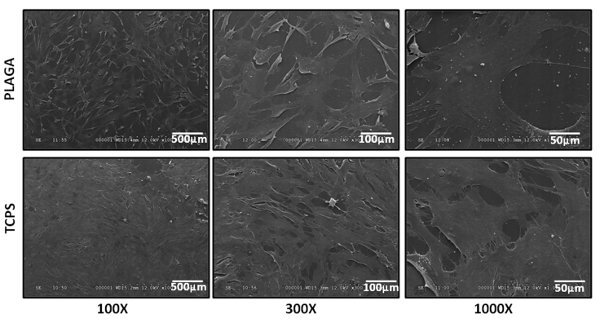 Figure 3. SEM micrographs of human Anterior Cruciate Ligament derived cells cultured on control TCPS and PLAGA (at 100X, 300X and 1,000X magnification). The cells adhered, grew and were confluent over the entire surface of the PLAGA and the control TCPS.
Figure 3. SEM micrographs of human Anterior Cruciate Ligament derived cells cultured on control TCPS and PLAGA (at 100X, 300X and 1,000X magnification). The cells adhered, grew and were confluent over the entire surface of the PLAGA and the control TCPS.
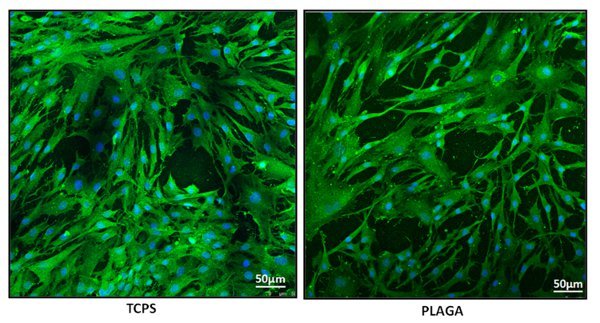 Figure 4. Immunofluorescence staining images captured using a confocal microscope (at 10X 3.1 zoom). Human Anterior Cruciate Ligament derived cells were stained with β-actin (green) and nuclear (blue) dye. The cells adhered, grew and exhibited a normal, non-stressed morphology on both the experimental (PLAGA) and control (TCPS) surfaces.
Figure 4. Immunofluorescence staining images captured using a confocal microscope (at 10X 3.1 zoom). Human Anterior Cruciate Ligament derived cells were stained with β-actin (green) and nuclear (blue) dye. The cells adhered, grew and exhibited a normal, non-stressed morphology on both the experimental (PLAGA) and control (TCPS) surfaces.
Discussion
The primary objective of this hACL/2D scaffold study was to use the obtained cells in a patch to augment primary repair of partial ACL tears. Nonoperative management of partial ACL tears may include a short period of immobilization, bracing, a progressive rehabilitation program, and regular follow-up evaluations 13,16,17. However, many studies show that conservative treatment in athletes has been associated with poor results and failure. Buckley et al. evaluated 25 patients with partial ACL tears at intermediate follow-up and found that only 44% of patients resumed sports at their pre-injury level, and 72% reported activity-related symptoms8. Bak et al. followed 56 patients with isolated partial ACL tears for a mean of 5.3 years and only 30% of patients resumed pre-injury activities10. Fritschy et al. found that 18 of 43 patients with partial ACL tears progressed to complete rupture by 5-year follow-up 11. These studies demonstrate a need for innovative techniques to treat and repair partial ACL tear.
The goal of this study was to develop a protocol that demonstrated a novel technique in which human ACL (hACL) cells were harvested, cultured and exhibited adherence to an engineered scaffold. This technique is a reproducible and reliable way to provide a potential cell source for a wide array of future investigations for ACL reconstruction. Unique to previous studies31,32, the hACL derived cells were obtained from surgical waste and represent non-mesenchymal hACL derived fibroblast cells.
In this study, non-mesenchymal hACL derived cells were isolated from tissue obtained during reconstructive procedures, expanded in vitro and grown on tissue engineered scaffolds. Cellular adhesion and morphology was then performed to confirm biocompatibility on scaffold surface. Future studies must offer further quantitative proliferation analysis, such as real time polymerase chain reaction (PCR) and fluorescence-activated cell sorting (FACS), to characterize the isolated cells. Additionally, quantitative proliferation analysis should be used in order to determine which class of biomaterials the isolated hACL cells grow best upon.
The lack of a quantitative analysis of these hACL cells is a major limitation of this study. However, this is a preliminary study and the goal of this study was to only isolate and expand the hACL derived cells. Future studies will involve cell proliferation studies and characterization of these cells using real time PCR and FACS. Another limitation of this study is that the amount of time required to culture the cells and incorporate them into the patch would require the patient to undergo two surgical procedures. Furthermore, the ability of these human derived ACL cells to survive in synovial fluid has not been established. Future studies must evaluate the ability of hACL to proliferate on a tissue engineered construct in the presence of synovial fluid.
This protocol will allow for repair of a partially torn ACL by means of suture and 2D scaffold. This protocol offers a delivery system for hACL fibroblasts to the wound site and simultaneously protects the cells from the synovial fluid. This could allow functional repair and healing of a partial tear to the ACL, avoiding the comorbidities associated with ACL reconstruction. This technique demonstrates promising results and future investigation will display the potential of this technique for ACL repair and reconstruction.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The authors would like to acknowledge the start-up fund and department of surgery research grant from Southern Illinois University, School of Medicine; and the Memorial Medical Foundation grant.
References
- Johnson RJ. The anterior cruciate ligament: A dilemma in sports medicine. Int J Sports Med. 1982;3:71–79. doi: 10.1055/s-2008-1026066. [DOI] [PubMed] [Google Scholar]
- Owings MF, Kozak LF. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stats. 1998;13:1–119. [PubMed] [Google Scholar]
- Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79:1556–1576. doi: 10.2106/00004623-199710000-00014. [DOI] [PubMed] [Google Scholar]
- Miyasaka KC, Daniel DM, Stone ML, Hirshman P. The incidence of knee ligament injuries in the general population. Am J Knee Surg. 1991;4:3–8. [Google Scholar]
- Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30 year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65–71. doi: 10.1177/0363546508325660. [DOI] [PubMed] [Google Scholar]
- Liljedahl SO, Lindvall N, Wetterfors J. Early diagnosis and treatment of acute ruptures of the anterior cruciate ligament: a clinical and arthrographic study of forty-eight cases. J Bone Joint Surg Am. 1965;47:1503–1513. [PubMed] [Google Scholar]
- Oensten M, Lysholdm J, Gillquist J. Suture of fresh ruptures of the anterior cruciate ligament: A 5 year follow up. Acta Orthop Scan. 1984;55 doi: 10.3109/17453678408992354. [DOI] [PubMed] [Google Scholar]
- Buckley SL, Barrack RL, Alexander AH. The natural history of conservatively treated partial anterior cruciate ligament tears. Am J Sports Med. 1989;17(2):221–225. doi: 10.1177/036354658901700212. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Mooar LA, Moorman CT, 3rd, McGinnis HG. Partial tears of the anterior cruciate ligament. Progression to complete ligament deficiency. J Bone Joint Surg Br. 1989;71(5):825–833. doi: 10.1302/0301-620X.71B5.2584255. [DOI] [PubMed] [Google Scholar]
- Bak K, Scavenius M, Hansen S, Norring K, Jensen KH, Jorgensen U. Isolated partial rupture of the anterior cruciate ligament. Long term followup of 56 cases. Knee Surg Sports Traumatol Arthrosc. 1997;5(2):66–71. doi: 10.1007/s001670050028. [DOI] [PubMed] [Google Scholar]
- Fritschy D, Panoussopoulos A, Wallensten R, Peter R. Can we predict the outcome of a partial rupture of the anterior cruciate ligament? A prospective study of 43 cases. Knee Surg Sports Traumatol Arthrosc. 1997;5(1):2–5. doi: 10.1007/s001670050015. [DOI] [PubMed] [Google Scholar]
- Sommerlath K, Odensten M, Lysholm J. The late course of acute partial anterior cruciate ligament tears. A nine to 15 year follow-up evaluation. Clin Orthop. 1992. pp. 281–2152. [PubMed]
- Barrack RL, Buckley SL, Bruckner JD, Kneisl JS, Alexander AH. Partial versus complete acute anterior cruciate ligament tears: the results of nonoperative treatment. J Bone Joint Surg Br. 1990;72(4):622–624. doi: 10.1302/0301-620X.72B4.2380216. [DOI] [PubMed] [Google Scholar]
- Murray MM. Current status and potential of primary ACL repair. Clin Sports Med. 2009;28(1):51–61. doi: 10.1016/j.csm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, et al. Single walled carbon nanotube composites for bone tissue engineering. J Orthop Res. 2013;9:1374–1381. doi: 10.1002/jor.22379. [DOI] [PubMed] [Google Scholar]
- Fruensgaard S, Johannsen HV. Incomplete ruptures of the anterior cruciate ligament. J Bone Joint Surg Br. 1989;71:526–530. doi: 10.1302/0301-620X.71B3.2722951. [DOI] [PubMed] [Google Scholar]
- Sandberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. J Bone Joint Surg Am. 1987;69:1120–1126. [PubMed] [Google Scholar]
- Lamar DS, Bartolozzi AR, Freedman KB, Nagda SH, Fawcett C. Thermal modification of partial tears of the anterior cruciate ligament. Arthroscopy. 2005;21(7):809–814. doi: 10.1016/j.arthro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Indelli PF, Dillingham MF, Fanton GS, Schurman DJ. Monopolar thermal treatment of symptomatic anterior cruciate ligament instability. Clin Orthop. 2003;407:138–147. doi: 10.1097/00003086-200302000-00021. [DOI] [PubMed] [Google Scholar]
- Smith DB, Carter TR, Johnson DH. High failure rate for electrothermal shrinkage of the lax anterior cruciate ligament: a multicenter follow-up past 2 years. Arthroscopy. 2008;24(6):637–641. doi: 10.1016/j.arthro.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101–1107. doi: 10.1007/s00167-006-0117-7. [DOI] [PubMed] [Google Scholar]
- Sekiya JK, Golladay GJ, Wojtys EM. Autodigestion of a hamstring anterior cruciate ligament autograft following thermal shrinkage: a case report and sentinel of concern. J Bone Joint Surg Am. 2000;82(10):1454–1457. doi: 10.2106/00004623-200010000-00012. [DOI] [PubMed] [Google Scholar]
- Farquharson-Roberts MA, Osborne AH. Partial rupture of the anterior cruciate ligament of the knee. J Bone Joint Surg Br. 1983;65:32–34. doi: 10.1302/0301-620X.65B1.6822597. [DOI] [PubMed] [Google Scholar]
- Rue JP, Ghodadra N, Bach BR., Jr Femoral tunnel placement in single-bundle anterior cruciate ligament reconstruction: a cadaveric study relating transtibial lateralized femoral tunnel position to the anteromedial and posterolateral bundle femoral origins of the anterior cruciate ligament. Am J Sports Med. 2008;36:73–79. doi: 10.1177/0363546507311093. [DOI] [PubMed] [Google Scholar]
- Carey JL, Dunn WR, Dahm DL, Zege SL, Spindler KP. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg. 2009;91:2242–2450. doi: 10.2106/JBJS.I.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979. pp. 279–283. [PubMed]
- Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligamentwith a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- Carter TR. Anterior cruciate ligament thermal shrinkage. Clin Sports Med. 2002;21:693–700. doi: 10.1016/s0278-5919(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Perry JJ, Higgins LD. Anterior and posterior cruciate ligament rupture after thermal treatment. Arthroscopy. 2000;16:732–736. doi: 10.1053/jars.2000.17981. [DOI] [PubMed] [Google Scholar]
- Cheng MT, Yang HW, Chen TH, Lee OK. Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif. 2009;42(4):448–460. doi: 10.1111/j.1365-2184.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, et al. Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cells Dev. 2012;21(6):859–872. doi: 10.1089/scd.2010.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]


