Abstract
Background
Impact of single versus multiple fms-like tyrosine kinase receptor 3 internal tandem duplication (FLT3-ITD) mutations on clinical outcomes of patients with acute myelogenous leukemia is not well studied particularly while simultaneously accounting for the quantitative mutation burden.
Methods
We performed a multi-variate analysis for overall (OS), event free (EFS) and remission duration (CRD) including numerical variation (single versus multiple) and quantitative mutant burden of FLT3-ITD as variables among other clinically relevant variables.
Results
Our analysis of a cohort of 1043 patients with AML showed that among patients with normal karyotype (NK-AML) and FLT3-ITD mutation, overall survival and event-free survival are not affected by the number of FLT3-ITD mutations but remission duration (CRD) is significantly longer in patients with multiple FLT3-ITD mutations (median CRD 86 weeks vs 34 weeks, p=.03).
Conclusion
In time to event analysis of patients with NK-AML and FLT3-ITD mutation, number of mutations and mutant burden should be considered among other factors.
Introduction
Multiple reports have confirmed the adverse impact of fms-like tyrosine kinase internal tandem duplication (FLT3-ITD) mutation on clinical outcomes among patients with acute myelogenous leukemia (AML) and normal karyotype (AML-NK).(1–6) High FLT3-ITD mutant level is associated with a poor outcome while clinical outcomes in patients with low mutant level may be similar to patients without FLT3-ITD mutation.(2;4)
Data on the number (one versus multiple) of FLT3-ITD mutants are conflicting. In a retrospective analysis involving 854 patients with AML (mostly 60 years of age or younger) treated in United Kingdom Medical Research Council (MRC) trials, the number of ITD mutations (0 vs. 1 vs. >2) replaced presence or absence of FLT3-ITD mutation as the most important independent variable predicting for relapse risk (RR) and disease free survival (DFS) in a multi-variate model, the higher mutant number being associated with worse outcome.(3) The number of ITD mutation was the most important factor after cytogenetic risk group, predicting for event-free survival (EFS) and overall survival (OS). Within the group of AML patients with FLT3-ITD mutation, RR was similar among patients with one versus two or more mutations. A later analysis involving a larger cohort of patients from the MRC group showed that quantitative mutation burden is more prognostic than number or size of ITD mutation.(2) Among pediatric patients presence of more than one ITD mutation was not clinically relevant.(7)
Methods
We retrospectively analyzed clinical outcome data on patients with newly diagnosed AML treated at MD Anderson Cancer Center from January 2003 to December 2009. EFS was calculated from the beginning of treatment until an event; defined as relapse, resistance to induction therapy or death. OS was calculated from the time of diagnosis until death. Complete remission duration (CRD) was calculated from CR until relapse or death in CR. Patients were censored at their last follow-up. Categorical and continuous variables were compared by the Fisher’s exact test and Wilcoxon test, respectively. Kaplan-Meier product-limit survival probability estimates of OS, EFS and CRD were calculated and log-rank tests were performed to compare the time to events. FLT3 mutation analysis and quantitation of mutant burden were done from genomic DNA extracted from bone marrow aspirate samples according to published methods.(8;9) Detection of ITD mutation was achieved by fluorescent-based PCR reactions and amplification of the juxta-membrane (JM) domain using fluorescent dye labeled primers that flanks ITD region. PCR product was subjected to fluorescent based capillary electrophoresis using 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
The allelic ratios for FLT3-ITD was calculated as a ratio of area under an ITD/mutant FLT3 peak to the area under total (mutant+wild-type) FLT3. The ratios were adjusted for the number of blasts in the aspirate as judged by a 500-cell manual differential count performed on the bone marrow aspirate smears to obtain blast normalized FLT3 mutation burden (FLT3 normalized).
The Cox proportional hazards regression model was used to evaluate the ability of prognostic variables at presentation including FLT3-ITD normalized mutation burden and number of FLT3-ITD (multiple vs. single) mutations to predict for OS, EFS, and CR duration.
Results and discussion
Data from1043 patients with newly diagnosed AML were analyzed. Five hundred thirty patients (51%) had AML-NK and 93 patients (18%) [59 with single ITD mutation and 34 with ≥2 ITD mutation (21 with 2 ITDs and 13 with 3 ITDs)] had FLT3-ITD mutation. Median FLT3-ITD normalized value was 0.41 (range, 0.01–1.09) and 0.52 (range, 0.02–2.35) for single and multiple ITD mutations respectively. Patient characteristics with single versus multiple FLT3-ITD are summarized in Table 1. Patients with single and multiple (≥2) ITD mutation were comparable in terms of age, performance status (PS), WBC and platelet counts and bone marrow blast percentage. OS (p= .59) (Figure 1A) and EFS (p=.53) were similar among patients with single or multiple ITD mutation.
Table 1.
Characteristics of patients with single versus multiple FLT3-ITD
| FLT3+ITD groups
|
||||
|---|---|---|---|---|
| Single N=59 (63.44%) |
Multiple N=34 (36.52%) |
p-value | ||
| Age (years) | Median (range) | 59 (19, 79) | 59 (17, 84) | .4 |
| WBC × 109/L | Median (range) | 12.9 (1.4, 166) | 17.4 (1, 161.5) | .53 |
| Platelet × 109/L | Median (range) | 54 (7, 302) | 40 (12, 295) | .36 |
| Bone marrow blast% | Median (range) | 72 (8, 95) | 60 (10, 96) | .6 |
| Creatinine (mg/dL) | Median (range) | 0.9 (0.5, 1.7) | 0.9 (0.6, 2) | .6 |
| FLT3-ITD normalized | Median (range) | 0.41 (0.01–1.09) | 0.52 (0.02–2.35 | .02 |
| Performance status | 0 | 11 (64.7%) | 6 (35.3%) | 1.0 |
| 1 | 38 (63.3%) | 22 (36.7%) | ||
| 2/3 | 10 (62.5%) | 6 (37.5%) | ||
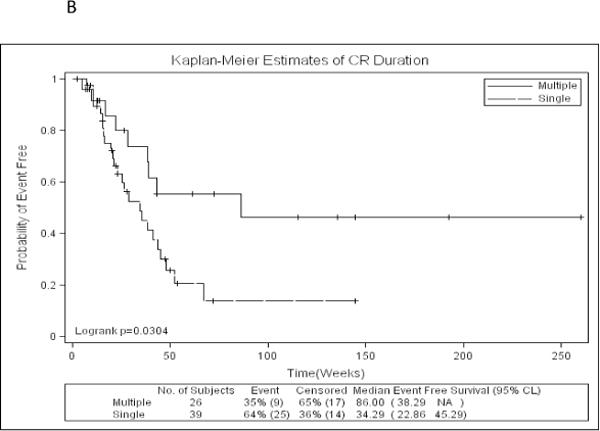
Figure 1.
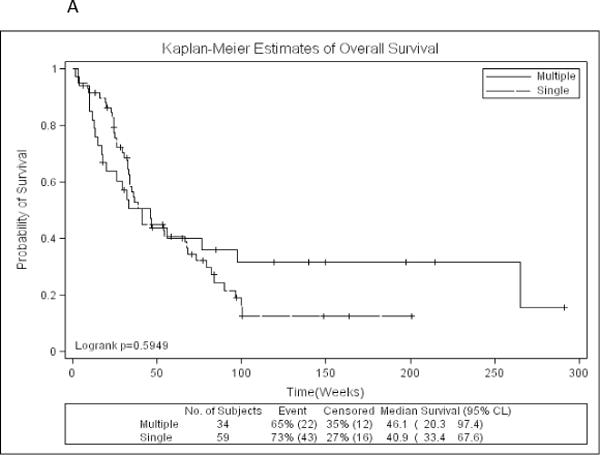
A: Kaplan-Meier estimates of overall survival by number of FLT3-ITD mutations
B: Kaplan-Meier estimates of complete remission duration by number of FLT3-ITD mutations
Of the 93 patients with AML and FLT3-ITD mutation, 65 (70%) achieved complete remission (CR) or CR with incomplete platelet recovery (CRp), CR rate being 66% in the single ITD group and 76% in multiple ITD group. Sixty-eight (83%) of all patients and 56 (86%) of the patients achieving CR were treated with cytarabine based induction. Time to CR was not different between single ITD and multiple ITD group. CRD was significantly longer in patients with multiple FLT3-ITD mutation compared to patients with single FLT3-ITD mutation (median 86 weeks vs. 34 weeks, p=.03) (Figure 1B). Seven (12%) patients with single FLT3-ITD and 2 (6%) patients with multiple FLT3-ITD mutations underwent stem cell transplant (SCT) in first remission. For this analysis, they were not censored at time of SCT. On uni- and multivariate analysis that included age, performance status, WBC and platelet counts among other variables, FLT3-ITD number (single vs. multiple) and FLT3 normalized were the only predictors for CRD (p=.004 and .02 respectively). As high FLT3-ITD mutant burden has been previously reported to be predictive of outcome we wanted to rule out the possibility that the patients with multiple ITD could have had a lower mutant burden (thus explaining the longer CRD). Our analysis on the contrary showed that patients with multiple ITD had a higher mutant burden than the ones with single ITD (p=.02). While older age and higher WBC counts were the predictors for shorter OS (p=.008 and .006 respectively), older age was the only predictor for EFS (p=.04).
While presence of single versus multiple ITDs does not seem to impact OS and EFS, a biological explanation for longer CRD in patients with multiple FLT3 ITD in our cohort is unclear. Multiple ITDs can develop if cells carrying FLT3-ITD mutation in one allele develop different sized ITD mutation in the wild-type allele. Alternately, different leukemic blast populations may carry different sized ITD mutations. Single cell PCR analysis may help to unravel this. Subclones of AML cells with multiple ITDs have been shown to have different engraftment potential in NOD-SCID mice with some clones engrafting and others failing to engraft and disappearing, suggesting that FLT3-ITD mutations can occur at different stages of leukemogenesis and may have differential sensitivity to chemotherapy.(10) Similarly observations of loss of FLT3 mutation at relapse of AML may indicate presence of some mutations in more mature leukemic cells rather than leukemic “stem” cell.(11) While remission rate is not considered to be influenced by presence of FLT3-ITD mutation, the impact of dose intensity on clinical outcomes among patients with FLT3-ITD mutation has not been analyzed in large data sets.
Conclusions
Clinical outcome analysis in patients with AML-NK and FLT3-ITD mutation needs to take in to account mutant allele burden, size and ITD number in addition to the categorical variable of presence and absence of mutation. If all these parameters turn out to be important in analysis of data pooled from large groups, they will need to be incorporated in stratification and randomization of patients in clinical trials involving FLT3 directed therapy.
Acknowledgments
FUNDING STATEMENT:
This article funded by National Institute of Health (P30 CA016672).
Footnotes
Contributions: G.B. collected and analyzed the data, drafted the brief report and provided clinical care to patients; HK, FR, SF, TK, JC coauthored the paper and provided clinical care to patients; SP, KP, RL, WQ, collected data, analyzed results and coauthored the paper; WQ performed statistical analysis and coauthored the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
References
- 1.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 2.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–84. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 3.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 4.Santos FP, Jones D, Qiao W, et al. Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer. 2011;117:2145–55. doi: 10.1002/cncr.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 6.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 7.Meshinchi S, Stirewalt DL, Alonzo TA, et al. Structural and numerical variation of FLT3/ITD in pediatric AML. Blood. 2008;111:4930–3. doi: 10.1182/blood-2008-01-117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Jones D, Medeiros LJ, Luthra R, Lin P. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. Br J Haematol. 2005;130:726–8. doi: 10.1111/j.1365-2141.2005.05666.x. [DOI] [PubMed] [Google Scholar]
- 9.Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Cheung AM, Chow HC, Kwong YL, Liang R, Leung AY. FLT3/internal tandem duplication subclones in acute myeloid leukemia differ in their engraftment potential in NOD/SCID mice. Leuk Res. 2010;34:119–22. doi: 10.1016/j.leukres.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Cloos J, Goemans BF, Hess CJ, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia. 2006;20:1217–20. doi: 10.1038/sj.leu.2404246. [DOI] [PubMed] [Google Scholar]


