Abstract
Tumefactive multiple sclerosis (TMS) is an unusual variant of demyelinating disease. TMS has a variable and unknown progression and presents with features similar to a neoplasm making the determination a diagnostic challenge to clinicians. This report presents one of the very few reported cases of isolated spinal cord TMS, and the second case to describe TMS of the lower spinal cord, given that the lesions are typically cervical. This case study presents a diagnostic approach based on clinical, laboratory, and imaging characteristics, as well as sheds some light on the response to therapy and disease evolution.
Keywords: Tumefactive multiple sclerosis, demyelinating disease, spinal cord, magnetic resonance imaging (MRI)
Introduction
Multiple sclerosis (MS) is the most common demyelinating disorder of the central nervous system (CNS). MS is diagnosed by establishing criteria including evidence of two separate areas of CNS demyelination including the brain, spinal cord, and optic nerves, with evidence the damage occurred at least one month apart, after exclusion of all other etiologies. The clinical and/or radiographic evidence of lesions is noted to be disseminated in both time and space (1,2). When demyelinating disease does not exhibit the classic presentation and its imaging findings are indistinguishable from a neoplasm, it is described as tumefactive multiple sclerosis (TMS) (3). TMS is a rare variant of MS with an estimated incidence of three cases per million individuals per year or one to two cases per 1000 cases of MS (4,5). The majority of previously reported cases in the literature describe intracranial TMS involvement (6). This report presents a unique case of TMS with isolated lower spinal cord involvement.
Case report
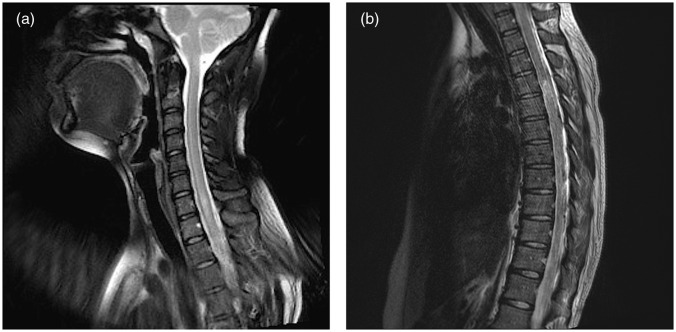
A 31-year-old woman with no significant past medical history had been in her usual state of good health when she presented with acute lumbar pain, bladder incontinence, and gait imbalance. The patient’s symptoms progressed into paraplegia with complete loss of function below the T4 level. Magnetic resonance imaging (MRI) demonstrated abnormal increased signal extending within the lower cervical to the upper thoracic spinal cord (Fig. 1).
Fig. 1.
(a) c-Spine sagittal T2: abnormal increased signal extending from the C6 level inferiorly into the upper thoracic cord; (b) t-spine sagittal T2: mild hyperintensity, faint enhancement, and mild cord expansion involving lower T5 to upper T8.
The patient received high-dose steroids and intravenous immunoglobulin therapy, and underwent physical rehabilitation. Two months later, the patient remained stable with no significant clinical improvement. On repeat MRI, the previously noted diffuse increased cervical and thoracic abnormalities significantly improved, with only small residual foci remaining (Fig. 2).
Fig. 2.
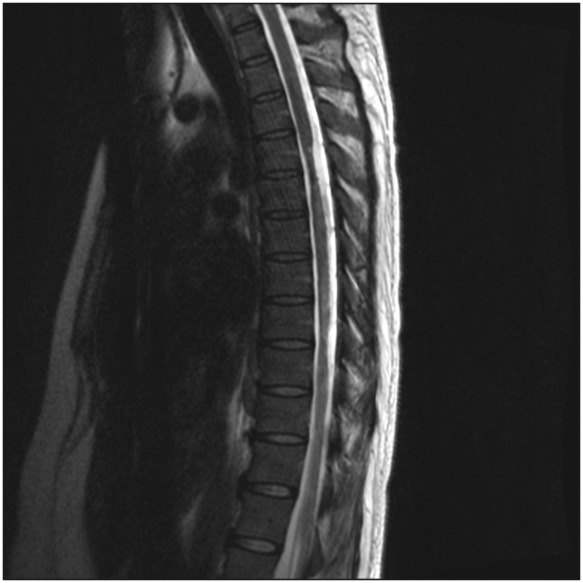
t-Spine sagittal T2: milder hyperintensity at T7 and T8, as well as focal cold atrophy at T4, T5, T8, and T9.
Six months following the initial presentation, the patient had an acute episode of upper thoracic pain and dysesthesias radiating to the ulnar aspect of both upper extremities. MRI of the spine demonstrated marked interval worsening of the previous cord abnormalities (Fig. 3). MRI of the brain revealed no abnormal findings, and spinal angiogram was negative for vascular malformations and dural fistulas. CT of the chest, abdomen, and pelvis showed no evidence of lymphoma. Cerebrospinal fluid (CSF) analysis revealed elevated protein and lymphocytic pleocytosis, with no neuromyeltis optica (NMO) IgG or neoplastic cytology. Autoimmune and infectious serologies including Sjogren, Lupus, Lyme, HIV, HTLV, and hepatitis were all unremarkable. A diagnosis of spinal TMS was established based on the combination of clinical, laboratory, and radiologic findings.
Fig. 3.
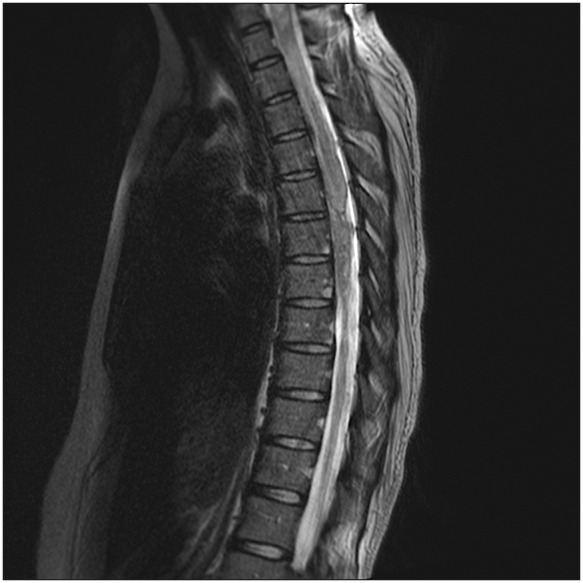
t-Spine sagittal T2: avid abnormal enhancement at T6 to T8, with a non-specific focal exophytic component at T7.
Discussion
TMS is a rare variant of MS, which can mimic a neoplasm, other demyelinating disorders, or even an infection. The current literature of TMS is limited to case reports, with the largest case series to date demonstrating a broad spectrum of radiographic features (7). Unlike typical MS plaques which appear as well-demarcated, homogenous, 5–10 mm ovoid lesions with no mass effect on MRI, imaging features of TMS include at least one lesion 2 cm or larger in diameter with mass effect, edema, and/or incomplete ring enhancement (1,7). Pathologically, TMS is identical to MS, and both demonstrate infiltrating foamy macrophages, reactive gliosis, myelin loss, and relative axonal preservation (8). While being a diagnostic challenge, the treatment of TMS is effective with high-dose steroid therapy being first-line treatment while plasma exchange and mitoxantrone demonstrating promising results (9–12).
The differential diagnosis of TMS lesions should primarily include CNS tumors and other idiopathic inflammatory demyelinating disorders (IIDD). The greatest challenge remains distinguishing TMS from neoplasms. Both disorders can exhibit diffuse enhancement, cord expansion, and edema. However, three observations are key to the diagnosis of TMS: (i) the presence of intracranial periventricular lesions; (ii) a relapsing-remitting course rather than a slow progressive trend; and (iii) response to steroid therapy on follow-up imaging. While this case presentation did not demonstrate intracranial findings, the recurrent progression, multifocality, negative CSF tumor cytology, and response to high-dose steroids established the diagnosis. CNS lymphoma can improve following steroid therapy; however, CSF serology, and chest, abdomen, and pelvic computed tomography (CT) imaging for the presence of lymphoma were negative. Other IIDDs associated with tumefactive demyelination are NMO, acute disseminated encephalomyelitis, acute hemorrhagic leukoencephalomyelitis, Balo concentric sclerosis, Marburg acute MS, and Schilder’s disease (7). While two-thirds of patients presenting with tumefactive demyelinating lesions have TMS, these other disorders should be considered, and are easily ruled out based on disease involvement and progression.
While there appears to be no unique radiologic difference between brain and spinal cord TMS lesions, diagnosis of TMS in the spinal cord is more difficult due to the limited use of perfusion-weighted MRI and spectroscopy in helping distinguish spinal cord demyelination from a neoplasm due to decreased tissue volume compared to the brain (6,13). In both locations, the clinical significance of recognizing TMS arises from sparing the patient from erroneous surgical resection and its concomitant high risk of morbidity, as well as from irradiation which is known to exacerbate demyelinating disease in addition to the risk of radiation necrosis and post-irradiation neoplasia (14). Clinical course, MRI evaluation, serology, and response to therapy are usually sufficient to make the diagnosis, and biopsy remains the gold standard of diagnosis in difficult cases.
In conclusion, TMS is a rare but important condition that should be considered in suspicious spinal cord lesions. It often presents as a diagnostic challenge, but accurate and early diagnosis is key in guiding appropriate treatment and optimal clinical outcomes. In addition to suggestive imaging clues such as relapsing-remitting tumor-like lesions, the total clinical picture should be considered when evaluating a patient for TMS.
Conflict of interest
None declared.
References
- 1.Given CA, Stevens BS, Lee C. The MRI appearance of tumefactive demyelinating lesions. Am J Roentgenol 2004; 182: 195–199 [DOI] [PubMed] [Google Scholar]
- 2.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–127 [DOI] [PubMed] [Google Scholar]
- 3.Dagher AP, Smirniotopoulos J. Tumefactive demyelinating lesions. Neuroradiology 1996; 38: 560–565 [DOI] [PubMed] [Google Scholar]
- 4.Poser S, Luer W, Bruhn H, et al. Acute demyelinating disease. Classification and non-invasive diagnosis. Acta Neurol Scand 1992; 86: 579–585 [DOI] [PubMed] [Google Scholar]
- 5.Paty DW, Oger JJ, Kastrukoff LF, et al. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology 1988; 38: 180–185 [DOI] [PubMed] [Google Scholar]
- 6.Yaghi S, Gokdin M, Sethi H. Tumefactive demyelination of the spinal cord. Acta Neurol Belg 2010; 110: 206–208 [PubMed] [Google Scholar]
- 7.Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 2008; 131: 1759–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paley RJ, Persing JA, Westwater JJ, et al. Multiple sclerosis and brain tumor: a diagnostic challenge. J Emerg Med 1989; 7: 241–244 [DOI] [PubMed] [Google Scholar]
- 9.Kepes JJ. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol 1993; 33: 18–27 [DOI] [PubMed] [Google Scholar]
- 10.Mao-Draayer Y, Braff S, Pendlebury W, et al. Treatment of steroid-unresponsive tumefactive demyelinating disease with plasma exchange. Neurology 2002; 59: 1074–1077 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez M, Karnes WE, Bartleson JD, et al. Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurology 1993; 43: 1100–1104 [DOI] [PubMed] [Google Scholar]
- 12.Jaffe SL, Minagar A. Demyelinating pseudotumor. Arch Neurol 2005; 62: 1466–1467 [DOI] [PubMed] [Google Scholar]
- 13.Haritanti A, Karathanasi E, Potsi S. Tumefactive multiple sclerosis: diagnostic study considering the differential diagnosis from other brain lesions. Aristotle University Medical Journal 2009; 36: 65–69 [Google Scholar]
- 14.Miller RC, Lachance DH, Lucchinetti CF, et al. Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys 2006; 66: 1178–1186 [DOI] [PubMed] [Google Scholar]