Abstract
Introduction
The task of the caregiver, especially a caregiving mother of a son with a chronic and fatal disease, may interfere with their quality of sleep, sexuality, and some hormone levels.
Aim
The aim of this study was to evaluate the sexual function and the quality of sleep of caregiving mothers of sons with Duchenne muscular dystrophy (DMD).
Methods
We evaluated 20 caregiving mothers of sons with DMD and 20 caregiving mothers of sons without any neuromuscular or chronic disease. All of them voluntarily responded to the evaluating questionnaires about their sexuality and their quality of sleep, and gave blood samples to evaluate their hormonal levels.
Main Outcome Measures
All mothers were evaluated using the questionnaire of Female Sexual Function Index (FSFI) and the Pittsburgh questionnaire (PSQI). The blood samples were tested to determine serum levels of testosterone, estradiol, follicle-stimulating hormone, luteinizing hormone, progesterone, adrenocorticotropic hormone, and cortisol.
Results
Caregiving mothers of sons with DMD had significantly lower scores in the FSFI questionnaire, suggesting a higher risk for sexual dysfunction. The PSQI demonstrated that these caregiving mothers present increased sleep latency, reduced sleep efficiency, daytime dysfunction, and poor sleep quality. Blood tests showed a rise in cortisol levels, which correlated with the compromised sexuality and quality of sleep.
Conclusions
This study indicates that caregiving mothers of sons with DMD show major risk for sexual dysfunction and a reduction in their quality of sleep mediated in part by the hormonal changes related to stress. Nozoe KT, Hachul H, Hirotsu C, Polesel DN, Moreira GA, Tufik S, and Andersen ML. The relationship between sexual function and quality of sleep in caregiving mothers of sons with Duchenne muscular dystrophy. Sex Med 2014;2:133–140.
Keywords: Caregivers, Duchenne Muscular Dystrophy, Sleep, Sexuality, Testosterone, Women
Introduction
Several factors may exert considerable effects upon sexual health. It should be noted that sexual health is characterized not only by the absence of any disease or dysfunction, but also by physical, mental, and social well-being related to sexuality [1]. Sleep deprivation (SD) is one major factor that may influence an individual's sex life 2–4. SD is capable of negatively affecting health, compromising several parameters, such as sexual satisfaction [4]. SD can impair the secretory patterns of different steroid hormones. In fact, studies have shown that SD affects concentrations of testosterone, estradiol, progesterone, adrenocorticotropic hormone (ACTH), and cortisol 5–7. Thus, the change of the hormonal profile induced by sleep disturbance could trigger a distinct response in sexual behavior.
It is widely recognized that caregivers of patients with chronic diseases suffer from SD [8,9] and a high demand is placed particularly on the caregiving mothers of sons with chronic diseases such as Duchenne muscular dystrophy (DMD). DMD is a recessive neuromuscular disease linked to the X chromosome. A mutation in the dystrophin gene produces a progressive and irreversible loss of the muscle contractile function. Patients demonstrate severe locomotion, respiration, and cardiovascular impairment [10]. These individuals become dependent on continuous care, which is generally provided by their mothers. Caregiving mothers dedicate a large part of their time caring for their sons, especially during the night as they suffer from movement impairment and need constant changes of position during sleep as well as possible adjustments of their ventilatory support. This leads to chronic SD for these caregiving mothers for an indefinite period of time. This SD may lead to several negative repercussions to their health, including in the reproductive system [11].
Female sexual satisfaction is complex and it is related to several factors, such as biological, emotional, psychosocial, and relationship factors. Caregiving mothers of sons with DMD (MCD group) undergo intense life modifications, following the progression of their sons' illness. Our hypothesis is that caregiving mothers of children with DMD would have a poorer quality of sleep than the study control group (CTRL), and this would lead to changes in hormone levels related to emotions and sexuality, resulting in an impairment in their sexual function. Therefore, the objective of the present study was to test this hypothesis by evaluating the sexual behavior of these caregiving mothers of sons with DMD, while also assessing their quality of sleep and its impact on their hormone levels and sexual life.
Materials and Methods
The present study refers to an observational, cross-sectional study, which was approved by the Ethical Council of Research of the Federal University of São Paulo (CEP #034176/2013) and registered on ClinicalTrials.gov (Identification NCT01921374). The research protocol was explained to the volunteers and their questions answered prior to the signing of the informed consent form by all participants.
Subjects
The sample of caregiving mothers of sons with DMD was recruited in the Center of Treatment of Neuromuscular Diseases, São Paulo, Brazil. These mothers attend the Center regarding the medical and physiotherapeutic care of their children. The CTRL group was constituted by mothers of healthy sons with no neuromuscular or chronic disease. In addition, to avoid interference of conflicting factors in the analysis, this group was selected in a paired fashion with the MCD group, in order to match age and body mass index.
Data Collection
Menstrual status was classified as pre- or postmenopausal. Postmenopausal status was based on the volunteers' responses regarding the date of last menstruation and was associated with the hormonal levels (follicle-stimulating hormone [FSH], luteinizing hormone [LH], progesterone, and estradiol) established through blood tests. Menopause was defined by amenorrhea for a period superior to 1 year, concomitantly with FSH and LH levels higher than 30 mIU/mL [12]. The classification of the premenopausal period was determined by means of the identification of the follicular phase, luteal phase, or amenorrhea state, in parallel with the verification of the FSH and LH levels below 30 mIU/mL. The follicular phase was correspondent to the first 12 days of the menstrual cycle; the luteal phase to the second half of the cycle; and the ovulatory phase to the 15th day of the cycle and the anovulatory phase of amenorrheic women with FSH and LH levels below 30 mIU/mL. Additionally, the volunteers were questioned about the use of any drugs (e.g., antidepressants and contraceptive oral drugs) and about previous illnesses, including depression.
Evaluation of Sexuality and Sleep
The Female Sexual Function Index (FSFI) questionnaire was used to evaluate sexual function. The FSFI evaluates women's sexual activity with her partner in the last 4 weeks. This instrument has been validated for the Portuguese language [13] and is composed of 19 questions, subdivided into 6 domains: desire (items 1 and 2), arousal (items 3–6), lubrication (items 7–10), orgasm (items 11–13), satisfaction (items 14–16), and pain (items 17–19). Each question has a Likert scale that varies from 0 to 5, which is added and multiplied according to each domain. At the end, the sum of all scores obtained in each domain results in a global score. For this questionnaire, the total score values ≤26.55 are correlated with probable risk for sexual dysfunction [14].
For sleep evaluation, we used the Pittsburgh questionnaire (PSQI), which is an instrument composed of 19 questions, subdivided into 7 domains with the purpose of evaluating the subjective quality of sleep, as well as the sleep disturbances occurring during a period of 1 month [15,16]. By means of this questionnaire's total score, it is possible to differentiate “good sleepers” (score ≤ 5) and “poor sleepers” (score > 5).
Hormonal Evaluation
The morning (around 7 am) following completion of the questionnaires, blood was collected for hormone analysis. A blood sample was obtained from the antecubital vein using vacutainer tubes. The samples were centrifuged immediately at 4°C. Serum and plasma aliquots were pipetted and processed for analysis. For all hormone analyses, chemiluminescence was used. Progesterone, LH, FSH, estradiol, and total testosterone levels were measured by using the acridinium ester chemiluminescence method (Advia Centaur, Siemens Healthcare Diagnostics, Deerfield, IL, USA). Cortisol and ACTH were also measured via the adamantyl dioxetane phosphate ester chemiluminescence method (Immulite 2000, Siemens Healthcare Diagnostics).
Statistical Analysis
For all statistical analyses, the SPSS® version 17.0.0 (SPSS, Inc., Chicago, IL, USA) was utilized. The variables were evaluated regarding the normality (Shapiro–Wilk test) and homogeneity (Levene test). When the distribution was demonstrated to be nonparametric, the data were standardized through Z-score. For sleep, sexual function, and hormones, we used the analysis of covariance test adjusted for menopause and contraceptive oral drugs. For clinical data, the t-test was used for comparison. For analysis of frequency, chi-square test was applied. The relationship between testosterone levels and sleep, sexual, and hormonal parameters were evaluated through Pearson correlation test.
The data are presented as mean ± standard error. Statistical significance was set to P ≤ 0.05.
Results
The main demographic characteristics of both groups are described in Table 1, showing that there were no statistical differences between the groups in relation to frequency of contraceptive drugs, as well as the frequency of women in menopause. In total, 20 MCD participated in the study; only one reported having no sexual intercourse, with 19 remaining for the statistical analysis of sexual evaluation. The CTRL group comprised 20 women with children without any neuromuscular or chronic diseases. In this group, only two informed having no sexual intercourse during the last 4 weeks, with 18 CTRL mothers remaining for statistical analysis. The results showed that the MCD group demonstrated impairment in the orgasm and total FSFI scores, suggesting compromised sexual function. As far as sleep was concerned, this group demonstrated impairment in sleep latency, subjective quality of sleep, sleep efficiency, and diurnal dysfunction, resulting in the compromising of the PSQI global score and indicating worse quality of sleep.
Table 1.
Clinical data showing age, body mass index, frequency of contraceptive use, and frequency of menopause between the groups
| CTRL (n = 18) | MCD (n = 19) | P | |
|---|---|---|---|
| Age (years) | 43.9 ± 1.2 | 43.4 ± 1.2 | NS |
| BMI (kg/m2) | 26.1 ± 1.1 | 27.6 ± 1.1 | NS |
| Contraceptive use (%) | 25 | 25 | NS |
| Menopause (%) | 25 | 30 | NS |
Analysis by t-test and chi-square test
BMI = body mass index; CTRL = control mothers; MCD = mothers of sons with Duchenne muscular dystrophy; NS = not significant
The majority of women in our study were married, with an average of age of 43. From the caregiving mothers, 70% were in premenopausal phase and 30% in postmenopausal phase. The CTRL group comprised 75% premenopausal and 25% postmenopausal women (Table 1).
Concerning the sexuality aspects (Table 2), it was observed that DMD caregiving mothers demonstrated higher sexual dysfunction (24.8 ± 1.44 vs. 29.11 ± 1.48, P < 0.05) and orgasm impairment (4.0 ± 0.33 vs. 4.99 ± 0.34, P ≤ 0.05), when compared with the CTRL group.
Table 2.
Adjusted mean ± standard error of sexual function components evaluated by Female Sexual Function Index (FSQI) questionnaire between the groups
| CTRL (n = 18) | MCD (n = 19) | P | |
|---|---|---|---|
| Desire | 3.87 ± 0.25 | 3.44 ± 0.25 | NS |
| Arousal | 4.49 ± 0.33 | 3.73 ± 0.32 | NS |
| Lubrication | 5.07 ± 0.35 | 4.18 ± 0.34 | NS |
| Orgasm | 4.99 ± 0.34 | 4.0 ± 0.33* | 0.05 |
| Satisfaction | 5.27 ± 0.30 | 4.54 ± 0.29 | NS |
| Pain | 5.41 ± 0.25 | 4.89 ± 0.24 | NS |
| Sexual function index | 29.11 ± 1.48 | 24.8 ± 1.44* | <0.05 |
P significant compared with the respective CTRL group
Analysis by ancova adjusted for menopause and contraceptive, with menopause and contraceptive as covariates
ancova = analysis of covariance; CTRL = control mothers; MCD = mothers of sons with Duchenne muscular dystrophy; NS = not significant
In relation to sleep (Table 3), it was also verified that the DMD's caregiving mothers present greater impairment of sleep quality, with alterations in terms of sleep latency (1.54 ± 0.21 vs. 0.76 ± 0.21, P < 0.05), sleep efficiency (0.95 ± 0.22 vs. 0.11 ± 0.23, P < 0.05), daytime dysfunction (0.58 ± 0.15 vs. 0.06 ± 0.15, P < 0.05), and subjective quality of sleep (1.47 ± 0.26 vs. 0.23 ± 0.27, P < 0.01). Indeed, Figure 1 illustrates that there was a higher frequency of poorly sleeping women in the DMD caregiving mothers group as compared with the CTRL group (χ2 = 15.0, degrees of freedom [df] = 1, P < 0.001, n = 37). However, we did not observe a significant association between the sexual function and the quality of sleep (χ2 = 1.4, df = 1, P > 0.05, n = 37), association demonstrating that the reduction in the quality of sleep of the caregiving mother's group was independent of those observed alterations in sexual function and vice versa.
Table 3.
Adjusted mean ± standard error of sleep quality components evaluated by Pittsburgh questionnaire (PSQI) between the groups
| CTRL (n = 18) | MCD (n = 19) | P | |
|---|---|---|---|
| Subjective sleep quality | 0.23 ± 0.27 | 1.47 ± 0.26* | <0.01 |
| Sleep latency | 0.76 ± 0.21 | 1.54 ± 0.21* | ≤0.05 |
| Sleep duration | 0.67 ± 0.18 | 1.15 ± 0.18 | NS |
| Habitual sleep efficiency | 0.11 ± 0.23 | 0.95 ± 0.22* | ≤0.05 |
| Sleep disturbances | 1.33 ± 0.13 | 1.53 ± 0.13 | NS |
| Use of sleeping medication | 0.01 ± 0.15 | 0.37 ± 0.15 | NS |
| Daytime dysfunctional | 0.06 ± 0.15 | 0.58 ± 0.15* | ≤0.05 |
| PSQI global score | 3.15 ± 0.70 | 7.59 ± 0.68* | <0.001 |
P significant compared with the respective CTRL group
Analysis by ancova adjusted for menopause and contraceptive, with menopause and contraceptive as covariates
ancova = analysis of covariance; CTRL = control mothers; MCD = mothers of sons with Duchenne muscular dystrophy; NS = not significant
Figure 1.
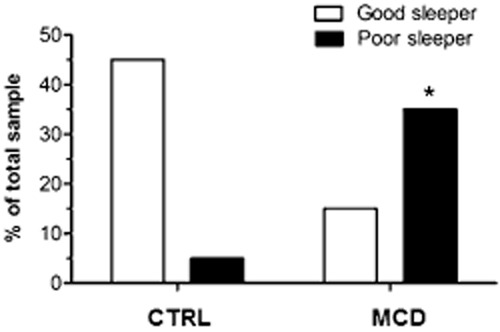
Frequency of poor sleeper and good sleeper in CTRL and MCD groups. Chi-square test (χ2 = 15.0, df = 1, P < 0.001, n = 37). *P < 0.001 compared with the respective CTRL group. CTRL = mothers in the control group; MCD = mothers of sons with DMD.
On the other hand, when the hormonal profiles were analyzed, there were marked differences only in the cortisol levels, with a significant increase in the MCD group in comparison with the CTRL group (15.4 ± 0.9 vs. 12.7 ± 0.97, P ≤ 0.05; Table 4). Results of the Pearson correlation tests examining total testosterone levels, sleep, and sexual and hormonal parameters are shown in Table 5. A significant negative correlation was found between testosterone levels and sleep latency (r = −0.46, P < 0.01, N = 40), PSQI global score (r = −0.44, P < 0.01, N = 40), and ACTH (r = −0.58, P < 0.001, N = 40).
Table 4.
Adjusted mean ± standard error mean of hormonal levels of testosterone, cortisol, and ACTH between the groups
| CTRL (n = 18) | MCD (n = 19) | P | |
|---|---|---|---|
| Testosterone | 28.5 ± 2.8 | 23.6 ± 2.7 | NS |
| ACTH | 34.4 ± 8.1 | 39.3 ± 7.9 | NS |
| Cortisol | 12.7 ± 0.97 | 15.4 ± 0.9* | ≤0.05 |
P significant compared with the respective CTRL group
Analysis by ancova adjusted for menopause and contraceptive, with menopause and contraceptive as covariates
ancova = analysis of covariance; ACTH = adrenocorticotropic hormone; CTRL = control mothers; MCD = mothers of sons with Duchenne muscular dystrophy; NS = not significant
Table 5.
Pearson's coefficient correlation between total testosterone levels and variables of sleep, sexuality, and hormones
| r | P | n | |
|---|---|---|---|
| Sleep latency | −0.46 | <0.01 | 40 |
| Diurnal dysfunction | −0.16 | NS | 40 |
| Sleep medication | 0.03 | NS | 40 |
| Subjective sleep quality | −0.28 | NS | 40 |
| Sleep duration | −0.04 | NS | 40 |
| Habitual sleep efficiency | −0.29 | NS | 40 |
| Sleep disturbances | −0.31 | NS | 40 |
| PSQI global score | −0.44 | <0.01 | 40 |
| Desire | −0.20 | NS | 37 |
| Arousal | −0.08 | NS | 37 |
| Lubrification | −0.18 | NS | 37 |
| Orgasm | −0.08 | NS | 37 |
| Satisfaction | −0.08 | NS | 37 |
| Pain | 0.08 | NS | 37 |
| Sexual function Index | −0.09 | NS | 37 |
| ACTH | −0.58 | <0.001 | 40 |
| Cortisol | −0.16 | NS | 40 |
ACTH = adrenocorticotropic hormone; PSQI = Pittsburgh questionnaire; NS = not significant
Discussion
In this study, caregiving mothers of sons with DMD demonstrated a higher level of impairment in terms of sexuality and quality of sleep when compared with the CTRL group. In particular, in agreement with the FSFI results, these mothers presented higher risk for sexual dysfunction (total FSFI ≤26.55) and orgasm difficulties. Moreover, it was observed that the quality of sleep of these women was affected, as they displayed alterations in their sleep latency and efficiency and were classified as poor sleepers more frequently. Increased cortisol levels were observed in the caregiving mothers and might be related to the sexual dysfunction and the worsening of the quality of sleep of those women, beyond worse sleep due to intense caring responsibilities.
Chronic diseases are not only obviously very stressful for the patient but also for their caregivers. Studies conducted with caregivers of children with chronic diseases have shown a clear association between high levels of stress and the worsening of the quality of sleep [17]. There are several studies with Alzheimer's [18], schizophrenia [19], and cancer patients' caregivers [20] that demonstrated the potential harm to health, such as stress level 21–23. Event stressors can activate the hypothalamic–pituitary–adrenal (HPA) axis, resulting in an increase of cortisol from the adrenal glands. Increased cortisol secretion can compromise the quality of sleep. Moreover, dysfunction of the HPA axis could also be the result of sleep disturbances, such as insomnia. Thus, these two factors might be related to the sleep and sexuality impairment observed in the MCD group, as those women are usually under a considerable stress, not only physically but also emotionally, in addition to the sleep impairment. This is a circular association that one factor reinforces the next one. In support of this point, it should be noted that we found elevated cortisol levels in the MCD group compared with the CTRL group. Another aspect relevant in relation to sleep in caregivers is the use of noninvasive ventilatory support. Previous studies have suggested that caregivers of children who used ventilatory support had compromised sleep in more than 60% of the cases. These caregivers complained of having their sleep disrupted by having to turn on the equipment or to turn off the alarm and having to wake up to verify their son's state throughout the entire night [24]. In this study, all women in the MCD group had sons at similar stages of illness; that is, at the stage where their respiratory tract had already been compromised and were using ventilatory support. Indeed, the worsening of their quality of sleep was demonstrated by decreased sleep latency and efficiency and daytime dysfunction captured in the present study.
Sleep impairment can affect the health, including the immune system [25] and sexual function [11]. In a previous translational study, Andersen et al. [11] demonstrated that rats that displayed loss of sleep showed reduction in their receptive behavior in relation to their sexual activity. In humans, a recent study showed that women who slept from 6 to 9 hours had a significantly more active sexual life and satisfaction compared with women who slept 6 hours or less [26]. Altogether, these data demonstrate the association between sleep and sexuality. Thus, the evaluation and quality of both aspects is very relevant. However, the present study did not find a relationship between sleep and sexual impairment. It is worth emphasizing that a relationship between SD and impairment of sexuality in caregivers is rare in the literature. It is known that SD might trigger the activation of the HPA axis and, in consequence, increase the secretion of cortisol [27], compromising the quality of sleep and sexual function. It should be pointed out that the MCD group had a high cortisol level when compared with the CTRL group. Thus, this observation of reduced sleep quality and impairment sexual function may be the result of other factors, such as stress. In the literature, there is a study demonstrating the negative effect of physical and psychosocial stress upon women's sexuality [28]. It should be pointed out that both acute and chronic stress are capable of inducing female sexual disturbances [29]. Chronic stress in particular can directly compromise female excitation and orgasm [30]. This observation corroborates our findings that caregiving mothers of sons with DMD presented high levels of cortisol that may result from chronic stress as well as self-reported orgasm and sexual dysfunction disorders.
Caregiver mothers are usually under a lot of stress, not only physically but also emotionally. As female sexuality involves markedly emotional aspects [31], it is susceptible to psychological and emotional problems. However, it should be noted that female sexual dysfunction (FSD) is considered a complex condition with multifactorial causes, associated with biological, cultural, psychological, social, and interpersonal factors [32]. Overall, FSD is classified in four categories: hypoactive sexual desire disorder, sexual arousal disorder, orgasmic disorder, and the sexual pain disorder [33]. FSD occurs in women both in premenopausal and postmenopausal stages. A woman's reproductive stage might influence some of the FSD's aspects, such as lubrication difficulties and the resulting pain [34]. However, when evaluating orgasm complaints, this problem is noted in both reproductive phases of women, encompassing approximately 20% of all women in general. The possible explanations for this finding of orgasm complaints in caregiver mothers are related with their function to care of a sick child. For example, Beck and Lopes [35] demonstrated sexual relation disturbances in 80% of caregivers of children with cancer. This observation was related to the lack of time and willingness to perform sexual intercourse. Alternatively, some authors have reported that caregivers declared feeling guilty for feeling pleasure or thinking about sex while their child was sick.
In addition, SD can induce or facilitate the appearance of mood disturbances [36], and this factor may influence the sexuality of these caregivers. Indeed, caregivers' sleep disturbances are the main result of emotional problems [37,38] and include higher latency and sleep fragmentation and reduction of the total time of sleep [39]. Pawl et al. [39] demonstrated that caregivers of children with cancer slept an average of 6 hours, thereby compromising their quality of sleep. In addition, several studies have shown that caregivers of children with epilepsy and diabetes demonstrated sleep disturbances, as well as frequent SD [8]. These observations corroborate the present findings of worse sleep latency and quality of sleep in the MCD group.
In the MCD group, sleep was possibly compromised for exactly the same reason (sleep disturbances for nocturnal care due to the use of noninvasive ventilatory support); once, most of the mothers in this group reported the same complaints. However, we could not confirm this information because the evaluation of sleep has a subjective nature, constituting therefore a real study limitation. It is important to note that this condition may persist for years, compromising not only the sleep but also the health and well-being of these caregiving mothers. Thus, it is fundamental that these caregiving mothers be oriented and followed up by a multidisciplinary team to lead them to a better health condition and maintain the necessary strength to enable them to continuously take good care of their sons, who are totally dependent on them. There are only a few studies with DMD caregivers [40,41] and none examine their sexual function. This study contributes to this field as it provides more significant data in relation to sexuality and quality of sleep of caregiving mothers of sons with DMD.
There are some limitations to this study that need to be addressed. One includes the small simple size. A second is the lack of assessment from the sexual partner and absence of objective assessment. This limitation also applies to the evaluation of psychological stress, such as depression, as the self-report measure is subjective. Depression, for instance, would need to be diagnosed with a clinical evaluation. Similarly, the sleep methods were also subjective (self-report questionnaires) and for this reason, an objective investigation of sleep (polysomnography) and a gynecological medical evaluation (to check and confirm the sexual dysfunction) would be necessary in future studies. However, despite the limitations of this study, we suggest that caregiving mothers of sons with DMD have impaired sleep quality and sexuality.
Conclusions and Future Directions
Taking care of a patient, more specifically, of a son with a chronic condition such as the DMD, may compromise the quality of sleep and sexual function of the caregiving mothers. As the number of informal caregivers and the impact upon them increases, the proper orientation and following up of these caregivers become indispensable in order to allow for better conditions of health and quality of life. Our findings may help to give guidance to caregiving mothers of children with chronic illness. However, additional research is still necessary to evaluate other factors that may influence sexuality and sleep, as these may have a multifactorial origin.
Acknowledgments
This work was supported by grants from Associação Fundo de Incentivo a Pesquisa (AFIP), CAPES, CNPq (M.L. Andersen and S. Tufik are recipients of the CNPq fellowship) and São Paulo Research Foundation (FAPESP) (grant #2012/08587-8 to K.T. Nozoe).
Conflict of Interest
The authors report no conflicts of interest.
Statement of Authorship
Category 1
-
Conception and Design
Monica L. Andersen; Karen T. Nozoe
-
Acquisition of Data
Monica L. Andersen; Sergio Tufik; Gustavo A. Moreira; Karen T. Nozoe
-
Analysis and Interpretation of Data
Monica L. Andersen; Helena Hachul; Sergio Tufik; Gustavo A. Moreira; Karen T. Nozoe; Camila Hirotsu; Daniel N. Polesel
Category 2
-
Drafting the Article
Monica L. Andersen; Helena Hachul; Karen T. Nozoe
-
Revising It for Intellectual Content
Monica L. Andersen; Helena Hachul; Sergio Tufik; Gustavo A. Moreira; Karen T. Nozoe; Camila Hirotsu; Daniel N. Polesel
Category 3
-
Final Approval of the Completed Article
Monica L. Andersen; Helena Hachul; Sergio Tufik; Gustavo A. Moreira; Karen T. Nozoe; Camila Hirotsu; Daniel N. Polesel
References
- 1.Domoney C. Sexual function in women: What is normal? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(suppl 1):S9–17. doi: 10.1007/s00192-009-0841-x. [DOI] [PubMed] [Google Scholar]
- 2.Tufik S, Andersen ML, Bittencourt LR, Mello MT. Paradoxical sleep deprivation: Neurochemical, hormonal and behavioral alterations. Evidence from 30 years of research. An Acad Bras Cienc. 2009;81:521–538. doi: 10.1590/s0001-37652009000300016. [DOI] [PubMed] [Google Scholar]
- 3.Basner M, Rao H, Goel N, Dinges DF. Sleep deprivation and neurobehavioral dynamics. Curr Opin Neurobiol. 2013;23:854–863. doi: 10.1016/j.conb.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen ML, Alvarenga TF, Mazaro-Costa R, Hachul HC, Tufik S. The association of testosterone, sleep, and sexual function in men and women. Brain Res. 2011;1416:80–104. doi: 10.1016/j.brainres.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez-Moctezuma J, Salazar ED, Retana-Marquez S. Effects of short- and long-term REM sleep deprivation on sexual behavior in male rats. Physiol Behav. 1996;59:277–281. doi: 10.1016/0031-9384(95)02127-2. [DOI] [PubMed] [Google Scholar]
- 6.Canchola E, Monroy E, Velazquez-Moctezuma J. REM sleep deprivation facilitates the estrogen effect on heterotypical sexual behavior in male rats. Physiol Behav. 1986;37:33–37. doi: 10.1016/0031-9384(86)90380-x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 8.Cottrell L, Khan A. Impact of childhood epilepsy on maternal sleep and socioemotional functioning. Clin Pediatr (Phila) 2005;44:613–616. doi: 10.1177/000992280504400709. [DOI] [PubMed] [Google Scholar]
- 9.Meltzer LJ, Moore M. Sleep disruptions in parents of children and adolescents with chronic illnesses: Prevalence, causes, and consequences. J Pediatr Psychol. 2008;33:279–291. doi: 10.1093/jpepsy/jsm118. [DOI] [PubMed] [Google Scholar]
- 10.Khan Y, Heckmatt JZ. Obstructive apnoeas in Duchenne muscular dystrophy. Thorax. 1994;49:157–161. doi: 10.1136/thx.49.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen ML, Alvarenga TA, Guindalini C, Perry JC, Silva A, Zager A, Tufik S. Paradoxical sleep deprivation influences sexual behavior in female rats. J Sex Med. 2009;6:2162–2172. doi: 10.1111/j.1743-6109.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- 12.Hachul H, Andersen ML, Bittencourt L, Santos-Silva R, Tufik S. A population-based survey on the influence of the menstrual cycle and the use of hormonal contraceptives on sleep patterns in São Paulo, Brazil. Int J Gynaecol Obstet. 2013;120:137–140. doi: 10.1016/j.ijgo.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D′Agostino R., Jr The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 14.Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. J Sex Marital Ther. 2003;29:39–46. doi: 10.1080/713847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, Miozzo IC, de Barba ME, Barreto SS. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011;12:70–75. doi: 10.1016/j.sleep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer LJ, Mindell JA. Impact of a child's chronic illness on maternal sleep and daytime functioning. Arch Intern Med. 2006;166:1749–1755. doi: 10.1001/archinte.166.16.1749. [DOI] [PubMed] [Google Scholar]
- 18.Richardson TJ, Lee SJ, Berg-Weger M, Grossberg GT. Caregiver health: Health of caregivers of Alzheimer's and other dementia patients. Curr Psychiatry Rep. 2013;15:1–7. doi: 10.1007/s11920-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 19.Awad AG, Voruganti LNP. The burden of schizophrenia on caregivers: A review. Pharmacoeconomics. 2008;26:149–162. doi: 10.2165/00019053-200826020-00005. [DOI] [PubMed] [Google Scholar]
- 20.Girgis A, Lambert S, Johnson C, Waller A, Currow D. Physical, psychosocial, relationship, and economic burden of caring for people with cancer: A review. J Oncol Pract. 2013;9:197–202. doi: 10.1200/JOP.2012.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg S, Morris P, Simmons RJ, Fowler RS, Levison H. Chronic illness in infancy and parenting stress: A comparison of three groups of parents. J Pediatr Psychol. 1990;15:347–358. doi: 10.1093/jpepsy/15.3.347. [DOI] [PubMed] [Google Scholar]
- 22.Roach MA, Orsmond GI, Barratt MS. Mothers and fathers of children with Down syndrome: Parental stress and involvement in childcare. Am J Ment Retard. 1999;104:422–436. doi: 10.1352/0895-8017(1999)104<0422:MAFOCW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Nereo NE, Fee RJ, Hinton VJ. Parental stress in mothers of boys with duchenne muscular dystrophy. J Pediatr Psychol. 2003;28:473–484. doi: 10.1093/jpepsy/jsg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews MM, Nielson DW. Technology dependent children in the home. Pediatr Nurs. 1988;14:111–114. 51. [PubMed] [Google Scholar]
- 25.Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R504–509. doi: 10.1152/ajpregu.00105.2007. [DOI] [PubMed] [Google Scholar]
- 26.Hess R, Conroy MB, Ness R, Bryce CL, Dillon S, Chang CC, Matthews KA. Association of lifestyle and relationship factors with sexual functioning of women during midlife. J Sex Med. 2009;6:1358–1368. doi: 10.1111/j.1743-6109.2009.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lac G, Chamoux A. Elevated salivary cortisol levels as a result of sleep deprivation in a shift worker. Occup Med (Lond) 2003;53:143–145. doi: 10.1093/occmed/kqg028. [DOI] [PubMed] [Google Scholar]
- 28.Bancroft J. The medicalization of female sexual dysfunction: The need for caution. Arch Sex Behav. 2002;31:451–455. doi: 10.1023/a:1019800426980. [DOI] [PubMed] [Google Scholar]
- 29.Ter Kuile MM, Vigeveno D, Laan E. Preliminary evidence that acute and chronic daily psychological stress affect sexual arousal in sexually functional women. Behav Res Ther. 2007;45:2078–2089. doi: 10.1016/j.brat.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Dunn KM, Croft PR, Hackett GI. Association of sexual problems with social, psychological, and physical problems in men and women: A cross sectional population survey. J Epidemiol Community Health. 1999;53:144–148. doi: 10.1136/jech.53.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson RA, Stevenson LD, Rupp HA, Kim S, Janssen E, James TW. Incorporating emotions specific to the sexual response into theories of emotion using the Indiana Sexual and Affective Word Set. Arch Sex Behav. 2011;40:59–78. doi: 10.1007/s10508-010-9669-1. [DOI] [PubMed] [Google Scholar]
- 32.Ghassamia M, Asghari A, Shaeiri MR, Safarinejad MR. Validation of psychometric properties of the Persian version of the Female Sexual Function Index. Urol J. 2013;10:878–885. [PubMed] [Google Scholar]
- 33.Basson R, Berman J, Burnett A, Derogatis L, Ferguson D, Fourcroy J, Goldstein I, Graziottin A, Heiman J, Laan E, Leiblum S, Padma-Nathan H, Rosen R, Segraves K, Segraves RT, Shabsigh R, Sipski M, Wagner G, Whipple B. Report of the international consensus development conference on female sexual dysfunction: Definitions and classifications. J Urol. 2000;163:888–893. [PubMed] [Google Scholar]
- 34.Aslan E, Fynes M. Female sexual dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:293–305. doi: 10.1007/s00192-007-0436-3. [DOI] [PubMed] [Google Scholar]
- 35.Beck ARM, Lopes MHBM. Caregivers of children with cancer: Aspects of life affected by the caregiver role. Rev Bras Enferm. 2007;60:670–675. doi: 10.1590/s0034-71672007000600010. [DOI] [PubMed] [Google Scholar]
- 36.Wehrens SMT, Hampton SM, Kerkhofs M, Skene DJ. Mood, alertness, and performance in response to sleep deprivation and recovery sleep in experienced shiftworkers versus non-shiftworkers. Chronobiol Int. 2012;29:537–548. doi: 10.3109/07420528.2012.675258. [DOI] [PubMed] [Google Scholar]
- 37.Brummett BH, Babyak MA, Siegler IC, Vitaliano PP, Ballard EL, Gwyther LP, Williams RB. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and noncaregivers. Health Psychol. 2006;25:220–225. doi: 10.1037/0278-6133.25.2.220. [DOI] [PubMed] [Google Scholar]
- 38.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: Contributing factors and treatment implications. Sleep Med Rev. 2007;11:143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawl JD, Lee S-Y, Clark PC, Sherwood PR. Sleep characteristics of family caregivers of individuals with a primary malignant brain tumor. Oncol Nurs Forum. 2013;40:171–179. doi: 10.1188/13.ONF.171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baiardini I, Minetti C, Bonifacino S, Porcu A, Klersy C, Petralia P, Balestracci S, Tarchino F, Parodi S, Canonica GW, Braido F. Quality of life in Duchenne muscular dystrophy: The subjective impact on children and parents. J Child Neurol. 2011;26:707–713. doi: 10.1177/0883073810389043. [DOI] [PubMed] [Google Scholar]
- 41.Pangalila RF, van den Bos GA, Stam HJ, van Exel NJ, Brouwer WB, Roebroeck ME. Subjective caregiver burden of parents of adults with Duchenne muscular dystrophy. Disabil Rehabil. 2012;34:988–996. doi: 10.3109/09638288.2011.628738. [DOI] [PubMed] [Google Scholar]


