Abstract
Introduction
Vaginal atrophy, which may affect up to 45% of postmenopausal women, is often associated with one or more urinary symptoms, including urgency, increased frequency, nocturia, dysuria, incontinence, and recurrent urinary tract infection.
Aims
To provide an overview of the current literature regarding cellular and clinical aspects of vaginal atrophy and response to treatment with local vaginal estrogen therapy.
Methods
PubMed searches through February 2012 were conducted using the terms “vaginal atrophy,” “atrophic vaginitis,” and “vulvovaginal atrophy.” Expert opinion was based on review of the relevant scientific and medical literature.
Main Outcome Measure
Genitourinary symptoms and treatment of vaginal atrophy from peer-reviewed published literature.
Results
Typically, a diagnosis of vaginal atrophy is made based on patient-reported symptoms, including genitourinary symptoms, and an examination that reveals signs of the disorder; however, many women are hesitant to report vaginal-related symptoms, primarily because of embarrassment.
Conclusions
Physicians in various disciplines are encouraged to initiate open discussions about vulvovaginal health with postmenopausal women, including recommended treatment options. Goldstein I, Dicks B, Kim NN, and Hartzell R. Multidisciplinary overview of vaginal atrophy and associated genitourinary symptoms in postmenopausal women. Sex Med 2013;1:44–53.
Keywords: Dyspareunia, Estrogen, Lower Urinary Tract Symptoms
Introduction
Vaginal atrophy is a common disorder in postmenopausal women that often occurs with urinary symptoms and causes considerable distress [1–3]. Typically, women with vaginal atrophy experience dryness, itching, irritation, burning, and dyspareunia. They also commonly present with one or more urinary symptoms, including urgency, increased frequency, nocturia, dysuria, incontinence, and recurrent urinary tract infection (RUTI). When any of these symptoms are left untreated, they may contribute to a lower quality of life marked by vaginal discomfort, pain, and sexual dysfunction [1–3].
Nonsexual and urologic aspects of vaginal atrophy have a significant psychosocial effect separate from that of dyspareunia, which has been discussed elsewhere [4,5]. RUTI interrupts daily functioning and reduces libido, which may negatively affect personal relationships and psychosocial health. Additionally, sex is more unpleasant with urinary urgency and the possibility of incontinence because women may experience fear of odor, embarrassment, shame, and loss of self-esteem [6]. Meanwhile, sleep loss from nocturia affects mood and social interactions, which may further affect personal well-being and relationships. Bothersome vulvovaginal symptoms may cause significant personal distress, while related sexual dysfunction may affect a woman's ego and life satisfaction [7]. Symptoms of vaginal atrophy may also remind women that they are aging, and the perception that their bodies are no longer responding or acting as they used to may create anxiety and depression. Furthermore, loss of elasticity to vulvovaginal tissues, thinning of the vaginal wall, and decreased vaginal lubrication may increase the risk for microtears or genital lesions during intercourse, which could allow easier transmission of sexually transmitted infections.
The burden of vaginal atrophy on the individual and on the population is greater than most physicians realize. Up to 45% of postmenopausal women experience symptoms of vaginal atrophy [8,9]. In the recent Vaginal Health: Insights, Views & Attitudes (VIVA) international survey of 3,520 women, the 45% of postmenopausal women who reported vaginal discomfort experienced a range of symptoms, including dryness (83%), dyspareunia (42%), involuntary urination (30%), soreness (27%), itching (26%), burning (14%), and pain (11%) [9]. Despite the high prevalence of such symptoms, only about 25% of affected women seek medical assistance [1]; reasons for not seeking medical help include embarrassment and the belief that their symptoms are an inevitable part of aging [4,8].
Aims
This article aims to provide an overview of the urogenital changes of menopause, the cellular effect of estrogen and its deficiency, the effect of local estrogen therapy on women with urogenital symptoms, the clinical management of vaginal atrophy, and the role various health care professionals (primary care physician, gynecologist, urologist, and sex therapist) can have in recognizing vaginal atrophy as a cause of urinary complaints in postmenopausal women.
Methods
Peer-reviewed publications were identified through a PubMed search using the search terms “vaginal atrophy,” “atrophic vaginitis,” and “vulvovaginal atrophy.” The search was completed through February 2012 and was limited to articles published in English. Relevant articles were identified based upon the expertise and clinical experience of the authors.
Main Outcome Measures
The main outcome measures for this study were genitourinary symptoms and treatment of vaginal atrophy from peer-reviewed published literature.
Results
Urogenital Changes of Menopause
Upon menopause, a decrease in estrogen production by the ovaries affects several of the urogenital tissues, including the vagina, urethra, bladder trigone, and pelvic floor musculature, all of which contain estrogen receptors (ERs) [2]. In the vagina, estrogen maintains the thickness of the muscularis and squamous vaginal epithelium, which is normally characterized by rugae, moisture and a pink color [2]. Estrogen also stimulates vaginal epithelial cells to produce glycogen. As these epithelial cells are shed into the vaginal lumen, glycogen is hydrolyzed to glucose and metabolized by native flora (lactobacilli) into lactic acid, which maintains vaginal pH at approximately 3.5–4.5 [2].
After the menopausal transition, the ovary ceases to synthesize estrogen. The subsequent decline in circulating estrogen results in fewer vaginal epithelial cells and reduced glycogen content per cell. As the main nutritional substrate of microbial flora in the vagina, decreased glycogen results in lower production of acidic metabolites (e.g., lactic acid) and a higher pH in the vaginal lumen. This alkaline milieu further hinders the survival of lactobacilli and permits the overgrowth of other species, including streptococci, staphylococci, coliforms, and diphtheroids. Reduced vaginal estradiol therefore results in an altered microbial environment and predisposes postmenopausal women to vaginal infections and/or RUTIs. Before menopause, reproductive cycling of 17β-estradiol also supports the elasticity of the tissues surrounding the urogenital tract by inhibiting proliferation of connective tissue, fragmentation of elastin, and hyalinization of collagen. After menopause, the reduction in 17β-estradiol leads to a loss of elasticity in the vulvovaginal tissues [2].
Estrogen deficiency is one of many factors, including age, which can influence the development of urinary symptoms, such as urgency, increased frequency, incontinence, and UTI [2,10]. Predisposing factors for lower urinary tract symptoms include history of UTI, vaginal colonization with Escherichia coli, diabetic peripheral neuropathy, hysterectomy, obesity, chronic constipation, and general chronic illness [11]. The effects of estrogen on particular urinary tract symptoms will be reviewed later in this article.
Cellular Effects of Decreased Estrogen
Although estrogen is a major regulator of vaginal epithelial growth, vaginal atrophy is not limited to being a vaginal mucosal condition, as suggested in the previous section. The vagina consists of three tissue layers: the muscularis, the lamina propria, and the vaginal epithelium. All three vaginal wall layers have ERs and are responsive to sex steroid hormones [12]. As discussed in the following paragraphs, ERs are present throughout the lower urinary tract and can regulate bladder smooth muscle contractility, as well as vaginal tissue perfusion, cellular and extracellular composition, overall structure and nerve density [13,14].
Aside from vaginal epithelial health, however, the mechanisms of action of estrogen in the female genitourinary tissues and the clinical effectiveness of estrogen supplementation in vaginal atrophy have been poorly characterized. The clearest evidence to suggest that estrogen regulates the growth and function of vascular and nonvascular smooth muscle in the subepithelial layers of the vagina, the lamina propria, and the muscularis, affecting vaginal wall perfusion and vaginal wall smooth muscle tone, is found in laboratory animal studies [13,14].
In an animal model of estrogen deficiency (ovariectomized rats), autonomic nerve density within the vagina also increased, even after normalizing for tissue atrophy [15]. Overall, the density of nerve fibers increases in estrogen-deficient animals, mostly because of an increase in sympathetic adrenergic, parasympathetic cholinergic, and sensory nociceptor nerves [15]. Systemic estrogen replacement reduces vaginal nerve density to a level comparable to that found in intact and normally cycling rats [15]. Because a major contributor to vaginal moisture is plasma transudate derived from the subepithelial vasculature, increased sympathetic innervation may cause vasoconstriction, leading to vaginal dryness and vaginal wall hypertonus. Furthermore, a greater density of sensory nociceptors may contribute to hypersensitivity and result in symptoms of pain, burning, and itching.
Expression of the ER is also regulated by native 17β-estradiol in the vagina. Estrogen-deficient animals express higher amounts of functional ER protein (specifically the ERα subtype) throughout the three layers of the vaginal wall, and a greater proportion of the receptor is found in the nuclear compartment, presumably as activated receptor [14,16]. This may serve as a homeostatic mechanism to buffer against changes in estrogen levels that may occur during the normal premenopausal ovarian cycle. Severe estrogen deficiency, as occurs in postmenopausal women, overcomes this protective mechanism and results in tissue atrophy.
In human vaginal tissue, ERα messenger RNA (mRNA), which may be used to estimate expression of the ERα protein, increases in postmenopausal women who are not receiving hormone therapy, whereas ERα mRNA in women using systemic hormone replacement therapy decreases to levels similar to those found in premenopausal women [17]. Postmenopausal women prescribed local estrogen therapy maintain elevated levels of vaginal ERα mRNA, presumably because of the absorption of lower levels of estrogen [17]. Therefore, it is not unreasonable to conclude that ERα regulation in humans may be similar to that in laboratory animal models of estrogen deficiency. The reciprocal regulation between circulating estrogen levels and ERα expression may serve as a rationale for using low doses of estrogen in the vagina in postmenopausal women to maximize the effects of local estrogen treatment.
Although expression of ERβ in the vagina has been documented, its role remains unclear. Upregulation of ERβ in women has been associated with various states of disease, including stress urinary incontinence [18] and vulval lichen sclerosus [19], although ERβ also mediates critical processes in normal tissue function and is important for maintaining health. Low ERβ expression in the vaginal tissue has been associated with genital prolapse in postmenopausal women [20], and a complete deficiency of ERβ leads to the onset of ulceration and atrophy in the bladder urothelium of female mice that lack the ERβ gene [21]. The complexity of ERβ signaling remains to be fully elucidated and is likely based on the interplay between multiple isoforms, most of which are not functional receptors but modifiers of receptor activity [22].
In addition to direct trophic effects on urogenital tissues, the association of estrogen withdrawal with increased urinary symptoms results from multiple additional factors. In laboratory studies, estradiol suppresses contraction of the rat detrusor smooth muscle, decreasing spasmodic activity [23], and inhibits the expression of rho-kinase, a key regulator of smooth muscle contraction in urethral smooth muscle cells [24]. Thus, estrogen deficiency can lead to increased contractility of bladder and urethral smooth muscle. Additionally, in healthy women, collagen turnover in urogenital tissue has been shown to increase in response to estradiol treatment [25], which suggests that estrogen deficiency may disrupt normal connective tissue metabolism. It should be emphasized that ERs were not detected in the striated muscle of the pelvic floor in women [26], suggesting that these voluntary muscles are not directly involved in the etiology of urinary symptoms caused by estrogen deficiency.
Estrogen is an important regulator of tissue growth and function in the lower urinary tract; however, testosterone plays an important role as well. Like ERs, the androgen receptor (AR) is expressed throughout the vaginal wall, the bladder, and the urethra [16,27]. Testosterone, independent of estradiol, has been shown to modulate vaginal wall contractility and perfusion and to upregulate AR in rats [13,16,28]. In addition, AR is localized to motoneurons innervating the urethral sphincter and the pubococcygeus muscle [29,30], suggesting that testosterone supplementation regimens may be somewhat effective in ameliorating urinary symptoms and urogenital tissue atrophy [31,32]. Because of the importance of estrogen and testosterone in maintaining vaginal health, intravaginal dehydroepiandrosterone, a precursor sex steroid that is converted directly to various androgens and eventually converted to estradiol in the vaginal epithelium and muscularis [10,33], is under investigation as an alternative to local estradiol therapy [34].
Clinical Effects of Local Estrogen Therapy for Vaginal Atrophy
On the tissue/organ level, exogenous estrogen increases blood flow, epithelial thickness, and secretions while decreasing pH [1]. These changes are reflected in positive clinical signs, including less vulvovaginal pallor and increased moisture. Moreover, patients receiving local vaginal estrogen therapy generally report less itching and irritation and reduced dyspareunia [35]. From a psychosocial perspective, a number of women using local estrogen therapy reported positive effects, including normalization of sex life, better quality of life, improvement in their relationship with their partner, feeling “less old,” higher self-esteem, and a better social life [8].
Generally, local vaginal estrogen products approved for the treatment of vaginal atrophy in the United States are not indicated for the treatment of RUTI, although urinary symptoms often improve after estrogen is restored to the vagina [11]. Significant evidence suggests a positive effect of local vaginal estrogen therapy on RUTI; however, the data supporting oral estrogen therapy are less convincing [36]. Some evidence supports the improvement of overactive bladder and urgency incontinence as a result of local vaginal estrogen therapy. Frequency, urgency, and/or number of incontinent episodes may improve [37], but recent analysis suggests that local treatment rather than systemic treatment is important [38]. Urodynamic parameters, such as number of uninhibited detrusor contractions and maximal cystometric capacity, may also improve [39].
Clinical Management of Vaginal Atrophy
Typically, a diagnosis of vaginal atrophy is made based on patient-reported symptoms and an examination that reveals signs of the disorder (Table 1; Figure 1). The diagnosis may be confirmed by vaginal pH test or vaginal maturation index assessment.
Table 1.
Common patient-reported symptoms and clinical signs of vaginal atrophy
| Vulvovaginal symptoms | Urinary symptoms | Clinical signs |
|---|---|---|
| • Dryness | • Urgency | • Pale, dry vulvar and vaginal mucosae |
| • Itching | • Increased frequency | • Reduction in volume of the labia |
| • Irritation | • Nocturia | • Scarcity of pubic hair |
| • Burning | • Dysuria | • Shortened and narrowed vagina |
| • Dyspareunia | • Incontinence | • Reduced or nonexistent rugae |
| • RUTI | • Erythema indicative of inflammation | |
| • Petechiae | ||
| • Psychosocial signs (e.g., decreased quality of life) |
RUTI = recurrent urinary tract infection
Figure 1.
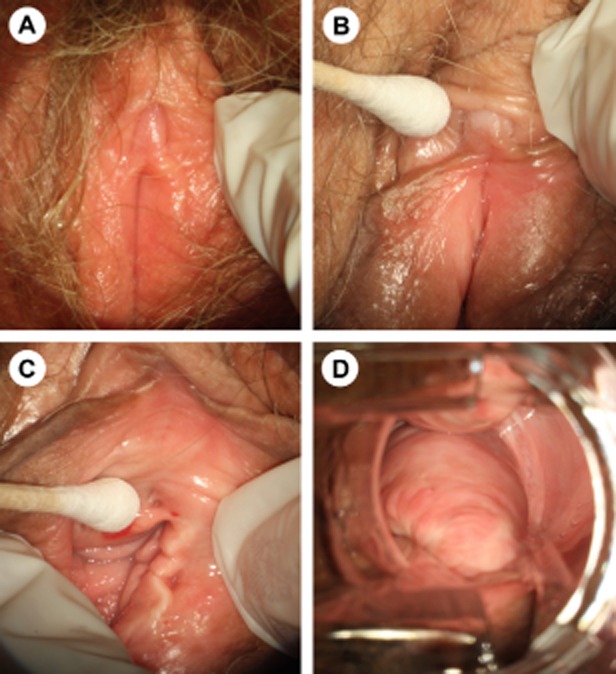
Atrophy of the vulva, clitoris, and vagina. (A) Vaginal atrophy is associated with pale, dry, shiny vulvar tissue and loss of adipose tissue in the labia majora and labia minora. (B) The prepuce and clitoris are often pale and reduced in size, while examination shows that (C) the introitus may be narrowed and friable. (D) In vaginal atrophy, the vaginal walls lack rugae and may be pale and/or erythematous.
First- and Second-Line Treatments
Vaginal atrophy may first be treated by over-the-counter vaginal moisturizers and lubricants. Sexual activity is also recommended, but only if it is comfortable. Some women are advised that continued sexual activity is important, but they are not told that sex should not be painful or uncomfortable. Continuing to engage in sexual activity when it causes significant pain or bother can result in local tissue damage and contribute to further sexual dysfunction. The North American Menopause Society (NAMS) recognizes that first-line treatments (moisturizers, lubricants, and continued sexual activity) are inadequate for some women, particularly those with moderate-to-severe vaginal atrophy [3]. For these cases, NAMS recommends low-dose, local vaginal estrogen therapy, which is effective and well tolerated (Table 2 [40–43]). NAMS recommends that clinical experience and patient preference determine the choice of therapy [3].
Table 2.
Recent clinical trials assessing effectiveness and endometrial safety of low-dose, local vaginal estrogen for treating symptoms of vaginal atrophy in postmenopausal women
| Formulation | Details of study | Relevant N | Efficacy end points and results | Endometrial safety end points and results |
|---|---|---|---|---|
| Vaginal tablet (10 mcg estradiol) | In a 52-week double-blind study, patients were randomly assigned to receive the vaginal tablet or a placebo tablet (2:1 assignment ratio) daily for 2 weeks, then twice weekly (Simon et al. [41]) | 205 (of 309) patients randomly assigned to receive estradiol; 164 (80%) of estradiol group completed; 70 (67%) of placebo group completed | • Vaginal cytology: improved VMI and MV | (analyzed with study below) |
| • Vaginal pH: improved | ||||
| • Most bothersome urogenital symptoms score: improved | ||||
| • Grading of vaginal health (secondary): improved | ||||
| Pooled analysis of above study and 52-week open-label study in which patients received the vaginal tablet daily for 2 weeks, then twice weekly (Simon et al. [42]) | 541 patients using estradiol; 456 patients completed trials; 443 had biopsy at week 52 | N/A | • Endometrial biopsy: two events of hyperplasia or carcinoma in 386 evaluable biopsy samples (0.52% incidence, similar to background) | |
| Vaginal ring | In a 12-month open-label study, patients were randomly assigned to receive the estradiol vaginal ring (releases 7.5 mcg estradiol daily) or a vaginal tablet (25 mcg estradiol, discontinued) daily for 2 weeks, then twice weekly (Weisberg et al. [43]) | 126 patients randomly assigned to receive vaginal ring | • Vaginal cytology: improved VMI and MV | • Endometrial thickness assessed by transvaginal ultrasound: no increase in average endometrial thickness |
| • Symptom-free rates of vaginal dryness and pruritus vulvae: improved | ||||
| • Vaginal burden of condition: improved | • Progestogen challenge test: no bleeding/spotting in vaginal ring group | |||
| • Urinary burden of condition: improved | ||||
| • Frequency of micturition: improved | ||||
| • Grading of vaginal health: improved | ||||
| CE vaginal cream | In the 12-week double-blind phase of this 52-week study, patients were randomly assigned to receive CE vaginal cream (0.3 mg CE) or placebo vaginal cream in a cyclical manner (daily for 21 days, then off for 7 days) or twice weekly. In the 40-week open-label phase, patients received CE vaginal cream according to their previous regimen (Bachmann et al. [40]) | 143 patients randomly assigned to CE cream on cyclic regimen; 140 patients randomly assigned to CE cream on twice-weekly regimen; 155 evaluable biopsies | • Vaginal cytology: improved VMI | • Endometrial thickness assessed by transvaginal ultrasound and endometrial biopsy: no hyperplasia or carcinoma |
| • Vaginal pH: improved | ||||
| • Severity of participant-reported, most bothersome symptom (vaginal dryness, itching, burning, or dyspareunia): improved |
CE = conjugated estrogens; MV = maturation value; N/A = not applicable; VMI = vaginal maturation index
Recently, a new oral treatment was approved in the United States. Ospemifene is an estrogen agonist/antagonist approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to menopause [44].
Formulations of Local Vaginal Estrogen
Four local vaginal estrogen products are approved in the United States for the treatment of vaginal atrophy: the conjugated estrogens cream [45], and an estradiol cream [46], vaginal ring [47], and vaginal tablet [48]. While vaginal estrogen preparations have been available for decades, the recent trend has been to use ultralow doses because this minimizes systemic exposure and associated risks. For example, the estradiol ring, containing a reservoir of 2 mg estradiol, releases approximately 7.5 mcg of estradiol per day [47], while the recommended estradiol vaginal tablet regimen provides even less, with only 10 mcg of estradiol twice a week after the initial 2-week phase of daily dosing has passed [48], and has been shown to have the lowest maximum annual delivered dose of the local vaginal estrogen products [49]. Notably, the 10-mcg estradiol vaginal tablet maintains systemic estradiol concentrations in the normal postmenopausal range [50]. The conjugated estrogens cream is also effective at a low dose: 0.5 g cream (equivalent to 0.3 mg conjugated estrogens) twice a week [40]. Lack of endometrial proliferation in response to low-dose vaginal estrogen treatment [40,42,43] obviates the need for coadministration of progesterone in women with an intact uterus.
A 2006 Cochrane review compared the efficacy of intravaginal estrogenic creams, pessaries, tablets, and rings with one another and with placebo in relieving the symptoms of vaginal atrophy, as well as the safety of each product. Overall, all intravaginal preparations appeared to be effective for the treatment of vaginal atrophy. However, one trial showed significant adverse effects associated with use of the conjugated equine estrogen cream when compared with estradiol tablets; these adverse events included uterine bleeding, breast pain, and perineal pain [35].
Recommendations for Breast Cancer Survivors
In patients with a history of hormone-dependent cancer, an oncologist should be consulted before hormonal treatment. Results of observational studies of use of topical vaginal estrogen in patients with a history of hormone-sensitive breast cancer have been favorable, but the lack of a large randomized trial prevents the making of general recommendations [51]. Although some patients may be comfortable with taking low doses of vaginal estrogen to manage severe vaginal atrophy, others may prefer to use nonhormonal methods [52,53]. Patients with a history of nonhormone-dependent cancer may be treated similarly to patients with no history of cancer.
The Role of Health Care Professionals
Generally, women are hesitant to report vaginal-related symptoms, primarily because of embarrassment [8]. The recent “women's voices in the menopause” survey [8] of 4,246 women 55–65 years of age found that only 30% of women with vaginal discomfort had spoken to a gynecologist about their symptoms and only 29% had spoken with a general practitioner. Strikingly, 30% of women with vaginal discomfort had not spoken to anyone about it, for reasons including, “I do not think other people want to hear about my vaginal problems,” “It makes me uncomfortable/embarrassed,” “It is private and does not concern others,” and “It's just part of growing older” [8]. Nearly one-third (31%) of women with vaginal discomfort expressed the preference that a physician initiate a discussion on the topic [8], which suggests that a similar percentage of women might be receptive to discussing vaginal discomfort and potential treatment options with their health care professional.
Primary Care Physician
In the VIVA study, 50% of women identified their primary care doctor as a source they had or would use for information on vulvovaginal symptoms and/or treatment options [9]. In the Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study, women ≥65 years were more likely to speak to their primary care physician (54%) than their gynecologist (30%) about their sexual problems [54]. Because many women are hesitant to report symptoms without prompting [8], primary care physicians are encouraged to ask postmenopausal women about any discomfort or lower urinary tract symptoms, including a history of RUTIs [55].
Gynecologist
Gynecologists play an important role in identifying vaginal atrophy in postmenopausal women. Routine gynecological exams may help identify symptoms of and diagnose vaginal atrophy. In the VIVA study, 46% of women identified their gynecologist as a source they had used or would use to understand vulvovaginal symptoms and/or treatment options [9]. As many women start experiencing perimenopausal symptoms around age 40, this may be a good time for gynecologists to begin conversations with women about changes in sexual health [56]. It has been recommended that gynecologists add questions about vulvovaginal and urinary symptoms to intake paperwork, directly question patients on symptoms, and reflexively question the patient on vulvovaginal and urinary symptoms based on findings of the physical exam [55].
Urologist
The urologist is in the unique position of being able to provide this advice because women may not feel comfortable visiting a physician for vaginal symptoms alone. The urologist should consider vaginal atrophy as a potential cause of urinary symptoms in a postmenopausal woman. Increased awareness that vaginal atrophy may be the underlying cause of urological symptoms is important for facilitation of proper diagnosis and treatment. Vaginal atrophy is a chronic condition; therefore, the urologist may wish to refer a patient with vaginal atrophy to a gynecologist for long-term management. In most moderate-to-severe cases of vaginal atrophy, prescribing local vaginal estrogen is the quickest and most effective way to relieve symptoms. After as few as 2 weeks of therapy [41], positive urogenital changes may be evident. The urologist may also provide several practical tips to menopausal women with vaginal atrophy. These include regular use of moisturizers; use of lubricants for sexual activity; avoidance of the use of any product near the vulva that may exacerbate symptoms, including scented soaps, lotions, or panty liners; regular sexual activity, as soon as it becomes manageable without pain or discomfort; and sex therapy, as described in the next section, to develop and implement strategies to increase sexual satisfaction and quality of life.
Sex Therapists
The nonphysical ramifications of pain, discomfort, and vaginal dryness during sexual activity may be addressed through sex therapy, a specialized form of counseling or psychotherapy designed to help individuals and couples with sexual problems [57]. Women may experience a variety of emotions as a result of vaginal atrophy, and a therapist can offer support to women struggling with urogenital discomfort, incontinence, aging, body image concerns, or low self-esteem. In couples therapy, a therapist can help the woman and her partner communicate about the changes to her body and how these changes can affect their relationship. The therapist can assist the couple in exploring a variety of sexual activities that may be less irritating to the vagina, such as oral sex, manual stimulation, sensual touch, and the use of vibrators. The therapist can also provide education regarding different types of lubricants (e.g., water based and silicone based) and help the couple find one that is enjoyable and does not irritate the vaginal tissue. The key to helping couples with vaginal atrophy is to devise manageable behavioral changes that will help them rediscover a satisfying sex life.
Women may not be aware that there is help for the nonphysical effects of vaginal atrophy, or they may be too embarrassed to ask for it. Health care professionals might consider directly asking their patients about the impact of vaginal symptoms on their patients' sexual relationships and offer referrals to interested patients.
Conclusions
Vaginal atrophy is a common condition that is under-recognized and undertreated, primarily because physicians and patients typically do not include vulvovaginal health issues in their discussions. For health care professionals, the challenge is to identify female patients whose urinary symptoms may be related to concurrent vaginal atrophy. The first step in making a difference in the quality of life for these women is for health care professionals to be aware that certain urinary complaints may be reflective of postmenopausal estrogen deficiency. Primary care physicians are encouraged to ask postmenopausal women about any discomfort or lower urinary tract symptoms, including a history of RUTIs, and gynecologists are encouraged to add questions about vulvovaginal and urinary symptoms to intake paperwork. The key to improved outcomes for menopausal women is the guidance of physicians who inquire about their nonurinary symptoms, such as vaginal dryness, itching, and dyspareunia, encourage open discussions about vulvovaginal health and recommended treatment options, including local vaginal estrogen therapy. Referral to sex therapists may help address the impact of vaginal atrophy on a woman's personal relationships and psychosocial health.
Acknowledgments
Editorial assistance was provided by Pamela Barendt, PhD, ETHOS Health Communications, Newtown, Pennsylvania, with financial assistance from Novo Nordisk, Inc, Princeton, New Jersey, in compliance with international guidelines on good publication practice. The authors received no remuneration of any kind for the development of this manuscript.
Conflict of Interest
Brian Dicks has nothing to disclose. Rose Hartzell has received research support from Emotional Brain, Palatin, and Trimel. Noel N. Kim is a consultant for Alagin Research LLC and Absorption Pharmaceuticals, and has received research funding from Pfizer and Astellas. Irwin Goldstein has received research support from Absorption Pharmaceuticals, Auxilium, BioSante, Emotional Brain, Endoceutics, Medtronic, Neogyn, Palatin, Repros, Slate, Target Health, and Trimel. He is a consultant for Apricus, Emotional Brain, Fabre-Kramer, Ironwood, Meda, Medtronic, Neogyn, Neotract, Slate, and Trimel, and he is on the Speakers Bureau for Abbott, Auxilium, Coloplast, Eli Lilly, Endo, and Slate.
References
- 1.Ibe C, Simon JA. Vulvovaginal atrophy: Current and future therapies (CME) J Sex Med. 2010;7:1042–1050. doi: 10.1111/j.1743-6109.2009.01692.x. [DOI] [PubMed] [Google Scholar]
- 2.Sturdee DW, Panay N on behalf of the International Menopause Society Writing Group. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13:509–522. doi: 10.3109/13697137.2010.522875. [DOI] [PubMed] [Google Scholar]
- 3.The North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:357–369. doi: 10.1097/gme.0b013e31805170eb. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein I. Recognizing and treating urogenital atrophy in postmenopausal women. J Womens Health (Larchmt) 2010;19:425–432. doi: 10.1089/jwh.2009.1384. [DOI] [PubMed] [Google Scholar]
- 5.Krychman ML. Vaginal estrogens for the treatment of dyspareunia. J Sex Med. 2011;8:666–674. doi: 10.1111/j.1743-6109.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 6.Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: A systematic review and meta-analysis. J Sex Med. 2012;9:34–43. doi: 10.1111/j.1743-6109.2011.02366.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein I, Alexander JL. Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women. J Sex Med. 2005;2(3 suppl):154–165. doi: 10.1111/j.1743-6109.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 8.Nappi RE, Kokot-Kierepa M. Women's voices in the menopause: Results from an international survey on vaginal atrophy. Maturitas. 2010;67:233–238. doi: 10.1016/j.maturitas.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Nappi RE, Kokot-Kierepa M. Vaginal health: Insights, views & attitudes (VIVA)—Results from an international survey. Climacteric. 2012;15:36–44. doi: 10.3109/13697137.2011.647840. [DOI] [PubMed] [Google Scholar]
- 10.Labrie F. DHEA, important source of sex steroids in men and even more in women. Prog Brain Res. 2010;182:97–148. doi: 10.1016/S0079-6123(10)82004-7. [DOI] [PubMed] [Google Scholar]
- 11.Ewies AA, Alfhaily F. Topical vaginal estrogen therapy in managing postmenopausal urinary symptoms: A reality or a gimmick? Climacteric. 2010;13:405–418. doi: 10.3109/13697137.2010.500748. [DOI] [PubMed] [Google Scholar]
- 12.Pessina MA, Hoyt RF, Jr, Goldstein I, Traish AM. Differential effects of estradiol, progesterone, and testosterone on vaginal structural integrity. Endocrinology. 2006;147:61–69. doi: 10.1210/en.2005-0870. [DOI] [PubMed] [Google Scholar]
- 13.Kim NN, Min K, Pessina MA, Munarriz R, Goldstein I, Traish AM. Effects of ovariectomy and steroid hormones on vaginal smooth muscle contractility. Int J Impot Res. 2004;16:43–50. doi: 10.1038/sj.ijir.3901138. [DOI] [PubMed] [Google Scholar]
- 14.Kim SW, Kim NN, Jeong SJ, Munarriz R, Goldstein I, Traish AM. Modulation of rat vaginal blood flow and estrogen receptor by estradiol. J Urol. 2004;172:1538–1543. doi: 10.1097/01.ju.0000137744.12814.2e. [DOI] [PubMed] [Google Scholar]
- 15.Ting AY, Blacklock AD, Smith PG. Estrogen regulates vaginal sensory and autonomic nerve density in the rat. Biol Reprod. 2004;71:1397–1404. doi: 10.1095/biolreprod.104.030023. [DOI] [PubMed] [Google Scholar]
- 16.Pessina MA, Hoyt RF, Jr, Goldstein I, Traish AM. Differential regulation of the expression of estrogen, progesterone, and androgen receptors by sex steroid hormones in the vagina: Immunohistochemical studies. J Sex Med. 2006;3:804–814. doi: 10.1111/j.1743-6109.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 17.Skala CE, Petry IB, Albrich SB, Puhl A, Naumann G, Koelbl H. The effect of hormonal status on the expression of estrogen and progesterone receptor in vaginal wall and periurethral tissue in urogynecological patients. Eur J Obstet Gynecol Reprod Biol. 2010;153:99–103. doi: 10.1016/j.ejogrb.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Soderberg MW, Johansson B, Masironi B, Bystrom B, Falconer C, Sahlin L, Ordeberg GE. Pelvic floor sex steroid hormone receptors, distribution and expression in pre- and postmenopausal stress urinary incontinent women. Acta Obstet Gynecol Scand. 2007;86:1377–1384. doi: 10.1080/00016340701625446. [DOI] [PubMed] [Google Scholar]
- 19.Taylor AH, Guzail M, Al-Azzawi F. Differential expression of oestrogen receptor isoforms and androgen receptor in the normal vulva and vagina compared with vulval lichen sclerosus and chronic vaginitis. Br J Dermatol. 2008;158:319–328. doi: 10.1111/j.1365-2133.2007.08371.x. [DOI] [PubMed] [Google Scholar]
- 20.Skala CE, Petry IB, Albrich S, Puhl A, Naumann G, Koelbl H. The effect of genital and lower urinary tract symptoms on steroid receptor expression in women with genital prolapse. Int Urogynecol J. 2011;22:705–712. doi: 10.1007/s00192-010-1327-6. [DOI] [PubMed] [Google Scholar]
- 21.Imamov O, Yakimchuk K, Morani A, Schwend T, Wada-Hiraike O, Razumov S, Warner M, Gustafsson JA. Estrogen receptor β-deficient female mice develop a bladder phenotype resembling human interstitial cystitis. Proc Natl Acad Sci U S A. 2007;104:9806–9809. doi: 10.1073/pnas.0703410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-β isoforms: A key to understanding ER-β signaling. Proc Natl Acad Sci U S A. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valeri A, Brain KL, Young JS, Sgaragli G, Pessina F. Effects of 17β-oestradiol on rat detrusor smooth muscle contractility. Exp Physiol. 2009;94:834–846. doi: 10.1113/expphysiol.2009.047118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning N, Lin G, Lue TF, Lin CS. Effects of estrogen, raloxifene, and levormeloxifene on the expression of Rho-kinase signaling molecules in urethral smooth muscle cells. Urology. 2010;76:1517. doi: 10.1016/j.urology.2010.07.470. e1516–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwall L, Carlstrom K, Jonasson AF. Different estrogen sensitivity of urogenital tissue from women with and without stress urinary incontinence. Neurourol Urodyn. 2009;28:516–520. doi: 10.1002/nau.20710. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein IT. The pelvic floor muscles: Muscle thickness in healthy and urinary-incontinent women measured by perineal ultrasonography with reference to the effect of pelvic floor training. Estrogen receptor studies. Neurourol Urodyn. 1997;16:237–275. doi: 10.1002/(sici)1520-6777(1997)16:4<237::aid-nau2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Sajjad Y, Quenby S, Nickson P, Lewis-Jones DI, Vince G. Immunohistochemical localization of androgen receptors in the urogenital tracts of human embryos. Reproduction. 2004;128:331–339. doi: 10.1530/rep.1.00227. [DOI] [PubMed] [Google Scholar]
- 28.Traish AM, Kim SW, Stankovic M, Goldstein I, Kim NN. Testosterone increases blood flow and expression of androgen and estrogen receptors in the rat vagina. J Sex Med. 2007;4:609–619. doi: 10.1111/j.1743-6109.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 29.Blanchet P, Yaici el D, Cayzergues L, Giuliano F, Jardin A, Benoit G, Droupy S. Identification of androgen receptors in the motoneurons of the external urethral sphincter in the spinal cord of female rats. Eur Urol. 2005;47:118–124. doi: 10.1016/j.eururo.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Cayzergues L, Yaici el D, Tabard SB, Jestin A, Blanchard P, Giuliano F, Bensadoun H, Jardin A, Benoit G, Droupy S. Morphological study of the spinal motoneurons controlling the urethral sphincter of female rats: Role of androgens in a menopausal model. J Urol. 2005;173:1022–1026. doi: 10.1097/01.ju.0000146269.43658.d3. [DOI] [PubMed] [Google Scholar]
- 31.Mammadov R, Simsir A, Tuglu I, Evren V, Gurer E, Ozyurt C. The effect of testosterone treatment on urodynamic findings and histopathomorphology of pelvic floor muscles in female rats with experimentally induced stress urinary incontinence. Int Urol Nephrol. 2011;43:1003–1008. doi: 10.1007/s11255-011-9938-5. [DOI] [PubMed] [Google Scholar]
- 32.Witherby S, Johnson J, Demers L, Mount S, Littenberg B, Maclean CD, Wood M, Muss H. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: A phase I/II study. Oncologist. 2011;16:424–431. doi: 10.1634/theoncologist.2010-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 34.Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dube R, Cote I, Labrie C, Lavoie L, Berger L, Gilbert L, Martel C, Balser J. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907–922. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 35.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD001500.pub2. CD001500. [DOI] [PubMed] [Google Scholar]
- 36.Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Obstet Gynecol. 2008;112:689–690. doi: 10.1097/AOG.0b013e318185f7a5. [DOI] [PubMed] [Google Scholar]
- 37.Cardozo L, Lose G, McClish D, Versi E. A systematic review of the effects of estrogens for symptoms suggestive of overactive bladder. Acta Obstet Gynecol Scand. 2004;83:892–897. doi: 10.1111/j.0001-6349.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 38.Cody JD, Richardson K, Moehrer B, Hextall A, Glazener CM. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD001405.pub2. CD001405. [DOI] [PubMed] [Google Scholar]
- 39.Simunic V, Banovic I, Ciglar S, Jeren L, Pavicic Baldani D, Sprem M. Local estrogen treatment in patients with urogenital symptoms. Int J Gynaecol Obstet. 2003;82:187–197. doi: 10.1016/s0020-7292(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann G, Bouchard C, Hoppe D, Ranganath R, Altomare C, Vieweg A, Graepel J, Helzner E. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause. 2009;16:719–727. doi: 10.1097/gme.0b013e3181a48c4e. [DOI] [PubMed] [Google Scholar]
- 41.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol. 2008;112:1053–1060. doi: 10.1097/AOG.0b013e31818aa7c3. [DOI] [PubMed] [Google Scholar]
- 42.Simon J, Nachtigall L, Ulrich LG, Eugster-Hausmann M, Gut R. Endometrial safety of ultra-low-dose estradiol vaginal tablets. Obstet Gynecol. 2010;116:876–883. doi: 10.1097/AOG.0b013e3181f386bb. [DOI] [PubMed] [Google Scholar]
- 43.Weisberg E, Ayton R, Darling G, Farrell E, Murkies A, O'Neill S, Kirkegard Y, Fraser IS. Endometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric. 2005;8:83–92. doi: 10.1080/13697130500087016. [DOI] [PubMed] [Google Scholar]
- 44.2013. Osphena (ospemifene) [prescribing information]. Florham Park, NJ: Shionogi Inc.; Febraury.
- 45. Premarin (conjugated estrogens) vaginal cream [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals Inc, part of Pfizer; May 2010.
- 46. Estrace (estradiol vaginal cream, USP, 0.01%) [prescribing information]. Rockaway, NJ: Warner Chilcott (US), LLC; July 2011.
- 47. Estring (estradiol vaginal ring) [prescribing information]. New York, NY: Pharmacia & Upjohn Company, Division of Pfizer Inc; Aug 2008.
- 48. Vagifem (estradiol vaginal tablets) [prescribing information]. Princeton, NJ: Novo Nordisk Inc; Nov 25 2009.
- 49.Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2011;17:403–408. doi: 10.1111/j.1524-4741.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 50.Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 μg 17β-estradiol vaginal tablets. Climacteric. 2010;13:219–227. doi: 10.3109/13697137.2010.483297. [DOI] [PubMed] [Google Scholar]
- 51.Dew JE, Wren BG, Eden JA. A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric. 2003;6:45–52. [PubMed] [Google Scholar]
- 52.Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. J Sex Med. 2011;8:549–559. doi: 10.1111/j.1743-6109.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- 53.Krychman ML, Katz A. Breast cancer and sexuality: Multi-modal treatment options. J Sex Med. 2012;9:5–13. doi: 10.1111/j.1743-6109.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 54.Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health (Larchmt) 2009;18:461–468. doi: 10.1089/jwh.2008.1133. [DOI] [PubMed] [Google Scholar]
- 55.Minkin M, Guess M. Diagnosis and treatment of the non–sex-related symptoms of vulvovaginal atrophy. Female Patient. 2012;37:33–41. [Google Scholar]
- 56.Reiter S. Barriers to effective treatment of vaginal atrophy with local estrogen therapy. Int J Gen Med. 2013;6:153–158. doi: 10.2147/IJGM.S43192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Althof SE. Sex therapy and combined (sex and medical) therapy. J Sex Med. 2011;8:1827–1828. doi: 10.1111/j.1743-6109.2011.02306.x. [DOI] [PubMed] [Google Scholar]


