Abstract
Cigarette smoking is a major risk factor for pulmonary Langerhans cell histiocytosis (pLCH) and lung cancer. Resolution of pLCH may occur spontaneously, after smoking cessation or other interventions. However, despite clinicoradiological resolution, residual pulmonary Langerhans cells may be present and may lead to recurrent disease. We report the first case of pLCH with a complete histological resolution.
Keywords: Histology, lung cancer, pulmonary Langerhans cell histiocytosis, smoking cessation
Introduction
The disparate group of diseases, now collectively referred to as Langerhans cell histiocytosis (LCH), includes diseases previously designated such as histiocytosis X, eosinophilic granuloma, and type II histiocytosis, and may involve the lung (pulmonary Langerhans cell histiocytosis, e.g. pLCH) [1]. In the largest published cohort of LCH patients [1], pLCH was present in 129/314 cases (41%) as part of a multisystem disorder and in 87 cases (28%) confined to the lungs. Approximately 88% of them were cigarette smokers. Natural history, diagnosis (with or without lung biopsy), and treatment were thoroughly described. However, no histological confirmation of suspected resolution was obtained. Furthermore, some cases showed recurrent disease. We present a case of a 62-year-old man with a biopsy-proven presence of pLCH and a histologically resolution. To the best of our knowledge, we describe the first case with no histological signs of residual disease.
Case Report
A 62-year-old Caucasian man was evaluated for shortness of breath and an acute swollen face and eyelids in October 2011 at the Emergency Department. The night before presentation, he fell out of his bed on his left side. He had a long history of nicotine abuse (approximately 30 pack-years). Physical examination showed subcutaneous emphysema of the thorax and in the neck. Computer tomography (CT) scan of the thorax showed a pneumomediastinum, a small pneumothorax, and costal fracture on the left side. Furthermore, multiple partly cavitating nodules were found in both lungs besides parenchymal emphysema (Fig. 1). Differential diagnosis at that time included metastasis, cavitating granulomatous diseases (vasculitis, pLCH), and pyogenic disease. A CT-guided biopsy of a cavitating lung nodule located in the left upper lobe was performed after placement of a chest tube. Analysis however revealed no classifying diagnosis, nor microorganisms could be cultured. Serologic testing for vasculitis (ANCA) was negative. Resolution of the pneumothorax, pneumomediastinum, and subcutaneous emphysema occurred within a few days and the patient was discharged. Within a week, the patient was readmitted with pleural empyema. He was treated with pleural drainage and intravenous antibiotics (flucloxacillin). Despite thorough treatment, he clinically deteriorated and was transferred for thoracotomy and surgical drainage. Along pleural decortication, surgical biopsies of the cavitated lung nodules located in the lower left lobe were obtained (Fig. 2). After two weeks of intravenous antibiotic treatment (flucloxacillin), he was discharged in good health. Pathological analysis of lung biopsies from the lower left lobe showed subpleural granuloma-like nodules with inflammatory cells, eosinophils, and Langerhans cells, identified by positive staining for CD1a (Fig. 3) and S100, characteristic for pLCH. There were no signs of microorganisms or malignancy. He discontinued smoking and further outpatient care was initiated. Nine months later, a new chest CT scan showed resolution of all cysts, nodules, and cavitating nodules (Fig. 4). However, one nodule in the right upper lobe increased in size and was therefore suspected for malignancy (Fig. 1, thin black arrow; Fig. 4). Positron emission tomography-CT scan was positive for this lesion without nodal or distant metastasis. A CT-guided biopsy revealed a primary adenocarcinoma of the lung for which a lobectomy including a systematic lymph node dissection was performed by video-assisted thoracoscopic surgery. Pathological analysis showed an adenocarcinoma of the lung with a maximal diameter of 1.9 cm, staged pT1aN0M0 adenocarcinoma. Thorough sampling throughout the whole right upper lobe showed no granuloma-like lesions nor mixed inflammatory infiltrates. Additional CD1a and S100 staining were all negative. Consequently, the radiological resolution of cavitating nodules was confirmed by negative staining for pLCH.
Figure 1.

Computed tomography scan of thorax showing bilateral subcutaneous emphysema, pneumomediastinum, pulmonary emphysema, and cavitating lung nodules in the right lung (black arrows). Also a more spiculated lung nodule is present in the right lung (thin black arrow).
Figure 2.

Computed tomography scan of thorax showing extensive left pleural empyema and a cavitating lung nodule in the compressed left lung (black arrow).
Figure 3.

Magnification 5×. Cluster of differentiation 1a immunohistochemical stain showing an intense membranous staining pattern of Langerhans cells in clusters.
Figure 4.
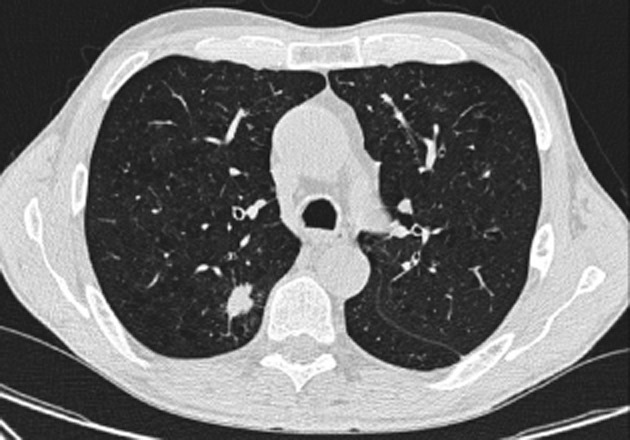
Computed tomography scan of the thorax showing complete radiological resolution of the cavitary lesions adjacent to the tumor.
Discussion
The diagnosis of pLCH requires a clinical, radiological, and preferably histological assessment [1]. Ultimately, pLCH has a good prognosis and more than 80% of patients achieve disease-free survival. However, in comparison with osseous LCH, the proportion of deaths was significantly greater in pLCH in a large cohort of patients [1]. Moreover, the proportion of a disease-free survival was significantly less in pLCH compared to osseous LCH [1]. Many patients in this cohort were cigarette smokers (88%) and only 3/87 patients were non-smokers. Therefore, confirmation of a classifying diagnosis by histology is required in pLCH, not only in the setting of a broad differential diagnosis but also in the differentiation between isolated pLCH or concomitant multisystem disease.
Pulmonary Langerhans cell histiocytosis follows a course that is difficult to predict. The early pathological lesion appears to be a bronchiolitis, which may progress into intraluminal fibrosis [2]. Cigarette smoking is a major risk factor for developing pLCH [3]. In the course of the disease, affected individuals may show progression and require aggressive treatment, including high-dose prednisone [1]. Alternatively, spontaneous resolution of pLCH after smoking cessation may occur, as assessed by clinical and radiological follow-up [1], [4]. However, in the present case, smoking cessation may not have been the exclusive reason for resolution of pLCH. Other interventions such as intravenous antibiotics or the interaction between pLCH and adenocarcinoma may have also affected the course of the disease. Bronchogenic carcinoma has been described previously in patients with pLCH [1]. Important risk factors include cigarette smoking and the propensity for pulmonary fibrosis [1], [2]. Coexistence of pLCH and bronchogenic carcinoma, particularly adenocarcinoma, has been found in lobectomy specimens previously [5]. In our patient, as may occur in pLCH patients, adenocarcinoma of the lung was found. However, in the lobectomy specimen, no remnants of previously detected pLCH were present. The present case stresses the importance of smoking cessation in pLCH not only in the development but also in its treatment.
Acknowledgments
The authors like to thank Klaas (K.G.) van der Ham for photographing all biopsy slides.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
References
- Howarth DM, Gilchrist GS, Mullan BP, et al. Langerhans cell histiocystosis: diagnosis, natural history, management, and outcome. Cancer. 1999;85:2278–2290. doi: 10.1002/(sici)1097-0142(19990515)85:10<2278::aid-cncr25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fukada Y, Basset F, Soler P, et al. Intraluminal fibrosis and elastic fibre degredation leading to lung remodelling in pulmonary Langerhans cell granulomatosis (histiocytosis X) Am. J. Pathol. 1990;137:415–424. [PMC free article] [PubMed] [Google Scholar]
- Hance AJ, Baseet F, Saumon G, et al. Smoking and interstitial lung disease. The effect of cigarette smoking on the incidence of pulmonary histiocytosis X and sarcoidosis. Ann. N. Y. Acad. Sci. 1986;465:643–656. doi: 10.1111/j.1749-6632.1986.tb18541.x. [DOI] [PubMed] [Google Scholar]
- Mogulkoc N, Veral A, Bishop PW, et al. Pulmonary Langerhans' cell histiocytosis. Radiologic resolution following smoking cessation. Chest. 1999;115:1452–1455. doi: 10.1378/chest.115.5.1452. [DOI] [PubMed] [Google Scholar]
- Colasante A, Poletti V, Rosini S, et al. Langerhans cells in Langerhans cell histiocytosis and peripheral adenocarcinomas of the lung. Am. Rev. Respir. Dis. 1993;148:752–759. doi: 10.1164/ajrccm/148.3.752. [DOI] [PubMed] [Google Scholar]


