Abstract
Intrapleural tissue plasminogen activator (tPA) and deoxyribonuclease (DNase) therapy is being increasingly employed as an alternative to surgical intervention for the treatment of complicated parapneumonic effusions and empyema. Published cases are limited to one randomized control trial and few case reports. No data exist on employing sequential or repeated courses of intrapleural tPA/DNase to aid evacuation of separate collections in patients' with a multiloculated pleural infection. This is the first report of successful use of sequential delivery of separate courses of intrapleural tPA/DNase to two noncommunicating infected pleural fluid collections within the same hemithorax of a patient. Our case confirms that prior treatment with tPA/DNase therapy does not preclude subsequent effective and safe use of this intrapleural treatment.
Keywords: Deoxyribonuclease, empyema, fibrinolytics, pleural infection, tissue plasminogen activator
Introduction
Pleural infection is common and is associated with high morbidity. Treatment involves antibiotic therapy and pleural fluid drainage; however, thick and loculated pus often prohibits drainage and may necessitate surgical intervention [1]. Initial management of multiloculated empyemas involves chest tube insertion to evacuate the largest collection. Further tube thoracostomy may be needed for separate locules if infection persists.
Intrapleural tissue plasminogen activator (tPA) and deoxyribonuclease (DNase) therapy has been shown in a randomized trial to enhance pleural fluid drainage in patients with complicated pleural infection, reducing length of hospitalization and need for surgery [2]. To date, outcomes from less than 50 published cases exist on intrapleural tPA/DNase therapy [2], [3]. No data exist on employing sequential or repeated courses of intrapleural tPA/DNase in the same patient.
We report the first successful treatment of multiloculated empyema by sequential delivery of two courses of intrapleural tPA/DNase to noncommunicating pleural pus collections within the same hemithorax of a patient.
Case Report
A previously well 17-year-old male presented with a productive cough, fever (38.4°C) and left-sided pleuritic chest pain. His chest radiograph (CXR) and subsequent computed tomography confirmed left lower lobe pneumonia and he was commenced on pipercillin/tazobactam. A repeat CXR and ultrasound on day (D) 5 identified a multiloculated pleural effusion. Analyses of the pleural aspirate confirmed a complicated parapneumonic effusion: pH 6.09, lactate dehydrogenase 71,300 U/L, protein 40 g/L but no microorganism was cultured. A chest tube (12F) was inserted under imaging guidance into the largest locule.
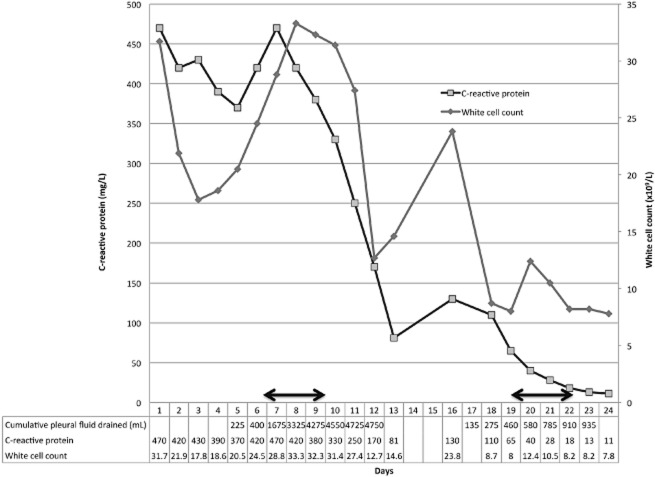
Although fever resolved, a rising leukocyte count and C-reactive protein (CRP) with residual pleural effusion on CXR suggested persistent infection (Figs. 1, 2). Antibiotic therapy was changed to meropenem. To facilitate drainage, tPA (10 mg) followed by DNase (5 mg) were instilled intrapleurally twice daily over 3 days, as per published protocols [2]. Prior to instillation, only 400 mL of pleural fluid had drained. Twenty-four hours after initiating tPA/DNase, 2075 mL was drained, and in total, 4050 mL were drained by 72 h. This was paralleled by a significant reduction in effusion size on CXR.
Figure 1.
White cell count, C-reactive protein and cumulative pleural fluid drained during hospital admission on days 1 to 13 and days 16 to 24. Horizontal arrows represent days of tissue plasminogen activator (tPA) and deoxyribonuclease (DNase) administration.
Figure 2.
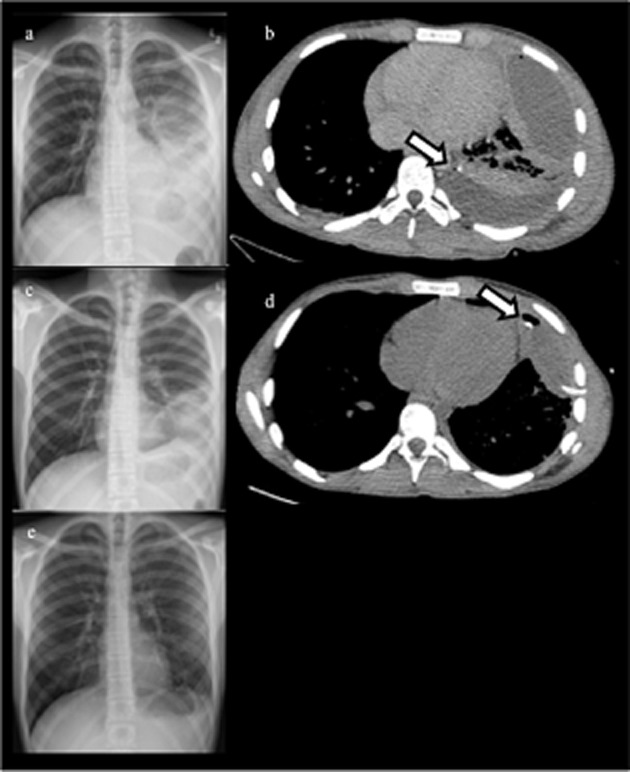
(a) Day 5 chest X-ray (CXR) – left lower lobe consolidation and effusion. (b) Day 5 computed tomography (CT) chest – multiloculated left effusion with intercostal catheter (ICC) in posterior locule (arrow) prior to first tPA/DNase treatment. (c) Day 17 CXR – opacity relating to persistent anterior locule. (d) Day 17 CT chest – ICC inserted in anterior locule (arrow). Posterior locule has been cleared following first tPA/DNase treatment. (e) CXR 4 weeks after completion of second tPA/DNase treatment.
A residual 5 × 7-cm anterolateral fluid collection, separate to the one treated with tPA/DNase was identified at D11 on pleural ultrasound. A therapeutic thoracentesis evacuated 300 mL of purulent fluid. His infection markers improved: white cell count 31.7 to 12.7 × 109/L; CRP 470 to 170 mg/L and he was discharged on D13 with home intravenous antibiotics.
He re-presented on D16 with fever, worsening leukocytosis and re-accumulation of the previously aspirated anterolateral collection. A chest tube (12F) was inserted and 310 mL of purulent fluid was drained over 48 h. Fluid culture remained negative. Computed tomography confirmed optimal placement of the chest drain but a residual collection persisted. Given the success from the first course of tPA/DNase, the family and patient elected to repeat intrapleural therapy over surgery. Six doses of tPA/DNase were administered to the second collection. A further 550 mL of pleural fluid was drained over 72 h with resolution of sepsis: the fever resolved and both leukocytosis (23.8 to 8.2 × 109/L) and CRP (130 to 13 mg/L) settled. No residual fluid was present on CXR or ultrasound when the tube was removed. The patient was discharged on oral antibiotics. He made an uneventful recovery and at 3-month follow-up had no respiratory symptoms or residual opacity on CXR.
Discussion
This is the first reported case of the use of successive courses of intrapleural tPA/DNase to separate noncommunicating pleural fluid collections. The initial course successfully drained the dominant collection with resolution of the infection indices. A separate and isolated locule was aspirated but recurred causing systemic infection. A second course of tPA/DNase targeting the residual collection successfully evacuated it with no complications.
Treatment of complicated parapneumonic effusion or empyema involves antibiotics and drainage of infected pleural fluid by tube thoracostomy. Inability to adequately evacuate the infected fluid, usually because of dense septation, is the commonest cause of ongoing infection, necessitating surgical drainage in 20–30% of patients [2], [4].
The combined use of tPA (to lyse adhesions) and DNase (to reduce pus viscosity) has been shown to improve outcomes [2], [3]. In a randomized trial, 96% of patients treated with tPA/DNase were cured without surgical intervention. All published cases of intrapleural tPA/DNase [2], [3] were treated at one site with one course (≤6 doses) of therapy. The maximal doses or duration of tPA/DNase remain undefined.
Patients with pleural infection often have multiloculated effusions. Insertion of separate chest tubes is sometimes needed to evacuate noncommunicating collections. The use of tPA/DNase can breakdown adhesions and minimize such a need. Nonetheless, in some patients (like this case), walled off collections are separated in distant parts of the pleural cavity. Our case showed for the first time that different noncommunicating collections can be treated separately with successive courses of tPA/DNase therapy without complications, and surgery avoided.
Concerns remain on the safety and optimal regime of tPA/DNase therapy. The bleeding rates of intrapleural fibrinolytics use have been low in all large published series [2], [4]. Given the short systemic half-life of fibrinolytics, repeated administration should not pose significantly higher bleeding risks. Unlike streptokinase, administration of tPA does not induce the formation of antibodies against the drug and will not prohibit further courses of therapy. Our case confirms that prior treatment with tPA/DNase therapy probably does not preclude effective use of this intrapleural therapy in the future.
Acknowledgments
Y. C. G. Lee is a National Health and Medical Research Council (NHMRC) Career Development Fellow and receives project grant funding from the NHMRC, New South Wales Dust Disease Board, Sir Charles Gairdner Research Advisory Committee, Westcare and the Cancer Council of Western Australia. N. Popowicz receives funding support from an Australian Postgraduate Award.
Disclosure Statements
Prof. Lee was a co-investigator of the MIST-2 study, which received unrestricted research funds from Roche UK.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
References
- Davies HE, Davies RJO, Davies CWH. Management of pleural infection in adults: British Thoracic Society pleural disease guideline. Thorax. 2010;65(Suppl. 2):ii41–ii53. doi: 10.1136/thx.2010.137000. [DOI] [PubMed] [Google Scholar]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N. Engl. J. Med. 2011;365(6):518–526. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- Simpson G, Roomes D, Reeves B. Successful treatment of empyema thoracis with human recombinant deoxyribonuclease. Thorax. 2003;58(4):365–366. doi: 10.1136/thorax.58.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. controlled trial of intrapleural streptokinase for pleural infection. N. Engl. J. Med. 2005;352(9):865–874. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]