Abstract
Key Clinical Message
Neurotoxic snake envenomation can result in respiratory failure and death. Early treatment is considered important to survival. Inexpensive, heat-stable, needle-free, antiparalytics could facilitate early treatment of snakebite and save lives, but none have been developed. An experiment using aerosolized neostigmine to reverse paralysis suggests how early interventions could be developed.
Keywords: Anticholinesterase, emergency medicine, neurotoxin, snakebite, toxicology
Lay Summary
Snakebite is arguably the most neglected of neglected tropical diseases. It is a disease of poverty with numbers of fatalities comparable to that of AIDS in some developing countries. Predominantly killing young and otherwise healthy individuals, neurotoxins paralyze their victims—resulting in death by respiratory failure. Most of these victims would likely survive with early access to emergency care. There is currently little funding to devise new approaches to address this problem. It is estimated that, for example, less than 25% of patients who die from snakebite in India do so in the hospital. That means more than 75% of patients who die from snakebite do not survive to receive treatments in the places best suited to treat them. The goal of our experiment was to take that first small step toward addressing this horrific problem and begin finding ways in which initial treatments can be brought to the victims of snakebite.
Anticholinesterases are a group of commonly used, heat–stable, and inexpensive drugs that have been used for decades to reverse chemically-induced paralysis in operating rooms and, in intravenous form, to treat snakebite when antivenoms are not available or not effective. They have also been used with experimental success in animal models. We argue that developing inexpensive, heat-stable, and easy-to-administer, topical antiparalytics could facilitate early treatment of snakebite and save lives. We report the first successful attempt to create a human model for neurotoxic paralysis specifically designed to test whether a nasally applied anticholinesterase could reverse paralysis caused by a drug that clinically mimics some critically important elements of neurotoxic envenomation. Our finding suggests a novel strategy for the development of field treatments—not just anticholinesterases—that could substantially decrease the worldwide burden of death and disability from neurotoxic envenomation and, inevitably, more complex types of envenomation.
Introduction
Bulbar palsies and neck weakness are life-threatening sequelae of some neurotoxic snakebites and are often followed by respiratory failure and death. A common mechanism between neurotoxic snakebite, nondepolarizing neuromuscular blocking agents and myasthenia gravis is interruption of the transmission of acetylcholine at the neuromuscular junction. Although the exact mechanisms by which these interruptions occur are many and varied, the final common cause of disability and death in neurotoxic envenomation is loss of acetylcholine transmission. In intravenous (IV) form, neostigmine, and other anticholinesterases are used as reversal agents for nondepolarizing curare-derived neuromuscular blocking agents. In addition, a trial of anticholinesterase therapy is recommended by the World Health Organization (WHO) in virtually all cases of confirmed or suspected neurotoxic snake envenomation. Interestingly, intranasal neostigmine has shown promise in the treatment of other neuromuscular junction maladies such as myasthenia gravis—with the bioavailability of topically applied neostigmine comparable to that of intravenous neostigmine. This led to the idea that a topically applied anticholinesterase might have utility in the early treatment of neurotoxic snakebite.
We used a continuous mivacurium infusion to induce neuromuscular blockade in a healthy volunteer, successfully mimicking many important elements of paralysis from snakebite neurotoxicity as evidenced by progressive bulbar deficits, neck and respiratory muscle weakness. A single application of atomized 6% neostigmine quickly improved clinical and acceleromyographic measures of neuromuscular blockade.
The demonstration that topical neostigmine can reverse weakness from a nondepolarizing neuromuscular blocking agent suggests a new avenue for research in the early treatment of neurotoxic snakebite. The systematic development of inexpensive, heat-stable, easy-to-administer, needle-free topical antiparalytics would facilitate early treatment of snakebite and eventually even more complex snakebite toxins by eliminating venipuncture. Reductions in the time between bite and treatment are considered universally important in the global challenge to reduce death and disability by snakebite. Our case study suggests a novel strategy fitting the specifications of a solution to the challenge of early drug delivery in the setting of neurotoxic envenomation that could save lives and eventually be developed to treat more complex envenomations.
Worldwide, snakebite causes hundreds of thousands of deaths annually, mainly in children and young adults in impoverished populations. In some developing countries, the death rate from snakebite is actually comparable to that from AIDS, with devastating personal, social, and economic impact [1–3]. Snakebite is a leading cause of accidental death in the developing world and remains a seriously underestimated public health problem [4,5].
The ability of anticholinesterase agents to reverse the symptoms and signs of myasthenia gravis was famously first described in a single case report by Dr. Mary Walker in 1935 [6–9]. Intravenously (IV) administered neostigmine is routinely used to reverse neuromuscular blockade by nondepolarizing muscle relaxants such as those originally derived from curare [10,11] and was first described as a promising or adjunct treatment for neurotoxic snakebite more than 40 years ago [12]. At present, IV administration of anticholinesterases such as edrophonium and neostigmine is considered first-line therapy in neurotoxic snakebite when antivenoms are not available or not effective [13–16].
The World Health Organization unequivocally recommends a trial of anticholinesterase in every patient with neurotoxic envenoming, “as it would be in any patient with suspected myasthenia gravis” [15,17]. Commonalities in mechanism of paralysis between snakebite and curare have been suspected for more than 120 years [18,19] and the pharmacologic and physiologic similarities between neurotoxic envenomation and myasthenia gravis were described at the microscopic level more than 40 years ago. In fact, I125-labeled snake neurotoxins were used to elucidate the mechanism of acetylcholine receptor loss in myasthenia gravis [20–24]. Neurotoxic snakebites, curare-derived nondepolarizing neuromuscular blocking agents, and myasthenia gravis all have in common interruption of the transmission of acetylcholine at nicotinic cholinergic receptors. Animal research strongly suggests that early treatment with parenteral anticholinesterase agents greatly reduces mortality from neurotoxic envenomation [25,26], while numerous studies and consensus reports agree that rapid transport and early treatment at capable facilities is a key to mortality reduction in human victims [13–16,27,28].
Anticholinesterase agents such as neostigmine are absorbed though the nasal epithelium, with bioavailability in animals [29] and healthy young adults comparable to that following intravenous administration [30]. Evidence for the general safety and even the safety of self-administered intranasal neostigmine to treat myasthenia gravis already exists [29–33], but has not been reported for use in the treatment of neurotoxic envenomation by snakes or any other animals whose neurotoxins typically kill by targeting the peripheral nervous system and paralyzing their victims' respiratory muscles [34]. Nor, to our knowledge, has this ever been reported in the field of anesthesia for the reversal of nondepolarizing neuromuscular blocking agents such as those derived from curare—but the importance of such an innovation in the field of surgery and anesthesia would likely be miniscule compared to its implications if developed to help improve outcomes in a global problem such as snakebite. Thus, this is the focus of our discussion in this manuscript.
Although antivenoms are sometimes available, even if the snake has been identified and corresponding antivenom exists, venomous bites often occur in remote locations far from population centers. Antivenoms themselves are expensive, need refrigeration, and are associated with significant morbidity, thus requiring significant expertise to administer and manage complications. Additionally, victims are often unable to reach a hospital in time to receive the needed treatment [35–37]. After more than 40 years of clinical experience with IV anticholinesterases, and in particular neostigmine and edrophonium, questions remain about its most effective use and even its efficacy in some neurotoxic envenomations [38–41], despite calls for further scientific study dating back more than 30 years [38]. In the only placebo-controlled study performed to date, the anticholinesterase edrophonium improved all clinical and electrophysiological measure in victims of bite by the Philippine cobra (Naja naja philippinensis) [13].
In this study we developed a human model of paralysis that mimics several important elements of neurotoxic snakebite, and then used it to test the hypothesis that neostigmine, administered by nasal spray, could reverse neuromuscular blockade with a drug (mivacurium) applied at a constant rate of infusion to insure that the effects seen were not the result of drug metabolism, but from the intervention drug.
Our findings suggest that the removal of the parenteral route to treat the most immediately life-threatening sequelae of neurotoxic and other complex forms of envenomation is plausible. If systematically and thoroughly developed in human, animal, and clinical models, the elimination of venipuncture could broaden access to life-saving treatments where they are needed most.
Materials and Methods
Ethics statement and general setting
The Committee on Human Research at the University of California, San Francisco, approved the study. A healthy 45-year-old male weighing 75.5 kg volunteered and gave written, informed consent. The subject was intensively monitored at all times by two anesthesiologists immediately before, during, and for five hours after the study. A third physician not responsible for either drug administration or airway protection collected data and made clinical assessments of bulbar muscle function. The study was conducted in the Anesthesiology Human Studies Research Laboratory at the University of California, San Francisco, that has intensive care capabilities.
Establishment and recording of neuromuscular block and drug administration
Mivacurium [Mivacron, Oslo, Norway], a curare-like nondepolarizing agent was chosen for the study because earlier studies conducted for other purposes suggested a clinical course that could simulate neurotoxic envenomation. Importantly stable, near steady-state blood concentrations can be reached rapidly compared to other drugs in its class, and its safety profile is good due to its rapid elimination and neuromuscular blockade was achieved by continuous infusion rather than bolus injection as is typical of envenomation [42–44]. Neuromuscular block was quantified by using the train-of-four (TOF) ratio at the left adductor pollicis (AP) muscle measured by acceleromyography and as described previously [43,44]. When the subject had significant oropharyngeal weakness and met electrophysiological criteria for Level 3 block [43,44] a single dose of 0.2 g mg IV glycopyrrolate was administered to prevent bradycardia. Five minutes later 6% neostigmine dissolved in sterile water [33] was administered using a primed atomizer (LMA MAD Nasal Device, LMA Corporation North America, San Diego, California). A total dose of 27.6 mg, 0.37 mg/kg with half the volume insufflated in each nostril was given [30,31,33,45] and the subject was left undisturbed for a total period of 10 min (Shaded area, Figure 1) except for acceleromyographic recording. After the final set of measurements, the mivacurium infusion was terminated and neuromuscular function was allowed to return spontaneously. Emergency equipment and drugs including IV neostigmine and edrophonium (for reversal of mivacurium block) and glycopyrrolate and atropine (for early treatment of neostigmine toxicity) were at the bedside at all times.
Figure 1.
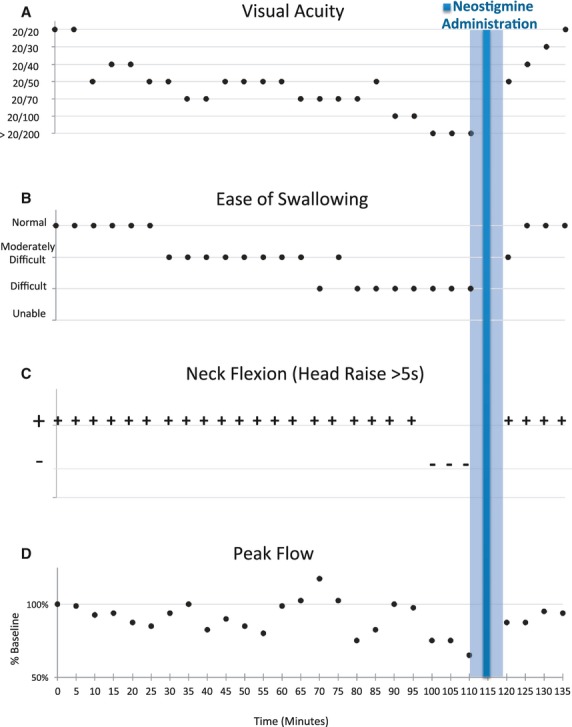
Clinical measures of muscle function are represented as a function of time with baseline measurements at Time 0 at the start of mivacurium infusion and ending at 135 min with the termination of the mivacurium infusion. Intranasal neostigmine was administered at 115 min after establishing the presence of clinically significant neuromuscular impairment and electrophysiologically stable neuromuscular blockade. Stable impairment and the constant mivacurium infusion rate allowed for pre- and postneostigmine administration comparisons as illustrated by: A) progressive loss and recovery of visual acuity and B) ease of swallowing were affected before late loss of C) neck flexion, and finally, D) decrement in peak flow, followed by almost complete recovery prior to terminating mivacurium after 135 min.
Clinical measures of muscle function
Clinical measures of muscle function emphasized those that would be seen in the setting of neurotoxic envenomation that could be readily measured in out-of-hospital settings. The clinical assessments of muscle function were as follows: visual acuity, ease of swallowing [43,44], ability to protrude the tongue [43,44], diction [43,44], and ability to raise the head completely off the bed for more than 5 sec (neck flexion) [43,44] with a postal scale (WeighMax, Industrial City, California, USA) placed under the subjects head to confirm complete elevation and peak respiratory flow measured using a Tru-Zone Peak Flow Meter (Monaghan, Plattsburg, New York). All clinical data were recorded every 5 min throughout the experiment and recorded separately by two physicians who did not communicate with each other or with the anesthesiologist managing the mivacurium infusion and acceleromyographic TOF ratio recordings. The subject was blinded to all clinical and acceleromyographic data as well as to the mivacurium infusion rate and the levels of neuromuscular blockade.
Data analysis
The mivacurium infusion was maintained with unchanged infusion rate, enabling the investigators to determine the characteristics of neostigmine effect [43]. Analysis of TOF ratios were made using TOFMON software (Schering-Plough Corporation, Kenilworth, NJ) and considered stable if the values obtained 10 min apart differed less than 5% and accounting for repeated measures [43,44]. Student's t-test results were calculated and reported as average values, standard deviation (SD), and 95% confidence intervals (95% CI) [43,44,46].
Results
During administration of the mivacurium, the subject experienced progressive weakness mimicking paralysis from neurotoxic envenomation, including loss of visual acuity, difficulty swallowing, jaw ptosis, tongue weakness, inability to flex the neck, and the beginnings of breathing difficulty. The subject was always fully awake and breathing without assistance under the partial, but stable, mivacurium-induced paralysis, and intranasally administered neostigmine quickly relieved all clinically important muscular deficits despite insuring constant pressure on synaptic function by mivacurium because of its constant infusion rate.
Figure 1 illustrates the time course of the experiment from the start of the mivacurium infusion (Time 0) to its termination at 135 min. Neurological deficits were stable within 100 min of the start of the mivacurium infusion and 15 min prior to neostigmine administration, whereas the stability of the neuromuscular blockade was established in the 10 min preceding neostigmine administration (105 min). Baseline visual acuity was 20/20 and became progressively worse until it exceeded 20/200 at the most advanced levels of neuromuscular blockade (Figure 1A). Steady improvement was documented following neostigmine administration. In previously reported experiments, loss of visual acuity was one of the first deficits noted and last to recover with mivacurium infusion, which has been attributed to weakness of the extraocular muscles [43]. The ability to swallow was progressively impaired relatively early in the course of the experiment starting at about 80 min and recovered fully by 10 min after neostigmine administration (Figure 1B).
Figure 1C shows that the ability to lift the head (neck flexion) off the bed for more than 5 sec was lost at 100 min and fully recovered 5 min later by the first test of neck flexion following neostigmine administration.
Peak flow (L/min) decreased from 100% of baseline to 72% of baseline (95% CI 64.72–78.61) and returned to an average of 91% of baseline after neostigmine administration (95% CI 85.24–97.26%) and was 95% of baseline by the termination of the mivacurium infusion (Figure 1D). At first, the subject did not feel as if breathing was impaired, but at the deepest levels of neuromuscular blockade he experienced difficulty wrapping his lips around the peak flow meter and two measurements had to be repeated to guarantee no air leak.
Acceleromyographic and clinical assessments of adductor pollicis muscle function are summarized in Table1. Briefly, the stimulating current was set 15 mA above threshold for the TOF device to detect thumb movement with final mAmps set at 39 mA based on a measured twitch threshold for the subject was 24 mA. The TOF ratio prior to neostigmine administration was stable at 0.56 and neostigmine was subsequently administered with the mivacurium infusion maintained at unchanged infusion rate of 2.5 ug/kg/min, enabling the investigators to determine the characteristics of the neostigmine effect. Neostigmine destabilized the adductor pollicis TOF ratio with preneostigmine with a peak improvement adductor pollicis TOF ratio of 0.70. Mean TOF ratios of 0.56 ± 0.02, range 0.51–0.58, and CI 95% 0.54–0.57, and postneostigmine administration 0.64 ± 0.03, range 0.61–0.70, and CI 95% (0.63–0.66).
Table 1.
Baseline clinical data in the first column are compared to stable level of neuromuscular blockade by mivacurium as measured by adductor pollicis TOF (train-of-four) ratios and clinical impairment represented in the second (middle) column, and the third column shows the clinical response to intranasal neostigmine. Intranasal neostigmine antagonized the neuromuscular blockade as measured by TOF ratios and also improved all clinical levels of muscle function prior to termination of the mivacurium infusion. A constant rate of mivacurium infusion combined with stabilized TOF ratio and clinical impairment made it possible to compare changes attributable to the administration of intranasal neostigmine
| Baseline | Stable Clinical Dysfunction + Stable Neuromuscular Blockade (TOF ratio) | Response to Intranasal Neostigmine | |
|---|---|---|---|
| Mivacurium Infusion Rate | 0 | 2.5 mcg/kg/min | 2.5 mcg/kg/min |
| Normalized TOF ratios (mean ± SD) | 1.00 | 0.56 ± 0.02 | 0.64 ± 0.03 |
| Range 0.51–0.58 | Range 0.61–0.70 | ||
| 95% CI (0.54–0.57) | 95% CI (0.63–0.66) | ||
| Visual Acuity | 20/20 | >20/200 | 20/20 |
| Ease of Swallowing | Easy | Difficult | Easy |
| Neck Flexion (Head Raise >5 s) | Easy (+) | Unable (-) | Easy (+) |
| Peak Flow | 100% | 72% | 91% |
| CI 95% (64.72–78.61) | CI 95% (85.24–97.26) | ||
| Jaw Ptosis | None | Present | None |
| Tongue Protrusion | Easy | Moderately Difficult | Easy |
| Diction | Normal | Poor | Improved |
There were no signs or symptoms of muscarinic-mediated toxicity while the mivacurium infusion was running, although the subject subsequently described the immediate and uncomfortable feeling of facial and lingual muscles “rearranging and tightening” within 5 min of receiving intranasal neostigmine. There was no stinging sensation or irritation or sense of swallowing the spray. There was no taste and no bronchospasm. No unanticipated effects occurred during the mivacurium infusion, but asymptomatic bradycardia was noted just prior to stopping the mivacurium infusion was easily reversed with IV glycopyrrolate, as were some symptoms concurrent with the episodes of bradycardia that included fasciculations and one brief episode of abdominal cramping.
Discussion
Administration of mivacurium to a healthy adult male under the closest monitoring conditions rapidly induced impairments of muscle function clinically, if not mechanistically, similar to those experienced by victims of neurotoxic envenomation. These abnormalities included progressive oropharyngeal muscle disability and loss of ability to flex the neck. Loss of visual acuity as a marker of extraocular muscle weakening, difficulty swallowing, jaw ptosis, neck weakness, and decreased peak respiratory flow. These effects were rapidly reversed by simple administration of neostigmine via nasal spray. These observations suggest that intranasal neostigmine may facilitate the treatment of the most immediately lethal aspects of many, but not necessarily all, types of neurotoxic envenomation, that is, neuromuscular weakness leading to airway obstruction, respiratory compromise, and death and might decrease or delay the need for ventilator support.
The high vascularity of the rhinopharynx and favorable bioavailability of a drug like neostigmine—when given by the intranasal route—suggests a strategy that could facilitate early treatment in the setting of neurotoxic envenomation by some snakes as well as weakness caused by residual neuromuscular blockade in the hospital setting [43,47]. Furthermore, it is also well-documented that the half-life of neostigmine given by the intranasal route is significantly longer than that given by IV [30], so it is possible that a single dose of intranasal neostigmine, other anticholinesterases, or drugs, not yet identified or discovered, could be given in the prehospital setting could last sufficiently long for the patient to protect his or her airway reflexes until arriving at a hospital or clinic adequately equipped to manage victims of snakebite [48]. In this experiment, the highest acceptable dose of neostigmine was administered [30,31,33,45], but it was only average for studies of bioavailability based on the weight of healthy human subjects [30]. We do not know if a lower dose of neostigmine would have been effective [30,31,33,45].
In our experiment, what suggested the long duration of neostigmine activity was the observation of continued signs of neostigmine activity, including bradycardia, abdominal cramping, and fasciculations several hours after withdrawal of mivacurium. Many drugs and devices have been developed for use by lay people specifically for life-threatening emergencies (e.g., epinephrine autoinjectors for anaphylaxis, nebulizers, and inhalers for asthma) and with proper development, a needle-free system to bridge the gap between field and hospital would likely save many lives, possibly more than in any other setting when one considers it is estimated that fewer than one-fourth of patients who die from snake bite die in the hospital [3]. We also suggest that the development of topical anticholinesterase–anticholinergic drug combinations would be a promisingly safe place to start because we are not aware of any serious complications having ever been reported as a result of IV neostigmine or edrophonium use in the setting of neurotoxic snakebite, even at higher than recommended doses [39].
Most likely, in practice, neostigmine, other anticholinesterases, or completely different drugs applied topically would be given at lower doses than the one we gave and the clinical response monitored with adjustments and repeated dosing made accordingly. Nevertheless, using our experiment as an example, intranasal neostigmine could be given as a mixture or nebulized with an equally inexpensive, heat-stable, nasally absorbed anticholinergic agent such as atropine [49]. IV atropine, like IV neostigmine, is already recommended for prevention and management of cholinergic crisis when anticholinesterases are given for neurotoxic envenomation [15]. Such a mixed prepackaging approach might improve envenomation outcomes where prehospital venipuncture is contraindicated [15] and it is imperative that such possibilities are examined in vitro, in animal and human clinical models in order to, ultimately, broaden access to care where it is needed most.
We tested and used the example of an anticholinesterase such as neostigmine because, in concept, it meets all the specifications for the type of drug suited to the geographic realities of the global snakebite epidemic: What is needed are heat stable and inexpensive, suggesting they would be potentially useful where access to highly perishable, expensive, and difficult to administer remedies are all barriers to treating populations that are at the highest risk of lethal snakebites. There are dozens of anticholinesterases (reversible and irreversible) and anticholinergic compounds that could be combined with or without permeation enhancers and other carriers to make drug delivery more predictably achieve desired plasma concentrations if simple aqueous solutions are not adequate [50]. Other drugs can be developed that address the complexities of snake venoms, but because a trial of anticholinesterase is already an unequivocal recommendation [15,17] in the setting of neurotoxic envenomation it was the most obvious place to begin this investigation into topical rather than parenteral treatments for candidate treatments using the strategy we suggest.
Estimates of snakebite incidence, prevalence, and mortality vary greatly because most victims are invisible to data collection, and data are based on statistical sampling and public health records that are difficult to assess [3,16,36]. Nevertheless, with an estimated 5 million bites per year and the estimated deaths numbering perhaps hundreds of thousands, WHO has designated snakebite a “Neglected Tropical Disease” since 2009 [4,16,51]. However, a systematic search has not disclosed a single funded human study of anticholinesterases in the setting of neurotoxic envenomation. This represents a lost opportunity to study the potential of inexpensive, heat-stable, and potentially safe agents in the setting of neurotoxic envenomation. If the need for needles, by whatever means, was removed from this equation, access to care could be broadened and time required to vital treatment decreased. In this communication, we describe a new method for studying medications that could be used to treat some types of neurotoxic envenomation and could offer some advantages over animal models, including the ability to assess clinically unique human features of neuromuscular dysfunction. There are dozens if not hundreds of combinations of anticholinesterases and anticholinergic agents that could be tried in simple combinations or with carriers to make delivery more predictable through the mucous membranes of the nose or eyes, for example. In addition to humans, these types of noninvasive treatments could be important in animal husbandry where animals are as often the victims of snakebite as humans [18].
Intranasal or otherwise topically applied anticholinesterases such as neostigmine—and other candidate drugs or drug combinations—could be delivered without advanced technical skill. The importance of the global snakebite problem and the potential for an efficient, effective solution warrants systematic investigation of topically applied anticholinesterases and other drugs as a potential first-line, in-the-field treatment strategy.
Acknowledgments
Mr. Jerry Harrison, especially, for vital logistic support and reading of the manuscripts, and Drs. Howard Jaffe, Michel Accad, David Lewin, David Wexler. Kimmi Hoang, Nathan Lewin, Christine Ring, Ms. Sunita Rao, Mr. Jim Davis, Mr. Dan Zidane, King-American Ambulance, and many others for their logistic and technical support of this project. We also thank Ms. Janet Harris, Dr. Terry Gosliner, and others at the California Academy of Sciences for their support and encouragement. Illustration of spectacled cobra courtesy of Ms. Isabella Kirkland.
References
- 1.Jha P, Kumar R, Khera A, Bhattacharya M, Arora P, Gajalakshmi V, et al. HIV mortality and infection in India: estimates from nationally representative mortality survey of 1.1 million homes. Br. Med. J. 2010;340:c621. doi: 10.1136/bmj.c621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girish KS, Kemparaju K. Overlooked issues of snakebite management: time for strategic approach. Curr. Top. Med. Chem. 2011;11:2494–2508. doi: 10.2174/156802611797633393. [DOI] [PubMed] [Google Scholar]
- 3.Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl. Trop. Dis. 2011;5:e1018. doi: 10.1371/journal.pntd.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson ID, Norris RL. The global snakebite crisis–a public health issue misunderstood, not neglected. Wilderness Environ. Med. 2009;20:43–56. doi: 10.1580/08-WEME-CON-263.1. [DOI] [PubMed] [Google Scholar]
- 5.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker MB. Case showing the Effect of Prostigmin on Myasthenia Gravis. Proc. R. Soc. Med. 1935;28:759–761. doi: 10.1177/003591573502800633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker MB. Myasthenia Gravis: a Case in which Fatigue of the Forearm Muscles could induce Paralysis of the Extra-ocular Muscles. Proc. R. Soc. Med. 1938;31:722. doi: 10.1177/003591573803100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker M. The treatment of myasthenia gravis. Med. Press. 1946;216:81–84. [PubMed] [Google Scholar]
- 9.Johnston JD. Mary Broadfoot Walker (1888-1974) J. Neurol. 2007;254:1306–1307. doi: 10.1007/s00415-007-0663-z. [DOI] [PubMed] [Google Scholar]
- 10.Lingle CJ, Steinbach JH. Neuromuscular blocking agents. Int. Anesthesiol. Clin. 1988;26:288–301. doi: 10.1097/00004311-198802640-00007. [DOI] [PubMed] [Google Scholar]
- 11.Buzello W, Diefenbach C. Curare and its successors. A 50-year's evolution. Anasthesiol Intensivmed Notfallmed Schmerzther. 1992;27:290–299. doi: 10.1055/s-2007-1000299. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee RN, Sahni AL, Chacko KA, Vijay K. Neostigmine in the treatment of Elapidae bites. J. Assoc. Physicians India. 1972;20:503–509. [PubMed] [Google Scholar]
- 13.Watt G, Theakston RD, Hayes CG, Yambao ML, Sangalang R, Ranoa CP, et al. Positive response to edrophonium in patients with neurotoxic envenoming by cobras (Naja naja philippinensis). A placebo-controlled study. N. Engl. J. Med. 1986;315:1444–1448. doi: 10.1056/NEJM198612043152303. [DOI] [PubMed] [Google Scholar]
- 14.Currie B, Fitzmaurice M, Oakley J. Resolution of neurotoxicity with anticholinesterase therapy in death-adder envenomation. Med. J. Aust. 1988;148:522–525. doi: 10.5694/j.1326-5377.1988.tb99464.x. [DOI] [PubMed] [Google Scholar]
- 15.WHO. 2010. pp. 106–107. Guidelines for the management of snake-bites in Southeast Asia.
- 16.Warrell DA. Snake bite: a neglected problem in twenty-first century India. Natl Med. J. India. 2012;24:321–324. [PubMed] [Google Scholar]
- 17.WHO. Guidelines for the Prevention and Clinical Management of Snakebite in Africa. Brazzaville, Congo: World Health Organization; 2010. pp. 87–88. [Google Scholar]
- 18.Fayrer J. An Address on the Nature of Snake-Poison; its Effects on Living Creatures, and the Present Aspect of Treatment of the Poisoned. Br. Med. J. 1884;1:205–210. doi: 10.1136/bmj.1.1205.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawgood BJ. Sir Joseph Fayrer MD FRS (1824-1907) Indian Medical Service: snakebite and mortality in British India. Toxicon. 1996;34:171–182. doi: 10.1016/0041-0101(95)00118-2. [DOI] [PubMed] [Google Scholar]
- 20.Drachman DB, Fambrough DM, Satyamurti S. Reduced acetylcholine receptors in myasthenia gravis. Trans. Am. Neurol. Assoc. 1973;98:93–95. [PubMed] [Google Scholar]
- 21.Fambrough DM, Drachman DB, Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973;182:293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- 22.Satyamurti S, Drachman DB, Slone F. Blockade of acetylcholine receptors: a model of myasthenia gravis. Science. 1975;187:955–957. doi: 10.1126/science.1145181. [DOI] [PubMed] [Google Scholar]
- 23.Drachman DB. Myasthenia gravis (second of two parts) N. Engl. J. Med. 1978;298:186–193. doi: 10.1056/NEJM197801262980404. [DOI] [PubMed] [Google Scholar]
- 24.Drachman DB. Myasthenia gravis (first of two parts) N. Engl. J. Med. 1978;298:136–142. doi: 10.1056/NEJM197801192980305. [DOI] [PubMed] [Google Scholar]
- 25.Flachsenberger W, Mirtschin P. Anticholinesterases as antidotes to envenomation of rats by the death adder (Acanthophis antarcticus) Toxicon. 1994;32:35–39. doi: 10.1016/0041-0101(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 26.Guieu R, Rosso JP, Rochat H. Anticholinesterases and experimental envenomation by Naja. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 1994;109:265–268. [PubMed] [Google Scholar]
- 27.Dash SC, Ghosh SK, Mathur DC, Jha GN, Prasad U, Grewal KS. Neurotoxic snake bite–dramatic recovery following neostigmine therapy. J. Assoc. Physicians India. 1976;24:535–537. [PubMed] [Google Scholar]
- 28.Sharma SK, Bovier P, Jha N, Alirol E, Loutan L, Chappuis F. Effectiveness of Rapid Transport of Victims and Community Health Education on Snake Bite Fatalities in Rural Nepal. Am. J. Trop. Med. Hyg. 2013;00 doi: 10.4269/ajtmh.12-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fossati A, Vimercati MG, Bandi GL, Formenti A. Pharmacokinetic study of neostigmine after intranasal and intravenous administration in the guinea pig. Drugs Exp. Clin. Res. 1990;16:575–579. [PubMed] [Google Scholar]
- 30.Broggini M, Benvenuti C, Botta V, Fossati A, Valenti M. Bioavailability of intranasal neostigmine: comparison with intravenous route. Methods Find. Exp. Clin. Pharmacol. 1991;13:193–198. [PubMed] [Google Scholar]
- 31.Ricciardi R, Rossi B, Nicora M, Sghirlanzoni A, Muratorio A. Acute treatment of myasthenia gravis with intranasal neostigmine: clinical and electromyographic evaluation. J. Neurol. Neurosurg. Psychiatry. 1991;54:1061–1062. doi: 10.1136/jnnp.54.12.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dooley JM, Goulden KJ, Gatien JG, Gibson EJ, Brown BS. Topical therapy for oropharyngeal symptoms of myasthenia gravis. Ann. Neurol. 1986;19:192–194. doi: 10.1002/ana.410190214. [DOI] [PubMed] [Google Scholar]
- 33.Sghirlanzoni A, Pareyson D, Benvenuti C, Cei G, Cosi V, Lombardi M, et al. Efficacy of intranasal administration of neostigmine in myasthenic patients. J. Neurol. 1992;239:165–169. doi: 10.1007/BF00833919. [DOI] [PubMed] [Google Scholar]
- 34.Mebs D. Venomous and Poisonous Animals. Boca Raton, Florida: CRC Press; 2002. p. 339. [Google Scholar]
- 35.Sharma SK, Chappuis F, Jha N, Bovier PA, Loutan L, Koirala S. Impact of snake bites and determinants of fatal outcomes in southeastern Nepal. Am. J. Trop. Med. Hyg. 2004;71:234–238. [PubMed] [Google Scholar]
- 36.Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: a review. PLoS Negl. Trop. Dis. 2010;4:e603. doi: 10.1371/journal.pntd.0000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma SK, Koirala S, Dahal G, Sah C. Clinico-epidemiological features of snakebite: a study from Eastern Nepal. Trop. Doct. 2004;34:20–22. doi: 10.1177/004947550403400108. [DOI] [PubMed] [Google Scholar]
- 38.Warrell DA, Looareesuwan S, White NJ, Theakston RD, Warrell MJ, Kosakarn W, et al. Severe neurotoxic envenoming by the Malayan krait Bungarus candidus (Linnaeus): response to antivenom and anticholinesterase. BMJ. 1983;286:678–680. doi: 10.1136/bmj.286.6366.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anil A, Singh S, Bhalla A, Sharma N, Agarwal R, Simpson ID. Role of neostigmine and polyvalent antivenom in Indian common krait (Bungarus caeruleus) bite. J. Infect. Prev. 2010;3:83–87. doi: 10.1016/j.jiph.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Chiappinelli V. K-neurotoxins and alpha-neurotoxins: Effect on neuronal nicotinic acetylcholine receptors. In: Harvey A, editor. Snake Toxins. New York: Pergamon press; 1996. pp. 233–258. [Google Scholar]
- 41.Singh G, Pannu HS, Chawla PS, Malhotra S. Neuromuscular transmission failure due to common krait (Bungarus caeruleus) envenomation. Muscle. Nerve. 1999;22:1637–1643. doi: 10.1002/(sici)1097-4598(199912)22:12<1637::aid-mus4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 42.Heier T, Caldwell JE, Sharma ML, Gruenke LD, Miller RD. Mild intraoperative hypothermia does not change the pharmacodynamics (concentration-effect relationship) of vecuronium in humans. Anesth. Analg. 1994;78:973–977. doi: 10.1213/00000539-199405000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Heier T, Caldwell JE, Feiner JR, Liu L, Ward T, Wright PM. Relationship between normalized adductor pollicis train-of-four ratio and manifestations of residual neuromuscular block: a study using acceleromyography during near steady-state concentrations of mivacurium. Anesthesiology. 2010;113:825–832. doi: 10.1097/ALN.Ob013e3181ebddca. [DOI] [PubMed] [Google Scholar]
- 44.Heier T, Feiner JR, Wright PM, Ward T, Caldwell JE. Sex-related differences in the relationship between acceleromyographic adductor pollicis train-of-four ratio and clinical manifestations of residual neuromuscular block: a study in healthy volunteers during near steady-state infusion of mivacurium. Br. J. Anaesth. 2012;108:444–451. doi: 10.1093/bja/aer419. [DOI] [PubMed] [Google Scholar]
- 45.Di Costanzo A, Toriello A, Mannara C, Benvenuti C, Tedeschi G. Intranasal versus intravenous neostigmine in myasthenia gravis: assessment by computer analysis of saccadic eye movements. Clin. Neuropharmacol. 1993;16:511–517. doi: 10.1097/00002826-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 46. Student's t-Test Calculator.
- 47.Brull SJ, Murphy GS. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth. Analg. 2010;111:129–140. doi: 10.1213/ANE.0b013e3181da8312. [DOI] [PubMed] [Google Scholar]
- 48.Curran R. A milestone change in practice: a call for widespread application of intranasal medication delivery in the prehospital environment. Emerg. Med. Serv. 2007;36:40–41. 43-46, 48-49 passim. [PubMed] [Google Scholar]
- 49.Rajpal S, Mittal G, Sachdeva R, Chhillar M, Ali R, Agrawal SS, et al. Development of atropine sulphate nasal drops and its pharmacokinetic and safety evaluation in healthy human volunteers. Environ. Toxicol. Pharmacol. 2009;27:206–211. doi: 10.1016/j.etap.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov. Today. 2002;7:967–975. doi: 10.1016/s1359-6446(02)02452-2. [DOI] [PubMed] [Google Scholar]
- 51.Chippaux JP. Estimating the global burden of snakebite can help to improve management. PLoS Med. 2008;5:e221. doi: 10.1371/journal.pmed.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]


