Abstract
Key Clinical Message
Ventricular arrhythmias induced by dasatinib are rare events, but physicians in charge of chronic myeloid leukemia patients should be aware of such potential complications and the need for regular ECG controls during treatment with tyrosine kinase inhibitors.
Keywords: Adverse drug reaction, BCR-ABL (breakpoint cluster region-abelson), chronic myeloid leukaemia, dasatinib, ventricular arrhythmia
Introduction
We investigated dasatinib treatment as the cause of a major aggravation of ventricular arrhythmia in a 54-year-old patient on second-line treatment with tyrosine kinase inhibitor (TKI) for the management of chronic phase chronic myeloid leukemia (CML). There was a high index of suspicion of a causal relationship between dasatinib and ventricular arrhythmia, but also for a nephritic syndrome and rhabdomyolysis developed by the patient during TKI therapy. A 7-day interruption of dasatinib showed a return to the baseline of the ventricular arrhythmia on a 24 h electrocardiogram (ECG), which is consistent with an adverse drug reaction. Although ventricular arrhythmias induced by dasatinib are rare events, this case emphasizes the need for regular ECG controls during treatment with TKI, and physicians in charge of CML patients should be aware of such potential complications.
In patients with ventricular premature beats (VPBs) on a resting electrocardiogram (ECG), but with no apparent heart disease, data suggest an approximately three-fold higher risk of sudden cardiac death at 7-year follow-up with no association with non-sudden death [1,2]. The prognostic significance and long-term mortality risk related to frequent VPBs remain a subject of debate [3–6]. In rare cases, very frequent VPBs may cause left ventricular dysfunction (LVD). However, patients with a left ventricular ejection fraction (LVFE) <40% with more than 20,000 VPBs in 24 h exhibited a significant improvement of LVFE after receiving anti-arrythmic drugs [7]. Non-sustained ventricular tachycardia (NSVT) is a common, but poorly understood arrhythmia. In patients without structural heart disease, NSVT did not predict a risk of higher mortality [8]. So far, only QT (measure between Q wave and T wave in the heart's electrical cycle) prolongation and LVD, but not VPBs or NSTV, have been described in association with dasatinib treatment, a second-generation tyrosine kinase inhibitor (TKI) used for first- or second-line treatment of chronic myeloid leukemia (CML) [9–11]. We report a case of aggravation of VPBs and NSVT arrhythmia in a patient treated with dasatinib (Sprycel®, Bristol-Myers Squibb, Baar, Switzerland) for CML.
Case history
In January 2011, a 54-year-old man from Cape Verde was diagnosed with high risk, chronic phase, positive BCR-ABL (breakpoint cluster region-Abelson) (Sokal score 2.4; Hasford score 1571; Eutos score 100) CML. He was treated with frontline nilotinib (Tasigna®, Novartis, Basel, Switzerland), a second-generation TKI [12]. He exhibited a complete haematological response at 3 months, but demonstrated treatment failure at 6 months with a minimal cytogenetic response (persistence of 80% of Philadelphia chromosome-positive metaphases) and a relatively high BCR-ABL/ABL ratio of 65% on the International Scale [13]. Treatment was also complicated by grade 2 mucositis (erythema and small foci of ulceration), neutropenia, and grade 3 serum creatine kinase elevation (>5 × upper limit of normal).
A mutation analysis showed a BCR-ABL resistant clone (Y253H) to nilotinib and he was immediately started on a dasatinib regimen (100 mg/day). The patient presented again a creatine kinase elevation with proximal limb myalgias accompanied by neutropenia. He developed also a nephritic syndrome with proteinuria (0.4 g/24 h). Clinical work-up consisted of a muscular (quadriceps femoris muscle) magnetic resonance imaging that revealed normal, a negative immunological screening for polymyositis, and a muscle biopsy compatible with drug-induced rhabdomyolysis based on the clinical history (biopsy was normal, apart from some muscular fibres in regrowth). In the absence of signs of severity, no renal biopsy was performed and it was suggested that the proteinuria was related to a drug-induced nephropathy. Since side-effects were mild to moderate, therapy with dasatinib was continued. However, the patient presented an aggravation (Fig1) of previously known ventricular arrhythmias (bi- and trigeminy and ventricular doublets and triplets [Fig2]) on an anatomically healthy heart to frequent severe VBPs (44% of QRS [deflection on electrocardiography from the Q wave to the S wave representing the ventricular depolarization] complexes/day) and NSVT (4992 episodes/day) confirmed on a 24 h ECG. There was no family history of sudden death or personal history of symptomatic arrhythmia. Based on serum troponin, chemistry panel, and urinary toxic screening, no ischemic, electrolytic, or toxic cause could be identified. At that time, the patient did not receive any other relevant medication. Of note, before the initiation of dasatiib treatment and during the ventricular arrhythmia episodes, cardiac ultrasound was performed with normal values observed, including no valve or structural anomalies, and normal ventricular ejection fraction.
Figure 1.
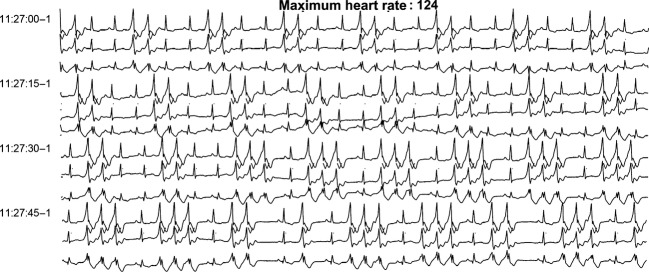
Rhythm strip after dasatinib initiation, 24 h electrocardiogram (ECG).
Figure 2.
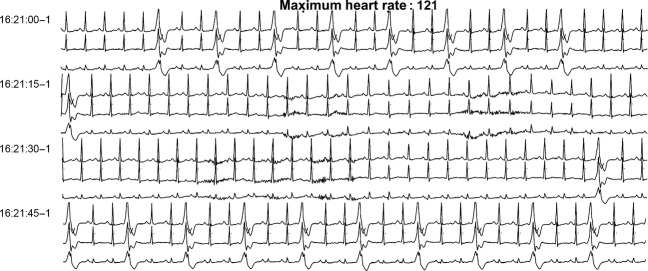
Baseline rhythm before any tyrosine kinase inhibitor (TKI) treatment, 24 h electrocardiogram (ECG).
Dasatinib treatment was interrupted for 7 days to ensure total body clearance (elimination half-life of 3–5 h) [14]. A 24 h ECG (Fig3) was then performed and showed a return to baseline values before the introduction of TKI and onset of ventricular excitability (17 episodes/day of NSVT and 22% of QRS complexes with VPBs). Dasatinib treatment was restarted and associated with an anti-arrythmic regimen of metoprolol and flecainide. The patient presented proximal limb myalgias after the first dose. A 24 h ECG (Fig4) was repeated and showed a return to a sinus rhythm without bi- or trigeminy and ventricular doublets and triplets, possibly due to metoprolol and flecainide therapy. The latter allowed to control temporarily the arrhythmia during dasatinib treatment until the patient proceeded to HLA (Human Leucocyte Antigen) identical sibling allogeneic hematopoietic stem cell transplantation 4 months after the start of dasatinib. Dasatinib treatment was interrupted before starting a reduced intensity conditioning regimen consisting of fludarabine, intravenous busulfan, and anti-thymoglobulin serum (Thymogobuline Genzyme®, Baar, Switzerland). At the time of transplant, a bone marrow examination showed a partial cytogenetic response with 25% Philadelphia-positive metaphases and the emergence of a new clone with trisomy 8 in the Philadelphia-negative metaphases, and a BCR-ABL/ABL ratio of 25%.
Figure 3.
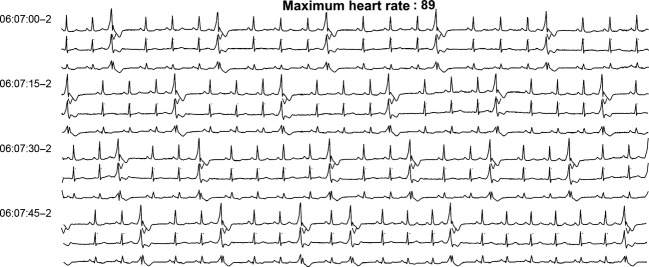
Rhythm strip after dasatinib withdrawal, 24 h electrocardiogram (ECG).
Figure 4.
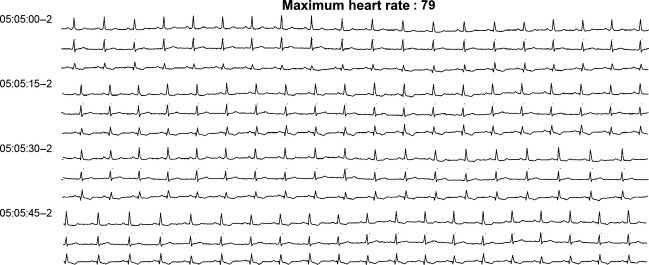
Rhythm strip after dasatinib rechallenge + metoprolol and flecainide therapy, 24 h electrocardiogram (ECG).
Following homograft, the patient showed a good clinical and paraclinical response. Dasatinib treatment was stopped and he has now been discharged from hospital.
Due to the observed response to dechallenge, the association of dasatinib to VPBs and NSVT arrhythmia was assessed as probable according to the World Health Organization Uppsala International Drug Monitoring Centre (WHO-UMC) system causality assessment [15] and the case was duly reported to the Swiss federal health authorities and the WHO-UMC.
Discussion
We describe a case of asymptomatic aggravation of VPBs and NSVT arrhythmia in a patient treated with dasatinib for CML. Dasatinib is a multityrosine kinase inhibitor active against BCR-ABL, kinases of the Src family (c-KIT, EPHA2, and platelet-derived growth factor [PDGF] receptor beta) with in vitro activity against imatinib-sensitive and resistant leukemic cell lines overexpressing BCR-ABL [16].
Tyrosine kinase inhibitors inhibit TK in cancerous and non-cancerous cells and their action on the latter is responsible for their adverse effects. The most frequent non-haematologic adverse effects associated with TKIs are gastrointestinal disorders and rash [17]. Cardiac adverse effects are poorly described in the published literature [9–11,18–20]. The general cardiotoxicity of TKI has been previously reviewed [17,21]. Adverse effects range from asymptomatic subclinical abnormalities, such as electrocardiographic changes and LVEF decline, to life-threatening events, such as congestive heart failure and acute coronary syndromes. In an observational study with patients treated with sunitinib or sorafenib for metastatic renal cell carcinoma, approximately 40% of patients showed ECG changes, arrhythmia, conduction disturbances, and QT prolongation. Half of these patients were asymptomatic [22].
Based on data from clinical studies and post-marketing experience, cardiac disorders are described as common (≥1/100 to <1/10) to uncommon (≥1/1000 to <1/100) for dasatinib [23]. TKI received approval on the basis of studies with relatively few patients, and no information is provided on the number of patients exposed to dasatinib, although the true frequency of cardiotoxicity could be under-recognized [21]. The United States Food and Drug Administration approval summary, which is based on safety data from 911 patients, reports two cases of patients with asymptomatic NSVT [24]. The database of the manufacturer of dasatinib records three cases of non-fatal arrhythmias, which appear to be poorly documented (unpublished data; information communicated by telephone to the authors by the manufacturer).
Of 2821 adverse drug reactions reported with dasatinib to the WHO-UMC, two other cases of ventricular tachycardia (VT) have been reported (data extracted 11 May 2012). However, the WHO-UMC database contains only spontaneous reports of adverse reactions from member countries, describing suspicions and observations of an unexpected or unwanted event, and these data cannot be used to calculate an incidence rate [25].
The patient described here shows monomorphic VPBs and NSVT, right bundle branch delayed conduction, and a right axis of LV ejection chamber origin. The mechanism of these VT on a healthy heart could be related to late potentials fired during phase 4 of action potential [26,27]. A rise in intracellular calcium and elevated circulating levels of catecholamines can promote the latter. Within the cell, the rise in cAMP (cyclic adenosine monophosphate) leads to an increase in intracellular calcium titer through the activation of protein-kinase A and the phosphorylation of the calcium channel. Any condition increasing cAMP has a pro-arrhythmic state. Thus, TKI therapy could lead to an increase in cAMP and exhibit pro-arrhythmic activity, although the mechanism remains unknown.
In summary, the available published literature suggests that TKI-associated cardiotoxicity may be a underestimated phenomenon. It seems that not all TKIs exert the same toxicity on the heart muscle, indicating that this is not a class toxic effect. However, the actual rates of cardiotoxicity are unknown as their detection is not included in most clinical trials. For this reason, it is difficult to know which TKI has minor cardiotoxicity. When comparing the clinical trials, it seems that dasatinib causes minor cardiotoxicity than others, but the present case emphasizes the need for closer rhythmic monitoring of patients treated with TKI for CML and we strongly recommend regular ECG controls. We recommend also that patients receiving TKI treatment should be aware about possible palpitations and dyspnoea and the need to inform their physician if these occur. Further clinical trials of TKIs including detection of cardiotoxicity are necessary to elucidate their potential to trigger this adverse event.
Consent
The patient has provided written consent for the case report to be published.
Acknowledgments
Thanks to Rosemary Sudan for the translation.
Conflict of Interest
None declared.
References
- 1.Chiang BN, Perlman LV, Fulton M, Epstein LD, Jr, Ostrander FH. Predisposing factors in sudden cardiac death in Tecumseh, Michigan. A prospective study. Circulation. 1970;41:31–37. doi: 10.1161/01.cir.41.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Packer M. Lack of relation between ventricular arrhythmias and sudden death in patients with chronic heart failure. Circulation. 1992;85(Suppl. 1):I50–I56. [PubMed] [Google Scholar]
- 3.Elkon KB, Swerdlow TA, Myburgh DP. Persistent ventricular ectopic beats: a long-term study. S. Afr. Med. J. 1977;52:564–566. [PubMed] [Google Scholar]
- 4.Rodstein M, Wolloch L, Gubner RS. Mortality study of the significance of extrasystoles in an insured population. Circulation. 1971;44:617–625. doi: 10.1161/01.cir.44.4.617. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N. Engl. J. Med. 1985;312:193–197. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 6.Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann. Intern. Med. 1992;117:990–996. doi: 10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 7.Barrett PA, Peter CT, Swan HJ, Singh BN, Mandel WJ. The frequency and prognostic significance of electrocardiographic abnormalities in clinically normal individuals. Prog. Cardiovasc. Dis. 1981;23:299–319. doi: 10.1016/0033-0620(81)90018-9. [DOI] [PubMed] [Google Scholar]
- 8.Fleg JL, Kennedy HL. Long-term prognostic significance of ambulatory electrocardiographic findings in apparently healthy subjects greater than or equal to 60 years of age. Am. J. Cardiol. 1992;70:748–751. doi: 10.1016/0002-9149(92)90553-b. [DOI] [PubMed] [Google Scholar]
- 9.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 10.Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 12.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J. Clin. Oncol. 2010a;28:424–430. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J. Clin. Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 15.WHO-UMC. 2012. The use of the WHO-UMC system for standardised case causality assessment [cited 07.02.2012]. Available at http://who-umc.org/graphics/24734.pdf (accessed 19 June 2013)
- 16.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 17.Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48:964–970. doi: 10.1080/02841860903229124. [DOI] [PubMed] [Google Scholar]
- 18.Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Pasquini R, Levy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R) Cancer. 2009;115:4136–4147. doi: 10.1002/cncr.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortes JE, Jones D, O'Brien S, Jabbour E, Ravandi F, Koller C, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J. Clin. Oncol. 2010b;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis J, Ahluwalia MS, Wetzler M, Wang E, Paplham P, Smiley S, et al. Reversible cardiotoxicity with tyrosine kinase inhibitors. Clin. Adv. Hematol. Oncol. 2010;8:128–132. [PubMed] [Google Scholar]
- 22.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 23.Bristol.Myers Squibb. 2010. Sprycel. Summary of product characteristics [cited 17.01.2012]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000709/WC500056998.pdf (accessed 19 June 2013)
- 24.Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin. Cancer Res. 2008;14:352–359. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 25.WHO-UMC. 2011. Caveat document accompanying statement to data released from the Uppsala Monitoring Centre, WHO Collaborating Centre for International Drug Monitoring. [cited 07.02.2012]; Available at http://www.who-umc.org/graphics/25300.pdf (accessed 19 June 2013)
- 26.Lerman BB, Stein K, Engelstein ED, Battleman DS, Lippman N, Bei D, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation. 1995;92:421–429. doi: 10.1161/01.cir.92.3.421. [DOI] [PubMed] [Google Scholar]
- 27.Lerman BB, Stein KM, Markowitz SM. Adenosine-sensitive ventricular tachycardia: a conceptual approach. J. Cardiovasc. Electrophysiol. 1996;7:559–569. doi: 10.1111/j.1540-8167.1996.tb00563.x. [DOI] [PubMed] [Google Scholar]


