Abstract
Objective
Febrile seizure (FS) is the most common form of childhood seizure disorders. FS is perhaps one of the most frequent causes of admittance to pediatric emergency wards worldwide. We aimed to identify a new, safe, and effective therapy for preventing FS recurrence.
Methods
A total of 115 children with a history of two or more episodes of FS were randomly assigned to levetiracetam (LEV) and control (LEV/control ratio = 2:1) groups. At the onset of fever, LEV group was orally administered with a dose of 15–30 mg/kg per day twice daily for 1 week. Thereafter, the dosage was gradually reduced until totally discontinued in the second week. The primary efficacy variable was seizure frequency associated with febrile events and FS recurrence rate (RR) during 48-week follow-up. The second outcome was the cost effectiveness of the two groups.
Results
The intention-to-treat analysis showed that 78 children in LEV group experienced 148 febrile episodes. Among these 78 children, 11 experienced 15 FS recurrences. In control group, 37 children experienced 64 febrile episodes; among these 37 children, 19 experienced 32 FS recurrences. A significant difference was observed between two groups in FS RR and FS recurrence/fever episode. The cost of LEV group for the prevention of FS recurrence is lower than control group. During 48-week follow-up period, one patient in LEV group exhibited severe drowsiness. No other side effects were observed in the same patient and in other children.
Interpretation
Intermittent oral LEV can effectively prevent FS recurrence and reduce wastage of medical resources.
Introduction
Febrile seizure (FS) is the most common form of childhood seizure disorders and accounts for 30% of seizures in children. FS is a benign condition. Children who have or have not suffered FS before their fifth birthday have similar academic and social successes.1 Nevertheless, FS is a traumatic experience and is a cause of panic for most parents. FS is also perhaps one of the most frequent causes of admittance to pediatric emergency wards worldwide.2 Considering that FS occurs in otherwise healthy children, an episode of generalized tonic–clonic convulsion represents an unfamiliar and terrifying event for most parents/caregivers. Very few parents will hesitate to dial 911 (120 in China) when witnessing a tonic–clonic seizure. These children are typically transported by emergency medical services to the emergency department. Patients who have experienced FS will present more emergently than febrile age-matched “controls” without seizure.3,4 Parental anxieties are typically high, and prehospital interventions such as intravenous line placements and supplemental oxygen can contribute to the child's distress while also consuming medical resources.
In previous years, interest has increased considerably in preventing FS and reducing its recurrence risk either by continuous treatment with antiepileptic drugs (AEDs) such as phenobarbital and valproic acid (VPA) or with intermittent treatment with a drug such as diazepam. Thus far, the evaluation of AEDs either administered continuously or intermittently during a febrile illness has been limited to old patients. Although phenobarbital, VPA, and primidone are considered effective in preventing the recurrence of FS when continuously administered,5 long-term treatment with such drugs is associated with a wide spectrum of adverse effects, including sedation, behavioral changes, gastrointestinal and hematologic toxicity, hypersensitivity reactions, and rare fatal hepatotoxicity with VPA in young children. Although the intermittent administration of benzodiazepines (e.g., diazepam and midazolam) at the onset of fever is effective in placebo-controlled trials,6 the effectiveness of this treatment is limited because sedative effects can mask the signs and symptoms of any evolving central nervous system infection.5,7,8 Considering that the potential toxicities associated with antiepileptic therapy outweigh the relatively minor risks associated with FS, the American Academy of Pediatrics does not recommend continuous antiepileptic therapy with phenobarbital or VPA and intermittent therapy with diazepam to prevent FS recurrences.9,5 If we can explore a drug that is not only effective in preventing FS recurrence but also safe, we can reduce the anxieties of parents/caregivers and the unnecessary wasting of medical resources.
Levetiracetam (LEV) is a novel AED with a unique mechanism of action that primarily involves interactions with the synaptic vesicle protein 2A.10,11 LEV has a favorable, dose-proportional pharmacokinetics in children,12,13 a relatively rapid onset of action (Cmax between 0.6 and 1.3 h), and a half-life of 6–8 h. In this study, we evaluated the efficacy and tolerability of intermittent LEV administration in preventing FS recurrence.
Subjects/Materials and Methods
Patients and study design
We performed a multicenter, randomized, controlled, 48-week follow-up parallel-group outpatient study in children with FS from five hospitals in China. The criteria for inclusion were as follows: children with a history of two or more episodes of FS within the last 6 months, at least one seizure recurrence within the last 2 weeks, and onset age between 3 months and 5 years. The participants were recruited from 31 October 2009 to 31 October 2011. The trial profile is summarized in Figure1. Another round of selection was performed in accordance with the further assessment of their conditions. The criteria for exclusion were as follows: episodes of previous seizures without fever, intracranial infections or head trauma, or current use of AEDs. The criteria for diagnosis of complex FS were FS duration longer than 15 min, repeated convulsions within the same day, and focal seizure activity or focal findings during the postictal period. Parents/caregivers were instructed to take a child's temperature immediately when the child appears ill or feverish, such as in cases of runny nose or nasal obstruction, hot flashes, sore throat, and constipation. Parents/caregivers were also instructed to administer promptly the study medication when the temperature indicates a fever. Patients in the LEV group received oral LEV at a dose of 15–30 mg/kg per day twice daily at the onset of fever (T > 37.5°C) for 1 week (therapy period), followed by dose tapering of 50% every 2 days until complete withdrawal at the second week (decrement period) (Fig.2). The parent/caregiver was instructed to administer any other antipyretic drug to their child when T > 38.5°C, with or without antibiotics as deemed appropriate by the attending pediatrician. The study was approved by the Medical Ethics Committee of the Chinese PLA General Hospital, Beijing, China. This study was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained from a parent/caregiver of each child before trial-related procedures were conducted.
Figure 1.
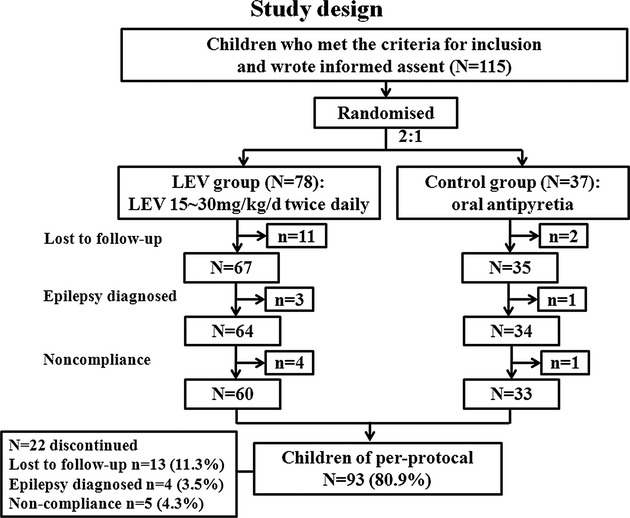
Trail profile: The 115 children who met the criteria for inclusion and signed the informed assent were divided into LEV group and control group randomly by 2:1 ratio. During the 48-week follow-up period, 13 children were lost to follow-up, four were diagnosed with epilepsy, five were noncompliant and discontinued their participation in the study, and 93 achieved the treatment goal at the end of the follow-up period.
Figure 2.
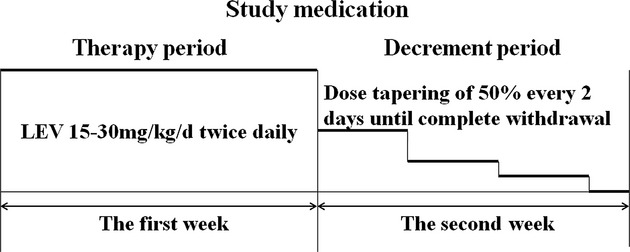
Study medication: Patient of LEV group received oral LEV 15–30 mg/kg per day twice daily at the onset of fever (T > 37.5°C) for 1 week (therapy period), then followed by dose tapering of 50% every 2 days until complete withdrawal at the second week (decrement period).
Randomization and masking
The sample size was obtained by calculating with SAS 9.1 (SAS, Cary, NC). A total of 115 patients were enrolled in the study. Randomization was computer generated with a block size of 3. The randomization code was managed by a centralized control. The center gathered and assigned the patients to the LEV and control groups in accordance with the mechanism of competitive enrollment. The responder of each site informed the study coordinator once a patient who met the criteria for inclusion signed the informed assent. Thereafter, the study coordinator opened the envelope corresponding to the order of the individual. The group wherein the patient was assigned was indicated inside the envelope.
Procedures
In accordance with the protocols, birth and development history, FS and seizure family history, liver and kidney function, neuroimaging (computer tomography [CT] or MRI), and electroencephalogram (EEG) (regular EEG or V-EEG) were taken. Pediatric neurology examination was performed outside an FS attack for all patients. Parents/caregivers were fully informed about the nature and management of FS. Follow-up observation tables were also distributed to the parents/caregivers for the recording of febrile and seizure events, temperature peaks, and adverse effects after medication at home. Parents/caregivers were contacted by telephone every 12 weeks to reinforce the study program. At each episode of febrile illness, parents/caregivers called the study site staff and provided all the necessary information about recurrence. The primary variable in efficacy was seizure frequency associated with febrile events and FS recurrence rate (RR) during the 48-week follow-up. The second variable in efficacy was the side effects associated with the drugs. These variables were analyzed at the end of the evaluation period, and the LEV and control groups were compared. For tolerability assessment, vital signs were assessed by multiparameter patient monitoring, including conditions such as mental state (e.g., changes in temper, lethargy), gastrointestinal symptoms (e.g., poor appetite, stomachache, vomiting), skin-related changes (e.g., rashes, pruritus), and body temperature observed at home. Upon admission to the study, parents/caregivers were instructed on what and how to observe vital signs. The parents/caregivers were assigned to assess treatment tolerability in accordance with the designed follow-up observation table. We measured the primary and secondary endpoints after all follow-up information on the patients were collected.
Statistical analysis
All results are presented for the intention-to-treat (ITT) population. All available data were used. Patients who discontinued the treatment early completed all end-of-study assessments. Descriptive statistics were used in the analysis of all efficacy, demographic, and baseline variables. Chi-square and Fisher's exact test were used to analyze the constituent ratio of gender, family convulsion history, FS type, number of patients lost to follow-up, diagnosed epilepsy, noncompliant cases, and temperature degree. Age of onset, visiting age, course of disease, and FS frequency before admittance to the study showed a skewed distribution, which was described with the median (Q1–Q3). The Wilcoxon rank-sum test was applied to compare the differences in these indexes between the LEV and control groups. Logistic regression analysis was performed to analyze seizure frequency associated with fever episodes and FS RR in the LEV and control groups. The odds ratio (OR) for FS recurrence relative to LEV with a 95% confidence interval (CI) was estimated. All statistical analyses were conducted by using SPSS 13.0 (SPSS Inc., Chicago, IL), and P < 0.05 was considered statistically significant. This study was registered as an International Standard Randomized Controlled Trial (No. ChiCTR-OCC-11001874). The formula for calculating the cost saving of LEV for the prevention of FS recurrence was described as follows:
where i is the FS frequency of one patient in 1 year, f1 is the one-time fee of LEV for the prevention of FS recurrence, f2 is the one-time fee for the prevention of FS recurrence in the control group (considering that no special medicine was used in the control group; thus, f2 was hypothesized as zero), and F is the medical costs produced after the failed prevention (direct medical costs = transport expenses of ambulance + outpatient or inpatient expenses; indirect medical costs = charge for loss of working time).
Results
Random sampling resulted in a sample size of 115 children (89 males and 29 females). From this sample, 78 children (61 males and 17 females) received oral LEV (15–30 mg/kg per day twice daily), whereas 37 children (25 males and 12 females) did not receive LEV in the primary analysis. The ranges of FS onset age, FS course, and visiting age in the LEV group were 3–55, 1–89, and 9–94 months, respectively. The medians (Q1–Q3) were 16 (11–22.75), 15 (9–27.5), and 33.5 (24–48.75), respectively. The range of FS frequency before enrollment was 2–15 times. The median (Q1–Q3) was 4 (3–5.75). Among the sample, 38.46% (30 of 78) had a family history of seizure disorder, 17.95% (14 of 78) were patients with complex FS, and 82.05% (64 of 78) were patients with simple FS.
In the control group, the ranges of FS onset age, FS course, and visiting age were 6–59, 0.5–43, and 12–79 months, respectively. The medians (Q1–Q3) were 22 (13–30), 12 (6–19), and 36 (25–45), respectively. The range of FS frequency before enrollment was 2–12 times. The median (Q1–Q3) was 3 (2–5). Among the sample, 21.62% (eight of 37) had a family history of seizure disorder, 24.32% (nine of 37) were patients with complex FS, and 75.68% (28 of 37) were patients with simple FS. Tables1, 2 summarize the demographics and characteristics of the sample.
Table 1.
Demographics and characteristics of the enrolled children.
| Variable | LEV group (N = 78) | Control group (N = 37) | Values | P value (two-sided) |
|---|---|---|---|---|
| Male/female | 61/17 | 25/12 | 1.5061 | 0.2542 |
| Onset age, months | ||||
| Range | 3–55 | 6–59 | ||
| M (Q1–Q3) | 16 (11–22.75) | 22 (13–30) | −2.6793 | 0.0074 |
| Visiting age, months | ||||
| Range | 9–94 | 12–79 | ||
| M (Q1–Q3) | 33.5 (24–48.75) | 36 (25–45) | −0.3273 | 0.7444 |
| Course of disease, months | ||||
| Range | 1–89 | 0.5–43 | ||
| M (Q1–Q3) | 15 (9–27.5) | 12 (6–19) | −1.8533 | 0.0644 |
| FS frequency before enrollment | ||||
| Range | 2–15 | 2–12 | ||
| M (Q1–Q3) | 4 (3–5.75) | 3 (2–5) | −2.0293 | 0.0424 |
| Family convulsion history | 30 | 8 | 3.2171 | 0.0912 |
| The type of seizures | ||||
| Complicated/simple | 14/64 | 9/28 | 0.6381 | 0.4602 |
χ2 value.
Fisher exact test.
Z value.
Wilcoxon rank-sums test.
Table 2.
The distribution of simple FS and complex FS.
| The type of FS | The number of patients | |
|---|---|---|
| LEV group | Control group | |
| Simple FS | 64 | 28 |
| Complex FS | 14 | 9 |
| Prolonged duration (>15 min) | 5 | 1 |
| Recurrent seizures within the same febrile illness over a 24-h period | 6 | 8 |
| Focal onset | 3 | 0 |
No significant differences were found in visiting age, disease cause, gender constitution (male/female), FS type (simple/complex), and patients with family convulsion history between the two groups (P > 0.05). However, a significant difference was found between the LEV and control groups (P < 0.05) in terms of FS onset age and FS frequency before enrollment. Hence, the FS onset age and FS frequency before enrollment were considered confounding factors for efficacy assessment.
EEG was performed on all children. No abnormal findings were found among the 86 children who underwent regular EEG. However, 21 abnormal findings were recorded from the 29 children who underwent V-EEG or active-EEG. The characteristics of abnormal EEG were summarized as bilateral leads paroxysmal or sporadic spikes and waves, slow waves, sharp waves, spike waves, or sharp waves during sleep. Eight children had abnormal birth histories, and two children had mild mental or motor retardation. Among the 100 children who underwent cranium imaging, 32 consented to MRI; a total of six abnormal findings were observed. Among the 68 children who underwent CT, one abnormal finding was observed. Table3 presents the essential information.
Table 3.
Essential information of the enrolled children.
| Investigations | No. of children | Abnormal findings | |
|---|---|---|---|
| EEG | 115 | ||
| Regular EEG | 86 | No | |
| V-EEG | 29 | 21 | |
| Bilateral leads paroxysmal or sporadic spike and waves, slow waves, sharp and waves, spike waves, or sharp waves during sleep | |||
| Cranium imaging | 100 | ||
| MRI | 32 | 6 | |
| Cornu posterius ventriculi lateralis lamellar long T2 signal with dilated lateral cerebral ventricle | |||
| Bilateral cerebral periventricle long T2 signal | |||
| Bilateral temporal lobe multiple punctiform long T2 signal | |||
| Right hippocampus long T2 signal | |||
| Right postoccipital neuroepithelial cyst | |||
| Left temporal pole arachnoid cyst | |||
| CT | 68 | 1 | |
| Cyst of pellucid septal cave | |||
| Liver function | 115 | No | |
| Birth history | 115 | 8 | |
| Low birth weight (SGA) | 3 | ||
| Premature infant associated with low birth weight | 5 | ||
| Developmental history | 115 | 2 | |
| Mild mental retardation IQ = 71 | 1 | ||
| Mild motor development retardation | 1 | ||
Thirteen children (11.3%) (11 in the LEV group and two in the control group) were lost to follow-up. Moreover, four children (3.5%) (three in the LEV group and one in the control group) discontinued their participation in the study because of diagnosed epilepsy, and five children (4.3%) (four in the LEV group and one in the control group) were noncompliant. No significant difference was found between the LEV and control groups in the constituent ratio (P > 0.05). This proportion of children did not affect the constituent ratio of the two groups (Table4).
Table 4.
The comparison of constituent ratio of discontinued patients.
| Discontinued reasons | LEV group (N = 78) | Control group (N = 37) | χ2 | P value1 (two-sided) |
|---|---|---|---|---|
| Lost/not lost | 11/67 | 2/35 | 1.893 | 0.218 |
| Epilepsy/nonepilepsy | 3/64 | 1/34 | 0.160 | 1.000 |
| Noncompliance | 4/60 | 1/33 | 0.502 | 0.656 |
Fisher exact test.
During the 48-week follow-up, ITT analysis showed 148 and 64 fever episodes in the LEV and control groups, respectively. The highest body temperatures were divided into four degrees: ≥40, 39–39.9, 38–38.9, and 37–37.9°C. No significant difference was found between the two groups in the constituent ratio of the maximum temperature (P = 0.064) (Table5).
Table 5.
The comparison of constituent ratio of fever episodes during follow-up.
| Temperature peak (°C) | LEV group (N) | Control group (N) | χ2 | P value1 (two-sided) |
|---|---|---|---|---|
| ≥40 | 1 | 3 | 6.791 | 0.064 |
| 39–39.9 | 46 | 24 | ||
| 38–38.9 | 91 | 30 | ||
| 37–37.9 | 10 | 7 |
Fisher exact test.
In the LEV group, 78 children experienced 148 febrile episodes. Among these 78 children, 11 experienced 15 FS recurrences. In the control group, 37 children experienced 64 febrile episodes. Among these 37 children, 19 experienced 32 FS recurrences during the 48-week follow-up, as indicated by the ITT analysis. The FS onset age and FS frequency before enrollment were divided into two stratifications. The ranges in the first and second stratifications of the FS onset age were <18 and ≥18 months, respectively. Before enrollment, the FS frequency had a range 2–4 times in the first stratification. The second stratification exhibited a range 5–15 times. The FS RR was 14.10% (11 of 78) in the LEV group and 51.35% (19 of 37) in the control group (P < 0.001) (Fig.3). The OR of FS RR with LEV treatment was 0.089, and the 95% CI was 0.029–0.268. The FS recurrence/fever episode was 10.14% (15 of 148) in the LEV group and 50.00% (32 of 64) in the control group (P < 0.001) (Fig.4). The RR of FS recurrence/fever episode with LEV treatment was 0.113, and the 95% CI was 0.055–0.233 (Tables6, 7).
Figure 3.
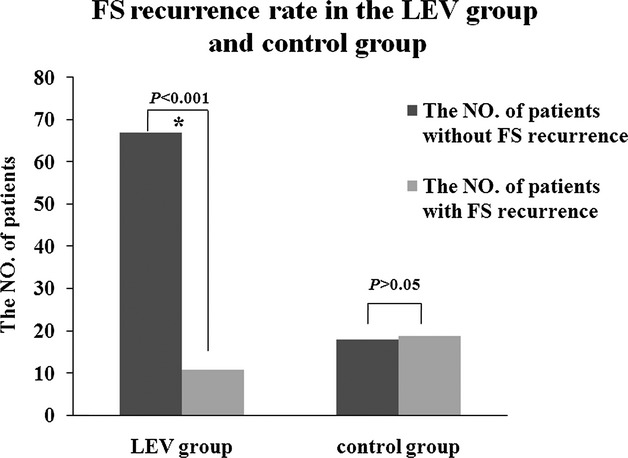
The FS recurrence rate in the LEV group and control group. The FS recurrence rate was 14.1% (11/78) in the LEV group and 51.4% (19/37) in the control group (P < 0.001).
Figure 4.
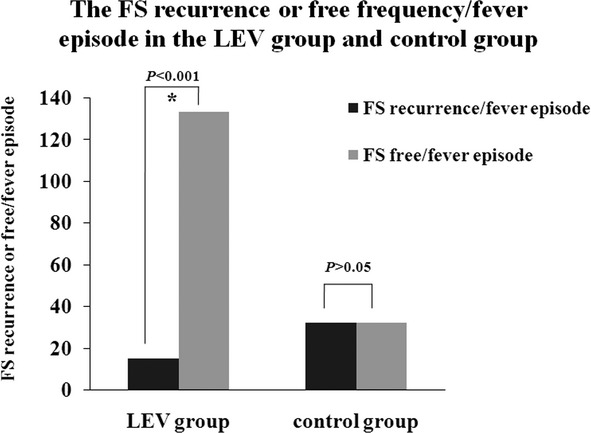
The FS recurrence or free frequency/fever episode in the LEV group and control group. The FS recurrence/fever episode was 10.14% (15/148) in the LEV group and 50.0% (32/64) in the control group (P < 0.001).
Table 6.
Logistic regression analysis of FS recurrent rate between LEV group and control group.
| Onset age (months) | FS frequency before enrolled | Group | The number of patients | χ2 | OR (95% CI) | P value (two-sided) | |
|---|---|---|---|---|---|---|---|
| Patients with FS recurrence | Patients without FS recurrence | ||||||
| <18 | 2–4 | LEV | 4 | 17 | 18.397 | 0.089 (0.029–0.268) | <0.001 |
| Control | 5 | 2 | |||||
| 5–15 | LEV | 6 | 15 | ||||
| Control | 4 | 2 | |||||
| ≥18 | 2–4 | LEV | 0 | 25 | |||
| Control | 8 | 10 | |||||
| 5–15 | LEV | 1 | 10 | ||||
| Control | 2 | 4 | |||||
OR, odds ratio; CI, confidence interval.
Table 7.
Logistic regression analysis of FS frequency/febrile episode between LEV group and control group.
| Onset age (months) | FS frequency before enrolled | Group | Fever episodes | χ2 | OR (95% CI) | P value (two-sided) | |
|---|---|---|---|---|---|---|---|
| FS frequency | FS-free frequency | ||||||
| <18 | 2–4 | LEV | 6 | 35 | 34.840 | 0.113 (0.055–0.233) | <0.001 |
| Control | 11 | 9 | |||||
| 5–15 | LEV | 7 | 53 | ||||
| Control | 5 | 3 | |||||
| ≥18 | 2–4 | LEV | 0 | 27 | |||
| Control | 11 | 13 | |||||
| 5–15 | LEV | 2 | 18 | ||||
| Control | 5 | 7 | |||||
OR, odds ratio; CI, confidence interval.
We calculated the cost saving of LEV for the prevention of FS recurrence in a child weighing 20 kg with FS. When presenting with fever, the one-time fee of LEV for the prevention of FS recurrence (f1) was 90 RMB; the dosage is 25 mg/kg and the outpatient and inpatient medical costs after failed prevention (F) were 998 and 5780 RMB on average, respectively, according to the formula described previously. LEV can save 308 RMB in the outpatient department and 2339 RMB in the inpatient department on average (Table8).
Table 8.
All kinds of medical costs and cost saving of LEV for prevention of FS recurrence one time in different center.
| All kinds of medical costs | Chinese PLA General Hospital | Jiang-Xi Children's Hospital | Beijing Children's Hospital | Chonging Children's Hospital | The Beijing New Century Children's Hospital | Average |
|---|---|---|---|---|---|---|
| Direct medical costs (RMB) | ||||||
| Transportation expenses of ambulance | 150 | 120 | 150 | 120 | 150 | 138 |
| Expenses of outpatient | 500 | 400 | 500 | 400 | 800 | 520 |
| Expenses of inpatient | 5000 | 3000 | 5000 | 3000 | 8000 | 4800 |
| Indirect medical costs (RMB) | ||||||
| Charge for loss of working time | ||||||
| Outpatient | 400 | 200 | 400 | 200 | 500 | 340 |
| Inpatient | 1000 | 600 | 1000 | 800 | 1500 | 998 |
| Fee of LEV for prevention of FS recurrence one time (RMB) | 90 | 90 | 90 | 90 | 90 | 90 |
| Cost saving of LEV for prevention of FS recurrence one time (RMB) | ||||||
| Outpatient | 329 | 197 | 329 | 197 | 488 | 308 |
| Inpatient | 2401 | 1483 | 2401 | 1563 | 3847 | 2339 |
On the basis of the complaints of the parents/caregivers of children who took the study medication during the course of fever, only one child experienced severe drowsiness after taking LEV once. Aside from this case, no other side effects were observed in the other children. Of the five noncompliant children, no one missed the dosage because of the side effects.
Discussion
FS is a convulsion associated with a significant rise in body temperature and pediatric emergency. However, an optimal strategy for preventing FS has not been established because the potential toxicities associated with antiepileptic therapy outweigh the relatively minor risks associated with FS. The patients who enrolled in the trial had at least two recent occurrences of FS, thus indicating that the patients have a high possibility for FS recurrence, which may cause accidental injuries and induce typically high panic among parents. Nevertheless, prophylactic therapy for FS recurrence should provide improved seizure protection but not at the expense of added toxicity and adverse effects. LEV has demonstrated good tolerability and efficacy against seizures as adjunctive therapy or monotherapy in children, including children aged 1 month to <4 years.14–18 In this study, we found that intermittent oral LEV can effectively prevent FS recurrence. With regard to safety, just one patient experienced drowsiness after taking LEV once. Nevertheless, we cannot tell whether the symptom was caused by the medicine or was merely one of the signs of fever because no side effects had previously occurred in this patient. Furthermore, no other adverse effects occurred in the other patients. Post hoc, intermittent LEV therapy was safe for FS patients at the same time.
The rationale behind the 2-week study design and the drug treatment is described as follows. Early and regular treatments for FS recurrence are consistently emphasized. However, 21% of the children experienced seizures prior to or within 1 h of the onset of fever, 57% had a seizure after 1–24 h of fever, and 22% experienced FS more than 24 h after the onset of fever.19 The fever of a child is not always recognized on time; this situation is one of the drawbacks of intermittent prophylaxis.20 FS is mostly caused by a variety of common infectious diseases. Acute upper respiratory tract infection or other viral illnesses, signs, and symptoms often last for 7–14 days, and the fever usually lasts 3–4 days.21–23 Given that the onset of symptoms typically occurs 1–3 days after viral or bacterial infection,23,24 we designed an LEV therapy period for 1 week when a child appeared ill or feverish (presenting runny nose or nasal obstruction, hot flashes, sore throat, and constipation). Parents/caregivers immediately took the child's temperature and administered the study medication promptly when febrile temperature was reached. The common mechanism of all AEDs is to inhibit paradoxical discharge from brain cells. Thus, unexpectedly stopping the medication may cause the abrupt removal of the inhibition of brain cells, thus making patients uncomfortable. Moreover, the V-EEG of some patients presented bilateral leads paroxysmal or sporadic slow waves, as well as sharp waves, spike waves, or sharp waves during sleep. Unexpectedly stopping the medication may adversely affect electrocerebral activity. To prevent the adverse effect caused by the sudden removal of LEV administration, the 1-week therapy was followed by a slow decrement period. A 1-week therapy period plus a 1-week decrement period is inconvenient for patients because the duration is long and noncompliance may be possible. Given that FS appears to occur consistently in the first 3 days, a comparison of the effects during a short medication period may be performed in the future.
The range of LEV dosage was set at 15–30 mg/kg per day taken twice daily. This range was large enough to be divided into high-dose and low-dose LEV groups. However, such dosage was designed for convenience in taking the medicine and in drug wastage because only 500 mg tablets are available in the Chinese market. Thus, the drug dose was not randomly allocated to the children in accordance with high or low doses. Further studies can improve the practical utility of the results through another randomized controlled trial test based on different LEV doses. The use of antipyretic medication cannot reduce the frequency of seizures.23 Therefore, we did not indicate what type of antipyretic should be taken at the onset of fever. The parents/caregivers could choose acetaminophen or ibuprofen to reduce fever and ensure patient comfort. The antipyretics used in this study worked as a pseudoplacebo but not as an active control, thus possibly introducing bias.
Given that intermittent oral LEV was effective in preventing FS recurrence and was safe for children, we proposed the use of intermittent oral LEV as preventive therapy. This approach may reduce the anxiety or panic of parents/caregivers, relieve pressure on the emergency department, and reduce the wastage of medical resources to a certain degree.
Acknowledgments
We would like to thank all physicians, children, and families of the children who provided clinical information. We also acknowledge the National Natural Science Foundation of China (Nos. 30770747, 81071036, 81200463, 81201013), the Beijing Municipal Natural Science Foundation (Nos. 7081002 and 7042024) and Zhejiang Provincial Natural Science Foundation of China (No. Y2100440).
Conflict of Interest
Foundation sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of report. There is no conflict of interest of any authors in relation to the submission. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Li-Ping Zou initiated the research program and supervised the project. Lin-Yan Hu, Li-Ping Zou, and Patrick Kwan wrote the manuscript. Lin-Yan Hu, Lei Gao, Jian-Min Zhong, Jian-Bo Zhao, Nong Xiao, Hong Zhou, Xiu-Yu Shi, Yu-Yie Liu, Jun Ju, Wei-Na Zhang, Xiao-Fan Yang, and Li-Ping Zou participated in recruitment of patients and data collection. Meng Zhao participated in statistical analysis. Patrick Kwan has received speaker's honoraria from GlaxoSmithKline and UCB Pharma, and has served on scientific advisory boards for GlaxoSmithKline and Eisai. Other coauthors report no disclosures.
References
- Sillanpää M, Suominen S, Rautava P, et al. Academic and social success in adolescents with previous febrile seizures. Seizure. 2011;20:326–330. doi: 10.1016/j.seizure.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Fetveit A. Assessment of febrile seizures in children. Eur J Pediatr. 2008;167:17–27. doi: 10.1007/s00431-007-0577-x. [DOI] [PubMed] [Google Scholar]
- Smith RA, Martland T, Lowry MF. Children with seizures presenting to accident and emergency. J Accid Emerg Med. 1996;13:54–58. doi: 10.1136/emj.13.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, King WD. Pediatric prehospital care in a southern regional emergency medical service system. South Med J. 1988;81:1473–1476. doi: 10.1097/00007611-198812000-00003. [DOI] [PubMed] [Google Scholar]
- Oluwabusi T, Sood SK. Update on the management of simple febrile seizures: emphasis on minimal intervention. Curr Opin Pediatr. 2012;2:259–265. doi: 10.1097/MOP.0b013e3283506765. [DOI] [PubMed] [Google Scholar]
- Offringa M, Newton R. Prophylactic drug management for febrile seizures in children. Cochrane Database Syst Rev. 2012;4:CD003031. doi: 10.1002/14651858.CD003031.pub2. . Available at http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003031.pub2. [DOI] [PubMed] [Google Scholar]
- Millar JS. Evaluation and treatment of the child with febrile seizure. Am Fam Physician. 2006;73:1761–1764. , 1765–1766. [PubMed] [Google Scholar]
- Daugbjerg P, Brems M, Mai J, et al. Intermittent prophylaxis in febrile convulsions: diazepam or valproic acid? Acta Neurol Scand. 1990;82:17–20. doi: 10.1111/j.1600-0404.1990.tb01581.x. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics, Subcommittee on Febrile Seizures. Clinical practice guideline-febrile seizures: guideline for the neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127:389–394. doi: 10.1542/peds.2010-3318. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Matagne A, Leclercq K, et al. SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology. 2008;54:715–720. doi: 10.1016/j.neuropharm.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain NB, Conry JA, Rodríguez-Leyva I, et al. Prospective assessment of levetiracetam pharmacokinetics during dose escalation in 4- to 12-year-old children with partial-onset seizures on concomitant carbamazepine or valproate. Epilepsy Res. 2007;74:60–69. doi: 10.1016/j.eplepsyres.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Pellock JM, Glauser TA, Bebin EM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia. 2001;42:1574–1579. doi: 10.1046/j.1528-1157.2001.41300.x. [DOI] [PubMed] [Google Scholar]
- Striano P, Coppola A, Pezzella M, et al. An open-label trial of levetiracetam in severe myoclonic epilepsy of infancy. Neurology. 2007;69:250–254. doi: 10.1212/01.wnl.0000265222.24102.db. [DOI] [PubMed] [Google Scholar]
- Andermann E, Andermann F, Meyvish P, et al. Efficacy and tolerability levetiracetam add-on therapy in patients with refractory idiopathic generalised epilepsy. Epilepsia. 2006;47:187. [Google Scholar]
- Labate A, Colosimo E, Gambardella A, et al. Levetiracetam in patients with generalized epilepsy and myoclonic seizures: an open label study. Seizure. 2006;15:214–218. doi: 10.1016/j.seizure.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Perucca E, Ryvlin P, et al. Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsy. Neurology. 2007;68:402–408. doi: 10.1212/01.wnl.0000252941.50833.4a. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Cerminara C, Domizio S, et al. Levetiracetam in absence epilepsy. Dev Med Child Neurol. 2008;50:850–853. doi: 10.1111/j.1469-8749.2008.03099.x. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Glauser TA. Febrile seizures. J Child Neurol. 2002;17:S44–S52. doi: 10.1177/08830738020170010601. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics, Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121:1281–1286. doi: 10.1542/peds.2008-0939. [DOI] [PubMed] [Google Scholar]
- Turner RB, Hayden GE. The Common Cold. In: Behrman RE, Kliegman R, Jenson HB, editors. Nelson textbook of pediatrics. 17th ed. Philadelphia, PA: Elsevier Science; 2003. pp. 1389–1390. [Google Scholar]
- Johnston MV. Seizures in Childhood. In: Behrman RE, Kliegman R, Jenson HB, editors. Nelson textbook of pediatrics. 17th ed. Philadelphia, PA: Elsevier Science; 2003. pp. 1993–2008. [Google Scholar]
- Upper respiratory tract infection. Available at http://en.wikipedia.org/wiki/Upper_respiratory_tract_infection (accessed 11 October 2013)
- Capovilla G, Mastrangelo M, Romeo A, et al. Recommendations for the management of “febrile seizures” Ad hoc Task Force of LICE Guidelines Commission. Epilepsia. 2009;50:2–6. doi: 10.1111/j.1528-1167.2008.01963.x. [DOI] [PubMed] [Google Scholar]


