Abstract
Objective
We sought to identify the prevalence of MRI features of disproportionately enlarged subarachnoid space hydrocephalus in possible idiopathic normal pressure hydrocephalus (DESH-iNPH) and to describe the clinico-radiological features and outcomes of a community-based investigation (The Vienna Trans-Danube Aging study).
Methods
Of the 697 inhabitants (all 75 years old), 503 completed extensive neurological examinations at baseline and were followed up every 30 months thereafter with MRIs, mini-mental state examination (MMSE), and the Unified Parkinson Disease Rating Scale-Motor Section (UPDRSM). The DESH-iNPH participant data were compared with the data from participants with Evans index ratios >0.3 (ex vacuo hydrocephalus), cerebral small-vessel diseases, and normal MRIs. The widening of perivascular space was also evaluated by MRI in these groups.
Results
Eight participants with DESH-iNPH (1.6%) and 76 with ex vacuo hydrocephalus (16.1%) at baseline were identified. The mean MMSE in DESH-iNPH, ex vacuo hydrocephalus, and normal MRIs was 26.4, 27.9, and 28.3, respectively, and the mean UPDRSM was 9.75, 2.96, and 1.87, respectively. After a 90-month follow-up, the mortality rates for DESH-iNPH, ex vacuo hydrocephalus, and normal MRIs were 25.0%, 21.3%, and 10.9%, respectively. The perivascular-space widening scores were significantly smaller in the DESH-iNPH cases, particularly at the centrum semiovale, compared to cerebral small-vessel disease and ex vacuo hydrocephalus cases.
Interpretation
The prevalence of DESH-iNPH was 1.6% for participants aged 75 years and revealed significantly lower MMSE and higher UPDRSM scores compared to the ex vacuo hydrocephalus and controls. Moreover, it is suggested that perivascular-space narrowing is a morphological and pathophysiological marker of DESH-iNPH.
Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is characterized by a clinical trial of gait disturbance, cognitive impairment, and urinary incontinence.1 iNPH has recently attracted attention as one of the few treatable causes of age-associated gait disturbance and cognitive impairment. Marmarou et al. revealed overall prevalence of suspected iNPH among patients residing in assisted-living and extended-care facilities ranged from ∼9% to 14%.2 Several recent studies have documented that the correct diagnosis of iNPH and good patient outcomes after shunt operations are supported by evidence-based guidelines for clinical diagnosis of iNPH and various brain imaging techniques,3,4 especially routine MRI evaluation of disproportionately enlarged subarachnoid space hydrocephalus.5–8 The aim of our study was to identify the prevalence of MRI features of disproportionately enlarged subarachnoid space hydrocephalus in possible iNPH (DESH-iNPH) and to describe the clinico-radiological features and outcomes of a community-based birth cohort investigation of The Vienna Trans-Danube Aging study (The VITA study). It is of note that iNPH brain or iNPH biopsy specimens often show cerebral small-vessel disease and Binswanger's disease pathologies as well as Alzheimer's disease pathology.9–11 DESH-iNPH participant data were also compared with data on cerebral small-vessel diseases. In cerebral small-vessel diseases, the typical vascular pathologies are ischemic white matter lesions (WML), multiple lacunes, and perivascular-space enlargement with fibro- and lipohyalinosis. These alterations ultimately result in Binswanger's disease.12–14 In this study, we evaluated not only ischemic WML scores and the number of lacunar infarcts but also the widening score of the perivascular space in DESH-iNPH and cerebral small-vessel diseases.
Methods
Study populations
The VITA study is a prospective cohort study of aging and dementia since 2000 organized by the Ludwig Boltzmann Institute of Aging Research and Danube Hospital. It was approved by the appropriate ethics committee.15,16 The participants were all 75-year-old inhabitants of the 21st and 22nd districts of Vienna, an area on the east shore of the Danube River. A total of 1920 individuals (765 males and 1155 females) who were born between May 1925 and June 1926 were identified; the birth data were extracted from official voting registries. At baseline, we investigated 697 inhabitants who agreed to participate, of whom 503 completed extensive neurological examinations, including MRI, the mini-mental state examination (MMSE), trail making tests A/B, and the Unified Parkinson Disease Rating Scale-Motor Section (UPDRSM), the Alzheimer's Criteria test from the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer's Disease and Related Disorders Association (ADRDA criteria). MMSE, trail making tests A/B, UPDRSM, and ADRDA criteria were performed after 30 and 60 months of follow-up and were compared with baseline data.
Clinical and MRI evaluations
DESH-iNPH was defined by disproportionate enlargement of the inferior subarachnoid spaces with tight high-convexity subarachnoid spaces and an Evans index ratio more than 0.3 on the baseline MRI.5–8 Enlargement and upward displacement of roof of Sylvian fissure7 and focally dilated sulci over the convexity or medial surface of the hemisphere5 was also included the additional supporting features. Data from DESH-iNPH participants, including MRI, MMSE, trail making test A/B, and UPDRSM, were compared with data from participants with Evans index ratios >0.3 (ex vacuo ventricular dilatation [VD]17), cerebral small-vessel diseases (diffuse WML and/or a scattering of lacunar infarcts), and normal MRIs. The term “cerebral small-vessel diseases” encompasses a range of features that are visible upon brain imaging, including lacunar infarcts, ischemic WML, microbleeds, and enlarged perivascular spaces. WML severity is evaluated from grades 0 to 4: 0, within normal limits; 1, punctuate; 2, early confluent; 3, confluent; and 4, diffuse. The severity of the lacunar state is evaluated from grades 0 to 3: 0, zero; 1, one to two lacunes; 2, three to four lacunes; and 3, more than five lacunes. The criteria for “MRI features on Binswanger's disease” include diffuse WML with a severity score of 3 or 4 and a scattering of multiple lacunes (more than five). The criteria for “MRI features on lacunar state” were defined as a scattering of lacunar infarcts (more than five) and WML with a severity score of 0 to 2. The criteria for “MRI features on diffuse WML” were defined as diffuse WML with a severity score of 3 or 4 and a severity of lacunar state from 0 to 2. The criteria for individuals with “normal MRI” are defined as diffuse WML with a severity score from 0 to 1 and a severity of lacunar state 0. Alzheimer's disease patients were diagnosed by interview assessments and using ADRDA criteria that corresponded to probable Alzheimer's disease. Parkinson's disease patients were diagnosed by their history of L-dopa administration and UPDRS evaluation.
MRI evaluation of perivascular space
In this study, we evaluated not only the severity scores of ischemic WML and the number of lacunar infarcts but also the perivascular space widening score on MRI. The widening score of the perivascular space was calculated by MRI evaluations modified from Kwee and Kwee18 and Patankar et al.19 The types of perivascular space widening were classified as the following three types: type I appears along lenticulostriate arteries and mostly in basal ganglia; type II appears along the paths of medullary arteries in the centrum semiovale; and type III appears in the brainstem (midbrain/pons) and hippocampus. Perivascular space widening score was evaluated in each type of perivascular space from grade 0 to 3 (0 = none, 1 = mild, 2 = moderate, and 3 = severe). Total perivascular space widening score was calculated by summing the three scores. Scheltens scale (medial temporal lobe atrophy) was also evaluated from baseline MRIs.20
Statistical analysis
The differences among UPDRSM, MMSE, trail making test A/B, and perivascular space widening score in DESH-iNPH, small-vessel disease, and normal MRIs groups were analyzed using the analysis of variance (ANOVA) with multiple comparisons. A P-value <0.05 was considered statistically significant.
Results
Prevalence and clinical features of DESH-iNPH and ex vacuo VD are summarized in Table 1. Eight participants with DESH-iNPH (1.6%) and 76 with ex vacuo VD (16.1%) were detected at baseline (Fig. 1). Two observers (I. A. and Y. S.) independently reviewed the MRI recordings from all participants of this study blinded to their clinical information. Interobserver agreements for MRI diagnosis of DESH-iNPH between two observers from 84 participants with Evans index ratio more than 0.3 were 89.3%. If DESH-iNPH with gait disturbance corresponded to possible iNPH, the prevalence of possible iNPH was 0.59%. The mean MMSE scores for DESH-iNPH, ex vacuo VD, and normal MRIs were 26.4, 27.9, and 28.3, respectively, and the mean UPDRSM scores were 9.75, 2.96, and 1.87, respectively. Longer performance time in trail making test A/B and highly frequent gait disturbance were also peculiar features of DESH-iNPH compared to ex vacuo VD and normal MRIs.
Table 1.
Prevalence and clinical features of DESH-iNPH, ex vacuo VD, and normal MRIs at baseline
| Prevalence at baseline | UPDRSM | MMSE | TMT-A | TMT-B | Hypertension | Gait disturbance | |
|---|---|---|---|---|---|---|---|
| Normal MRIs | 112 (22.3%) | 1.87 | 28.3 | 48.3 | 159.4 | 56.3% | 4.5% |
| DESH-iNPH | 8 (1.6%) | 9.75* | 26.4* | 102.1* | 262.9 | 75.0% | 37.5% |
| Ex vacuo VD | 76 (15.1%) | 2.96 | 27.9 | 47.4 | 161.6 | 64.5% | 9.2% |
DESH-iNPH, MRI features of disproportionately enlarged subarachnoid space hydrocephalus in possible idiopathic normal pressure hydrocephalus; ex vacuo VD, ex vacuo ventricular dilatation; UPDRSM, Unified Parkinson Disease Rating Scale-Motor Section; MMSE, mini-mental state examination; TMT-A and B, trail making tests A and B.
Significant as compare to those in normal MRIs and ex vacuo VD (P < 0.05).
Figure 1.
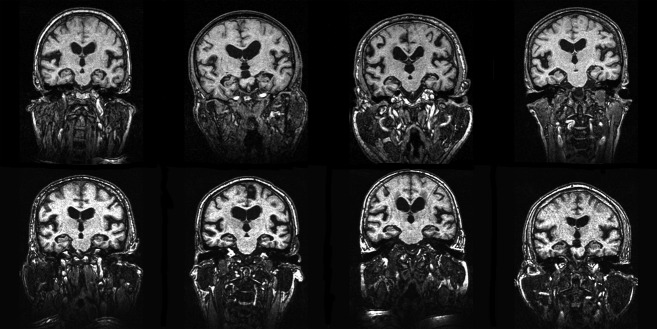
T1-weighted MRI images from eight participants with DESH-iNPH at baseline. DESH-iNPH, MRI features of disproportionately enlarged subarachnoid space hydrocephalus in possible idiopathic normal pressure hydrocephalus.
Table 2 shows comorbidity, outcomes, and MRI findings at baseline and at the 30- and 60-month follow-ups in eight participants with DESH-iNPH. Alzheimer's disease, MRI features on Binswanger's disease, and those on diffuse WML patients were comorbid in 25%, 12.5%, and 12.5% of DESH-iNPH cases, respectively. Multiple lacunes and periventricular and deep/subcortical WML more than grade 1 were observed in 63%, 88%, and 50% of DESH-iNPH cases, respectively. The DESH-iNPH and VD continued in all cases, and the severity of WML and the number of lacunes increased in three of eight cases during the 60 months of follow-up. Two of eight cases with DESH-iNPH (25%) and 16 with ex vacuo VD (21.3%) died during the 90-month follow-up period. Eight of 14 MRI features on Binswanger's disease patients (57.2%), nine patients with Alzheimer's disease (52.9%), four patients with Parkinson's disease (33.3%), and six patients with MRI features on lacunar state (26.1%) also died. Only three deaths were confirmed in the MRI features on diffuse WML group (15%), and 12 of 110 (10.9%) control cases with normal MRIs were confirmed.
Table 2.
Outcomes, MRIs, and comorbidities at baseline and 30- and 60-month follow-ups
| Outcome BL/30/60 | DESH-iNPH features | Evans index | PVWM | DWM | LS | Comorbid diseases | |
|---|---|---|---|---|---|---|---|
| 1 | BL/F/F | Yes/yes/yes | 0.32/0.31/0.35 | 1/2/2 | 0/1/1 | 1/1/1 | No |
| 2 | BL/F/N | Yes/yes/− | 0.38/0.39/− | 2/2/− | 0/1/− | 0/0/− | AD at 30 months follow-up |
| 3 | BL/F/F | Yes/yes/yes | 0.35/0.34/0.37 | 3/3/3 | 2/2/2 | 1/1/1 | No |
| 4 | BL/F/D | Yes/yes/− | 0.33/0.32/− | 3/3/− | 2/3/− | 2/3/− | AD at 30 months follow-up |
| 5 | BL/F/N | Yes/yes/− | 0.30/0.31/− | 2/2/− | 2/1/− | 0/0/− | No |
| 6 | BL/N/D | Yes/−/− | 0.39/−/− | 2/−/− | 1/−/− | 1/−/− | No |
| 7 | BL/F/F | Yes/yes/yes | 0.34/0.33/0.35 | 3/3/3 | 3/3/3 | 2/2/3 | BD & meningioma at BL |
| 8 | BL/F/F | Yes/yes/− | 0.31/0.31/− | 2/2/− | 1/1/− | 1/1/− | No |
BL, baseline; F, follow-up MRI; N, no follow-up MRI; D, died; AD, Alzheimer's disease; BD, Binswanger's disease; DESH-iNPH, MRI features of disproportionately enlarged subarachnoid space hydrocephalus in possible idiopathic normal pressure hydrocephalus; PVWM, periventricular white matter lesions; DWM, deep white matter lesions; LS, lacunar state.
Table 3 shows UPDRS, MMSE, and trail making test A/B at baseline and at the 30- and 60-month follow-ups in DESH-iNPH. During 60 months of follow-up, additional three participants with DESH-iNPH showed gait disturbance or worsening of total performance scores in UPDRSM. Trail making test A/B also deteriorated in two of these participants. MMSE was not worsened except for the two cases with both Alzheimer's disease and MRI features on Binswanger's disease.
Table 3.
UPDRS, MMSE and TMT-A/B at baseline and 30- and 60-month follow-ups
| Outcome BL/30/60 | UPDRSM gait | UPDRSM posture | UPDRSM chair | UPDRSM motor | MMSE | TMT-A | TMT-B | |
|---|---|---|---|---|---|---|---|---|
| 1 | BL/F/F | 0/0/1 | 0/0/0 | 0/0/0 | 5/9/8 | 26/26/26 | 68/69/− | 364/371/− |
| 2 | BL/F/N | 1/1/− | 0/0/− | 1/1/− | 7/21/− | 24/25/− | 58/83/− | 172/382/− |
| 3 | BL/F/F | 1/1/− | 0/1/− | 0/1/− | 12/29/− | 27/28/− | 75/147/− | 266/527/− |
| 4 | BL/F/D | 3/−/− | 2/−/− | 2/−/− | 40/−/− | 28/24/− | 363/319/− | 600/600/− |
| 5 | BL/F/N | 0/1/− | 0/1/− | 0/0/− | 2/15/− | 23/25/− | 47/−/− | 81/−/− |
| 6 | BL/N/D | 0/0/− | 0/0/− | 0/0/− | 4/−/− | 29/−/− | 69/−/− | 170/−/− |
| 7 | BL/F/F | 0/0/0 | 0/1/1 | 0/0/1 | 5/20/− | 24/25/22 | 82/73/− | 332/−/− |
| 8 | BL/F/F | 0/0/0 | 0/0/0 | 0/0/0 | 3/4/2 | 30/30/30 | 55/60/− | 118/130/− |
BL, baseline; F, followed up/tested; N, not tested (home visit, telephone interview, or refusal); D, died; DESH-iNPH, MRI features of disproportionately enlarged subarachnoid space hydrocephalus in possible idiopathic normal pressure hydrocephalus; UPDRSM, Unified Parkinson Disease Rating Scale-Motor Section; MMSE, mini-mental state examination; TMT-A and B, trail making tests A and B.
Perivascular space widening score, WML, Evans index, and Scheltens scale (medial temporal lobe atrophy) at baseline MRI are summarized in Table 4. Perivascular space widening score was significantly smaller in DESH-iNPH participants compared to both patients with cerebral small-vessel diseases and ex vacuo VD. DESH-iNPH showed the smallest perivascular space, particularly at the centrum semiovale, compared to those in ex vacuo VD and normal MRIs. Perivascular spaces in the DESH-iNPH brains were tight along with subarachnoid space tightness in the high convexities. Medial temporal lobe atrophy, which was revealed using the Scheltens scales, was prominent in DESH-iNPH, along with enlarged basal cisterns and VD, and significant compared to those in ex vacuo VD and normal MRIs (P < 0.05).
Table 4.
Perivascular space widening score, WML, Evans index, and Scheltens scale at baseline MRIs
| Perivascular space widening score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type I | Type II | Type III | Total | Periventricular WML | Deep WML | Evans index | Scheltens scale | |
| Normal MRIs | 0.89 | 0.79 | 0.63 | 2.30 | 0.29 | 0.4 | 0.27 | 0.13# |
| DESH-iNPH | 0.88 | 0.44* | 0.75 | 2.06** | 2.5# | 1.5 | 0.34 | 1.25# |
| Ex vacuo VD | 1.59 | 1.53 | 1.03 | 4.10 | 1.1 | 1.2 | 0.32 | 0.34# |
| MRI-BD | 1.96 | 1.21 | 0.86 | 4.04 | 3 | 3.7 | 0.31 | 0.62 |
| MRI-dWML | 1.53 | 1.47 | 0.71 | 3.68 | 3 | 3 | 0.28 | 0.40 |
| MRI-LS | 1.59 | 1.43 | 1.16 | 4.18 | 0.9 | 1.2 | 0.28 | 0.39 |
| Parkinson's D | 1.08 | 1.17 | 0.67 | 2.92 | 1.1 | 0.6 | 0.31 | 0.08 |
| Alzheimer's D | 1.15 | 1.27 | 0.62 | 3.04 | 1.3 | 1.2 | 0.31 | 0.67 |
Types of perivascular space widening: Type I appears along lenticulostriate arteries and mostly in basal ganglia; Type II appears along the paths of medullary arteries in the centrum semiovale; Type III appears in the brainstem (midbrain/pons) and hippocampus. DESH-iNPH, MRI features of disproportionately enlarged subarachnoid space hydrocephalus in possible idiopathic normal pressure hydrocephalus; MRI-BD, MRI-dWML, and MRI-LS = MRI features on Binswanger's disease, MRI features on diffuse WML, and MRI features on lacunar state, respectively.
Significant compared to those in ex vacuo VD, MRI-dWML, and MRI-LS, and
in ex vacuo VD, MRI-BD, and MRI-LS (P < 0.05).
Significant compared to those in ex vacuo VD and normal MRIs (P < 0.05).
Discussion
The prevalence of DESH-iNPH was 1.6% for participants aged 75 years in our study. If MRI features of iNPH with gait disturbance corresponded to possible iNPH,6,7,21 the prevalence of possible iNPH was 0.59%. In DESH-iNPH, the MMSE scores were significantly lower compared to those in normal MRIs and the UPDRSM scores were significantly higher those in ex vacuo VD and normal MRIs. Iseki et al. reported nearly the same prevalence in a Japanese cohort study as in our VITA study.22 In two age groups (61 and 70 to 72 years), the prevalence of DESH-iNPH and those with clinical features of iNPH (possible iNPH) were 1.5% and 0.5% (1.6% and 0.6% in the VITA study), respectively. A recent cohort study in the western region of Sweden also revealed that 2% of inhabitants aged 70–79 years showed the radiologic signs of iNPH including DESH-iNPH features; these results are consistent with those in Iseki's study and our own.23 Thus, the prevalence of DESH-iNPH in elderly people aged around 70 or 75 years is approximately the same and a considerably high worldwide, ranging from 1.5% to 2.0%. In addition, in our study, the participants with DESH-iNPH showed significantly low MMSE scores and high UPDRSM scores compared to those in the ex vacuo VD case and control groups at baseline. Among eight participants with DESH-iNPH, only three of them had a gait disturbance at baseline, however, additional three participants showed gait disturbance or worsening of total performance scores in UPDRSM and one died during 60 months of follow-up. The MRI features of iNPH, VD, and severity of WML also continued or deteriorated in all eight cases during 60 months of follow-up. These results indicate a significant role for the MRI-based scheme of iNPH features in the radiological diagnosis and pathomorphological aspects of iNPH.7 However, it remains unknown whether participants with DESH-iNPH all go on to develop clinical features of iNPH or not. Further study is needed to clarify this issue.
What mechanism of intracranial fluid hydrodynamics becomes predominantly impaired in an iNPH brain? Is it an alternative pathway for interstitial fluid via small vessels and/or lymphatic perivascular drainage24–26? Alternatively, is it the classical pathway for cerebrospinal fluid (CSF) via the arachnoid villi/lateral canal and venous sinuses27? Perhaps both of these factors play a role, along with developmental and/or late adulthood disease process.28,29 Bradley et al. proposed a hypothesis that iNPH could result from decreased CSF resorptive capacity by arachnoid villi, leading to benign external hydrocephalus in infancy, followed by deep white matter ischemia in late adulthood (the more hydrophilic environment increasing resistance to CSF flow through the extracellular space).29 We preliminary reported that immunogold high-molecular albumin leaked out of the basement membrane and extracellular space, in and around the cerebral small vessel in iNPH biopsy specimens.30 No study, however, has demonstrated the relationship between iNPH and perivascular space derangement.
It is suspected that the pathologies of perivascular space and cerebral small vessels have a common base.13,31 According to recent fine structural studies, perivascular space and subarachnoid space are separated from one another by the pia mater, and the intra-cerebral artery is covered with a sheath of perivascular space running entirely from the subarachnoid space to the brain parenchyma.32–34 Perivascular space enlargement and vascular ectasia are common findings in aged brains.35 Moreover, perivascular space expansion is often accompanied by mild WM rarefaction and Binswanger's disease pathology accelerated in and around perivascular space.36 Binswanger's disease brains showed significant collagenosis, T-cell infiltration, microglial activation, and derangement in axonal transport in and around perivascular space compared to brains with a small number of lacunes and control brains.11
It is of note that iNPH brain or iNPH biopsy specimens often show cerebral small-vessel disease and Binswanger's disease pathologies as well as Alzheimer's disease pathology.9–11 Our VITA study revealed comorbidities of these disorders (>50%) at age 75. Even with these comorbidities, however, Binswanger's disease is the predominant type of subcortical vascular dementia, showing distinct pathomorphology of hypertensive small artery disease12,13 and Alzheimer's disease has definite diagnostic criteria with A-beta and tau pathological staging.37 We previously reported that postsurgical evaluation in iNPH patients without Binswanger's disease showed significant improvement in Evan's index, mean total scores for WML, MMSE, and urinary disturbance compared to patients with MRI features on Binswanger's disease.30 It has also been reported that moderate to severe Alzheimer's disease pathology in cortical biopsy specimen is associated with worse baseline cognitive performance and diminished postoperative improvement on iNPH symptom severity scales compared to patients lacking pathology.37
In this study, the perivascular space widening score was significantly smaller in DESH-iNPH patients compared to both patients with cerebral small-vessel diseases and ex vacuo VD. Thus, perivascular space in DESH-iNPH becomes tight, particularly at the centrum semiovale, along with a narrowed CSF space at high convexity and periventricular WML. The major route for CSF drainage in healthy human adults appears to be into venous blood via arachnoid villi/granulations or into capillary walls and perivascular/paravenous pathways of the brain parenchyma along with interrelation between CSF and interstitial fluids of the extracellular space.28 Moreover, some lymphatic CSF drainage may occur via nasal mucosa and spinal nerve roots.26,28,38 However, the number of volumes of interstitial fluids that drain into the CSF in healthy and iNPH brains is not well known. Gadolinium injected into the cerebral cortex of normal young rats spreads into the white matter and enters the ventricles, but this fluid movement is impaired in hydrocephalus.39 Moreover, in the acute stage of hydrocephalus, CSF passes into the periventricular white matter (because of impaired drainage of CSF from the ventricles) and causes interstitial edema.27,28 This process might be caused by the lack of efficient perivascular drainage of interstitial fluids in the white matter compared to that in the gray matter.40 There is an upregulation of aquaporin-4 in hydrocephalus suggesting that CSF is absorbed into the blood from the edematous periventricular white matter.41 Much attention has been paid recently that cerebral amyloid angiopathy in cortical and leptomeningeal arteries blocks the flow of interstitial fluid along the wall of arteries in the white matter with consequent derangement of the perivascular space, deposition of beta-amyloid, and Alzheimer's disease pathology.42 These processes may explain why perivascular space becomes narrow (this process occurs predominantly at the centrum semiovale), why periventricular white matter hyperintensity in MRI is shunt-responsive and considerably reversible in iNPH brains,6 and why iNPH brain or iNPH biopsy specimens often show Alzheimer's disease pathology.37
It has been suggested that perivascular space narrowing at the centrum semiovale is a significant radiological and pathomorphological marker of iNPH brains and might further accelerate the dysfunction of lymphatic perivascular drainage of CSF. Further study will be needed to confirm the pathoetiological significance of perivascular space narrowing in iNPH brains.
Author Contributions
Dr. Ichiro Akiguchi was responsible for the conception of the study and design, acquisition of data, and analysis and interpretation. Dr. Yoshitomo Shirakashi, Dr. Susanne Jungwirth, and Dr. Wolfgang Krampla acquired the data; Dr. Herbert Budka and Dr. Peter Fischer supervised the study; Dr. Yuko Watanabe, Dr. Toshiyuki Watanabe, Dr. Akihiko Shiino, Dr. Mihoko Ogita, and Dr. Yasuhiro Kawamoto analyzed and interpreted the data.
Conflict of Interest
None declared.
References
- 1.Adams R, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with normal cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273:117–126. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- 2.Marmarou A, Young HF, Aygok GA. Estimated incidence of normal pressure hydrocephalus and shunt outcome in patients residing in assisted-living and extended-care facilities. Neurosurg Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.4.2. [DOI] [PubMed] [Google Scholar]
- 3.Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S4–S16. doi: 10.1227/01.neu.0000168185.29659.c5. [DOI] [PubMed] [Google Scholar]
- 4.Ivkovic M, Liu B, Ahmed F, et al. Differential diagnosis of normal pressure hydrocephalus by MRI mean diffusivity histogram analysis. AJNR Am J Neuroradiol. 2013;34:1168–1174. doi: 10.3174/ajnr.A3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitagaki H, Mori E, Ishii K, et al. CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR Am J Neuroradiol. 1998;19:1277–1284. [PMC free article] [PubMed] [Google Scholar]
- 6.Akiguchi I, Ishii M, Watanabe Y, et al. Shunt-responsive parkinsonism and reversible white matter lesions in patients with idiopathic NPH. J Neurol. 2008;255:1392–1399. doi: 10.1007/s00415-008-0928-1. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto M, Ishikawa M, Mori E, Kuwana N Study of INPH on neurological improvement (SINPHONI) Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res. 2010;7:18. doi: 10.1186/1743-8454-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida N, Nagata N, Toda H, et al. Association of lipocalin-type prostaglandin D synthase with disproportionately enlarged subarachnoid-space in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2014;11:9. doi: 10.1186/2045-8118-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koto A, Rosenberg G, Zingesser LH, et al. Syndrome of normal pressure hydrocephalus: possible relation to hypertensive and arteriosclerotic vasculopathy. J Neurol Neurosurg Psychiatry. 1977;40:73–79. doi: 10.1136/jnnp.40.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bech RA, Waldemar G, Gjerris F, et al. Shunting effects in patients with idiopathic normal pressure hydrocephalus; correlation with cerebral and leptomeningeal biopsy findings. Acta Neurochir (Wien) 1999;141:633–639. doi: 10.1007/s007010050353. [DOI] [PubMed] [Google Scholar]
- 11.Golomb J, Wisoff J, Miller DC, et al. Alzheimer's disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry. 2000;68:778–781. doi: 10.1136/jnnp.68.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Román GC, Erkinjuntti T, Wallin A, et al. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 13.Akiguchi I, Tomimoto H, Suenaga T, et al. Alterations in glia and axons in the brains of Binswanger's disease patients. Stroke. 1997;28:1423–1429. doi: 10.1161/01.str.28.7.1423. [DOI] [PubMed] [Google Scholar]
- 14.Román G. Cerebral small-vessel disease: what lies beyond the early years? Neurology. 2011;76:684–685. doi: 10.1212/WNL.0b013e31820eb127. [DOI] [PubMed] [Google Scholar]
- 15.Fischer P, Jungwirth S, Krampla W, et al. Vienna Transdanube Aging “VITA”: study design, recruitment strategies and level of participation. J Neural Transm Suppl. 2002;62:105–116. doi: 10.1007/978-3-7091-6139-5_11. [DOI] [PubMed] [Google Scholar]
- 16.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 17.Ohno N, Miyati T, Mase M, et al. Idiopathic normal-pressure hydrocephalus: temporal changes in ADC during cardiac cycle. Radiology. 2011;261:560–1565. doi: 10.1148/radiol.11101860. [DOI] [PubMed] [Google Scholar]
- 18.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27:1071–1086. doi: 10.1148/rg.274065722. [DOI] [PubMed] [Google Scholar]
- 19.Patankar TF, Mitra D, Varma A, et al. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard CG, Burton EJ, Barber R, et al. NINDS AIREN neuroimaging criteria do not distinguish stroke patients with and without dementia. Neurology. 2004;63:983–988. doi: 10.1212/01.wnl.0000138435.19761.93. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa M, Hashimoto M, Kuwana N, et al. Guidelines for management of idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 2008;48(suppl):S1–S23. doi: 10.2176/nmc.48.s1. [DOI] [PubMed] [Google Scholar]
- 22.Iseki C, Kawanami T, Nagasawa H, et al. Asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on MRI (AVIM) in the elderly: a prospective study in a Japanese population. J Neurol Sci. 2009;277:54–57. doi: 10.1016/j.jns.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Jaraj D, Rabiei K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82:1449–1454. doi: 10.1212/WNL.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27:145–165. doi: 10.1007/s10143-004-0326-9. [DOI] [PubMed] [Google Scholar]
- 25.Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 26.Orešković D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev. 2010;64:241–262. doi: 10.1016/j.brainresrev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Yamashima T. Functional ultrastructure of cerebrospinal fluid drainage channels in human arachnoid villi. Neurosurgery. 1988;22:633–641. doi: 10.1227/00006123-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 29.Bradley WG, Jr, Bahl G, Alksne JF. Idiopathic normal pressure hydrocephalus may be a “two hit” disease: benign external hydrocephalus in infancy followed by deep white matter ischemia in late adulthood. J Magn Reson Imaging. 2006;24:747–755. doi: 10.1002/jmri.20684. [DOI] [PubMed] [Google Scholar]
- 30.Shirakashi Y, Akiguchi I, Watanabe Y, et al. Clinicoradiologic and pathological studies on the relationship between Binswanger's disease and idiopathic NPH: does the pathogenesis of the two diseases overlap? Brain Pathol. 2010;20(suppl 1):23. [Google Scholar]
- 31.Doubal FN, MacLullich AM, Ferguson KJ, et al. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–454. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997;191:337–346. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wuerfel J, Haertle M, Waiczies H, et al. Perivascular spaces – MRI marker of inflammatory activity in the brain? Brain. 2008;131:2332–2340. doi: 10.1093/brain/awn171. [DOI] [PubMed] [Google Scholar]
- 35.Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- 36.Akiguchi I, Tomimoto H, Wakita H, et al. Cytopathological alterations and therapeutic approaches in Binswanger's disease. Neuropathology. 1999;19:119–128. doi: 10.1046/j.1440-1789.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton R, Patel S, Lee EB, et al. Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann Neurol. 2010;68:535–540. doi: 10.1002/ana.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoesmith CL, Buist R, Del Bigio MR. Magnetic resonance imaging study of extracellular fluid tracer movement in brains of immature rats with hydrocephalus. Neurol Res. 2000;34:111–116. doi: 10.1080/01616412.2000.11741045. [DOI] [PubMed] [Google Scholar]
- 40.Carare RO, Bernardes-Silva M, Newman TA, et al. Solute, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries. Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 41.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23:2929–2936. doi: 10.1111/j.1460-9568.2006.04829.x. [DOI] [PubMed] [Google Scholar]
- 42.Roher AE, Kuo YM, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol Med. 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]


