Abstract
Saffold cardiovirus, a newly discovered human cardiovirus, has close similarity with Theiler's murine encephalomyelitis virus (TMEV) which can cause a chronic demyelinating encephalomyelitis in mice. In this study, we tested whether Saffold cardiovirus infection of the brain is associated with multiple sclerosis (MS). Autopsy white matter samples from 19 MS and 9 normal brain donors were tested by polymerase chain reaction. All were negative. Paired cerebrospinal fluid and serum samples from 24 MS patients and 27 controls were tested for Saffold cardiovirus-specific oligoclonal bands, two patients and two controls reacted positive. We conclude that an association between Saffold cardiovirus and MS is highly improbable.
Introduction
The possible relation between multiple sclerosis (MS) and viruses has a long-standing history. In spite of extensive searches, data that reach beyond an association have not been produced.1 The search for causal viruses is fuelled by epidemiological data suggesting an infectious trigger and by the enigmatic specificity of unique oligoclonal IgG bands (OCB) that are found in the cerebrospinal fluid of ∼95% of MS patients and reflect intrathecal IgG synthesis.2,3 Several of the OCBs have antiviral reactivity as can be found in response to central nervous system (CNS) infections. Moreover, a substantial proportion of the B cells in the CNS of MS patients have characteristics of antibody secreting plasma blasts, suggesting ongoing antigenic stimulation and inflammation.4 The latter is concordant with a chronic infection of which autoimmunity may be a consequence.5
Another reason for revisiting the subject is the discovery of Saffold cardiovirus (SAFV) which is the first human virus species belonging to the genus Cardiovirus.6 Cardioviruses are well-known pathogens in rodents and Theiler's murine encephalomyelitis virus (TMEV) is a prototype mouse cardiovirus that can cause a chronic encephalomyelitis with demyelination showing many similarities with MS (reviewed in reference 7).
SAFV has a close genetic similarity with Theiler's like cardioviruses of rodents and has therefore also been named Human Theiler's like Cardiovirus (HTCV).8 By analogy, a relationship of SAFV infection with MS should be considered. So far, only one group has studied whether SAFV RNA sequences can be detected in cerebro spinal fluid (CFS) samples from MS patients but with negative results.8
Previously, we have shown that SAFV infections in humans are ubiquitous and occur early in life.9,10 Up to now, no clear illness has been attributed to SAFV infections and the virus has rarely been detected in CSF.8,11–13 Here, we present an in-depth search for SAFV RNA sequences in brain samples from MS patients and whether the unique OCB in CSF of MS patients do recognize SAFV antigens.
Materials and Methods
Patients and specimens
Postmortem white matter brain tissue from 19 MS patients and 9 brain donors without brain disease was obtained from the Netherlands Brain Bank (http://www.brainbank.nl). Both MS patients and donors had given informed consent for brain autopsy and for use of tissue and clinical information for research purposes. The quality of brain tissue was assessed by measurement of pH and of RNA integrity measured on an Agilent 2100 Bioanalyser (Agilent Technologies, San Diego, CA) and expressed in RNA Integrity Numbers (RIN).14 In the brain samples used for this study the pH ranged between 6.1 and 7.2. None of the specimens had a RIN value below six (indicating substantial RNA breakdown). RIN values ≥6 were previously shown not to affect RT-qPCR efficiency for reference genes (unpublished observations). Clinical diagnoses of MS were confirmed by a neurologist (Prof. C. H. Polman, VUmc, Amsterdam, the Netherlands). The MS brain donors (2 males and 17 females) had a median age of 62 years (range 38–84). Seventeen of the 19 MS brains contained still active white matter lesions (HLA DR+ and/or PLP+ macrophages), 12 of the 19 MS brains showed even more than 50% active lesions.15,16 From 6 of the 19 MS patients, thick cryostat sections of active MS lesions were directly tested by polymerase chain reaction (PCR). All other specimens were normal appearing white matter (NAWM). The nine white matter specimens from donors without a brain disease were derived from one male and eight females with a median age of 61 years, range 41–82.
Paired CSF and serum samples drawn for diagnostic routine were available from the Neurology Department in Nijmegen. The samples were from 24 MS patients, 7 males and 17 females, with a median age of 42 years, range 24–66, and from 27 patients with other illnesses (7 males and 19 females, median age 41 years, range 32–59). For one control subject information on gender and age is lacking. Permission for anonymous testing of these samples fulfilled the terms of the ethical code “Goed Gebruik” of the Netherlands Federation of Medical Scientific Societies (Federa).
Virus antigens
For antigen production, a SAFV type 3 strain was grown in HeLa cells as reported earlier.9 The culture supernatant was concentrated by Amicon filtration. The final preparation had a protein concentration of 65 mg/mL and a virus titer of 3x10e11.
Real-time PCR
Brain tissue samples were homogenized by bead beating (Roche Diagnostics, Almere, The Netherlands), and nucleic acid isolation, copyDNA synthesis, and real-time PCR (50 cycles of 95°C for 15 sec and 60°C for 45 sec) were performed essentially as described previously,17 using SAFV-specific primers (GCTGGGGTTGCACCGCTA and GAGCCTCTGCGGCCAAA) and a hydrolysis probe (6FAM-ACAGCAGTGGATCTTATCCACGGGGC-BBQ). Positive and negative controls for isolation, reverse transcription, and PCR were included in each run.17 The detection limit was less than 10 infectious units/mL.
Immunoblotting technique
Immunoblotting was performed, essentially as described by Sindic et al.18 Briefly, equal amounts of IgG (250 ng) purified from paired CSF and blood samples, were separated by isoelectric focusing. Next, the agarose gels were blotted on a sheet of nitrocellulose that was pretreated by saturation with viral antigen (65 mg/sheet) and blocking with Phosphate Buffered Saline (PBS) containing 3% Bovine Serum Albumin. After washing, the blots were incubated with alkaline phosphatase-conjugated goat anti-human IgG at a 1:2500 dilution and stained with nitro blue tetrazolium.
Results
Detection of SAFV genomic RNA in brain lesions from MS patients
After invasion of the CNS, a virus can either behave as an immunological trigger of autoimmunity and disappear (“hit-and-run” model) or establish a low-grade persistent infection accompanied by inflammation, demyelination, and autoimmunity as found in animal models of MS.7,19 To test for the option of a low-grade chronic infection, autopsy samples of brains from 19 MS patients (17 with active lesions on microscopy) were tested by a highly sensitive RT-PCR for SAFV genomes. All samples reacted negative (data not shown). Nine additional brain samples without signs of MS or another inflammatory process that were tested for comparison were also negative. Thus, we concluded that a chronic SAFV infection of the brain in MS is highly unlikely.
Virus-specific immunoblotting
In case of the “hit-and-run” option, a neuro-invasive infection will probably leave an immunological imprint behind of intrathecal IgG antibodies.20 CSF was analyzed for intrathecal synthesis of SAFV antibodies by means of the immunoblotting technique that tests unique OCB for their reactivity with the virus. We tested paired CSF and blood samples from 25 MS patients and 27 patients without a neuro-inflammatory condition. As illustrated in the Figure 1, some CSF samples showed unique reactivity with SAFV antigens, whereas no reactivity was found in serum. In total, 2 of 25 CSF samples from MS patients and 2 of 27 control CSF samples showed SAFV antigen reactivity, refuting a direct association of SAFV infection with MS.
Figure 1.
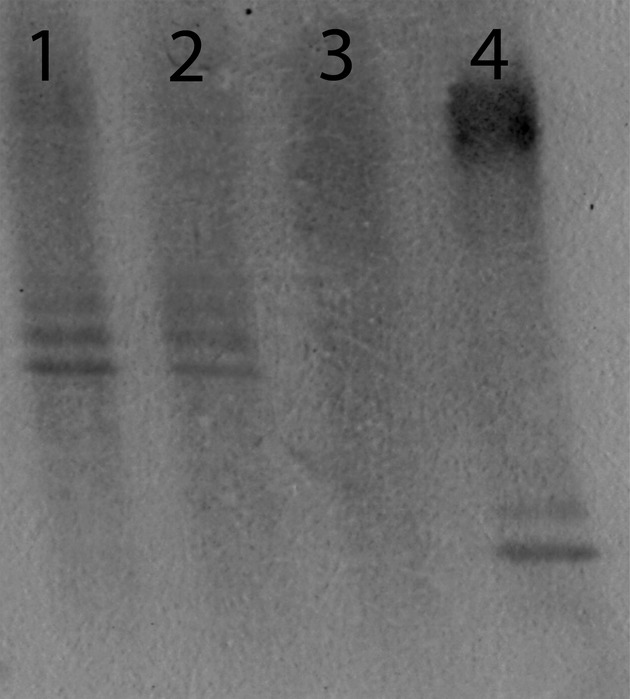
Immunoblot staining for SAF virus-specific oligoclonal IgG bands in two multiple sclerosis (MS) patients. The figure shows paired serum and CSF oligoclonal IgG blots from two MS patients: lanes 1 (serum) and 2 (CSF) show a mirror pattern of SAF virus-specific IgG bands; lanes 3 (serum) and 4 (CSF) from a second MS patient show unique SAF virus-specific IgG bands in CSF only.
Discussion
Although SAFVs have only recently been discovered, serologic epidemiology showed that infections are ubiquitous and acquired early in life.9,10 Ubiquity suggests moreover that SAFVs must have a long-standing history of infecting mankind. The large majority of SAFV infections are asymptomatic or mild and a clear disease association still awaits discovery.11 Little is yet known about the pathophysiology of SAFV infections other than that the infections are ubiquitous, mostly pass unnoticed, but sporadically present with a serious CNS infection.12,13 This picture shows close similarity with TMEV infections of mice, which are widespread in nature and cause very sporadically an MS-like chronic demyelinating illness, actually only when genetically susceptible mice become infected by a unique variant of TMEV.7 By analogy, a relationship of MS with SAFV infection could have been the rare outcome of a chronic encephalomyelitis caused only by particular SAFV strains and only when occurring in genetically predisposed individuals. In this manner, a ubiquitous virus (SAFV) could have been the cause of MS, fulfilling a condition of monocausality: one virus, one disease, as discussed by Lipton and colleagues.19
However, in our study we found no support for a chronic SAFV infection in MS brains. We applied a highly sensitive RT-PCR and tested selected brain specimen from 19 autopsy-confirmed MS patients, 12 of 19 specimens were highly selected samples from areas with inflammation. None reacted positive, making the chance of having missed a slowly ongoing chronic encephalomyelitis very small: its existence in MS must be quite improbable. These findings are corroborated by a negative PCR in CSF samples from 40 MS patients reported earlier.8
Considering that a chronic SAFV encephalomyelitis may have died out by gain of an adequate intracerebral immune response, one may expect that an antibody imprint will still be detectable as has been demonstrated for herpes simplex virus encephalitis.20 To test this possibility, we applied a specific immunostaining technique to detect SAFV antigen-specific OCB. In 2 of 25 MS patients we found indeed evidence for intrathecal IgG OCB that reacted with SAFV antigens, but such OCB were also found in 2 of 27 controls. Thus, SAFV-specific OCB can sometimes be detected in CSF but without a clear association with MS. Because of anonymous testing of the paired serum and CSF samples we were not informed on the medical history of the positive patients.
In conclusion, evidence for SAFV infection of the brain was not found and even an association of MS with a past SAFV infection was not found. We conclude that, although one cannot exclude an etiological role of SAFV infections in MS from a single study, a monocausal relationship, for example, by chronic infection of the brain, will be improbable.
Author's Contributions
Jochem Galama and Frank van Kuppeveld: design of the study, overall supervision, and writing of the manuscript. Jan Zoll: supervision of immunoblotting and contribution to writing of the manuscript. Kjerstin Lanke: supervision of virology and virus culture and contribution to writing of the manuscript. Arjan de Jong: design of SAFV PCR and supervision of testing and contribution to writing of the manuscript Jeroen Melief: selection of autopsy samples and contribution to writing of the manuscript. Inge Huitinga: selection of autopsy samples, neuropathology, and contribution to writing of the manuscript. Marcel Verbeek: selection of CSF and serum samples, supervision of CSF analyses, and contribution to writing of the manuscript.
Conflict of Interest
None declared.
References
- 1.Brahic M. Multiple sclerosis and viruses. Ann Neurol. 2010;68:6–8. doi: 10.1002/ana.22057. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens GP, Bennett JL, Lassmann H, et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann Neurol. 2009;65:639–649. doi: 10.1002/ana.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 5.Zinkernagel RM. Antiinfection immunity and autoimmunity. Ann N Y Acad Sci. 2002;958:3–6. doi: 10.1111/j.1749-6632.2002.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 6.Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J Clin Microbiol. 2007;45:2144–2150. doi: 10.1128/JCM.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahic M, Bureau J-F, Michiels T. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Annu Rev Microbiol. 2005;59:279–298. doi: 10.1146/annurev.micro.59.030804.121242. [DOI] [PubMed] [Google Scholar]
- 8.Chiu CY, Greninger AL, Kanada K, et al. Identification of cardioviruses related to Theiler's murine encephalomyelitis virus in human infections. Proc Natl Acad Sci USA. 2008;105:14124–14129. doi: 10.1073/pnas.0805968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoll J, Erkens-Hulshof S, Lanke K, et al. Saffold virus, a human Theiler's-like cardiovirus, is ubiquitous and causes infection early in life. PLoS Pathog. 2009;5:c1000416. doi: 10.1371/journal.ppat.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galama J, Lanke K, Zoll J, et al. Seroepidemiology of Saffold cardiovirus type 2. Emerg Infect Dis. 2011;17:1572–1573. doi: 10.3201/eid1708.101953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himeda T, Ohara Y. Saffod virus a novel human cardiovirus with unknown pathogenicity. J Virol. 2012;86:1292–1296. doi: 10.1128/JVI.06087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drexler JF, Baumgarte S, Eschbach-Bludau M, et al. Human cardioviruses, meningitis, and sudden infant death syndrome in children. Emerg Infect Dis. 2011;17:2313–2315. doi: 10.3201/eid1712.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen AC, Böttiger B, Banner J, et al. Serious invasive Saffold virus infections in children, 2009. Emerg Infect Dis. 2012;18:7–12. doi: 10.3201/eid1801.110725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durrenberger PF, Fernando S, Kashevi SN, et al. Effects of antemortem variables on human brain mRNA quality: a BrainNet Europe study. J Neuropathol Exp Neurol. 2010;69:70–81. doi: 10.1097/NEN.0b013e3181c7e32f. [DOI] [PubMed] [Google Scholar]
- 15.Van der Valk P, Amor S. Preactive lesions in multiple sclerosis. Curr Opin Neurol. 2009;22:207–213. doi: 10.1097/WCO.0b013e32832b4c76. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickx DA, Koning N, Schuurman KG, et al. Selective upregulation of scavenger receptors in and around demyelinating areas in multiple sclerosis. J Neuropathol Exp Neurol. 2013;72:106–118. doi: 10.1097/NEN.0b013e31827fd9e8. [DOI] [PubMed] [Google Scholar]
- 17.van Kuppeveld FJM, de Jong AS, Lanke KH, et al. Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ. 2010;340:c1018. doi: 10.1136/bmj.c1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sindic CJ, Monteyne Ph, Laterre EC. The intrathecal synthesis of virus-specific oligoclonal IgG in multiple sclerosis. J Neuroimmunol. 1994;54:75–80. doi: 10.1016/0165-5728(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 19.Lipton HL, Liang Z, Hertzler S, Son K-N. A specific viral cause of multiple sclerosis: one virus, one disease. Ann Neurol. 2007;61:514–523. doi: 10.1002/ana.21116. [DOI] [PubMed] [Google Scholar]
- 20.Vandvik B, Sköldenberg B, Forsgren M, et al. Long-term persistence of intrathecal virus-specific antibody responses after herpes simplex virus encephalitis. J Neurol. 1985;231:307–312. doi: 10.1007/BF00313707. [DOI] [PubMed] [Google Scholar]


