Abstract
Key Clinical Message
Intracranial Hodgkin lymphoma (HL) is considered so atypical that an intracranial space-occupying lesion in a patient with known HL should be thoroughly investigated to rule out a second disease process.
Keywords: Central nervous system (CNS), cerebral mass, Hodgkin lymphoma, relapse, unusual presentation
Introduction
Central nervous system (CNS) involvement in Hodgkin lymphoma (HL) is uncommon, previously reported as occurring in 0.2–0.5% of patients with systemic HL, but more recently reported as occurring at a frequency of less than 0.02% [1–7]. Most reports of CNS involvement in HL are case reports, and the infrequency of this disease entity makes noting patterns in disease characteristics, information on prognosis, and therapeutic decision making difficult. We describe a patient with systemic HL who presented with relapse of disease in the CNS.
Case Presentation
A 41-year-old Caucasian male, who was previously well, presented in December 2010 to the Clinical Haematology Unit, Department of Medicine, at Chris Hani Baragwanath Academic Hospital, Soweto, Johannesburg, with a history of progressive right neck and chest swelling, significant weight loss, intermittent night sweats, fatigue and pruritus for the last 6 months. He was a smoker with a nine pack year history, no past medical history, and no significant family history. His HIV status was negative. On examination he had bulky right supraclavicular lymphadenopathy, no other peripheral lymphadenopathy, no hepatosplenomegaly, and normal neurological function. Cervical lymph node biopsy revealed the presence of nodular sclerosis HL. Immunohistochemistry confirmed CD15 and CD30 positivity in the Reed-Sternberg cells. A bone marrow biopsy showed no evidence of lymphoma, however, there were areas concerning for granulomata. The Ziehl-Neelsen stain highlighted acid fast bacilli and polymerase chain reaction (PCR) was subsequently positive for Mycobacterium genus. Staging Computed Tomography (CT) was performed and showed evidence of enlarged right cervical and supraclavicular nodes as well as multiple enlarged nodes bilaterally in the superior mediastinum with a widest diameter of 11 cm. CT brain was normal. A positron emission tomography (PET) was not performed at this time as the procedure was not readily available at our institution. On the basis of the clinical assessment, radiological imaging and negative bone marrow biopsy, he had Ann Arbor/Cotswold Stage 2B (X) disease. The patient was initiated on anti-tuberculosis multi-agent treatment (Rifampicin, Isoniazid, Ethambutol and Pyrazinamide) which he received for a period of 9 months, and was given four cycles of ABVD chemotherapy (Adriamycin, Bleomycin, Vinblastine, Dacarbazine (DTIC)). After showing an inadequate response to the initial chemotherapy, the regimen was changed to BCVPP (Carmustine (BCNU), Cyclophosphamide, Vinblastine, Procarbazine, and Prednisone). In September 2011, following on high-dose chemotherapy, an autologous stem cell transplant was performed, as a restaging CT showed persistence of mediastinal lymphadenopathy. Mantle radiotherapy was administered, although the patient defaulted follow-up having received a total dose of only 24 Gy.
After a period of defaulting, the patient re-presented in June 2012 with pain in the right neck and head, and persistent right supraclavicular swelling, and was given a further four cycles of combination chemotherapy. In October 2012, he reported weakness of his right arm, focal seizures of the right hand, episodes of dysarthria, and continued pain in his right neck and head. A CT Brain revealed a large, well circumscribed mass in the periphery of the left parietal lobe, with heterogeneous enhancement and significant surrounding cerebral edema and mass effect resulting in midline shift (Fig.1). A differential diagnosis of a primary CNS neoplasm, metastasis and HL was entertained. Further characterization on magnetic resonance imaging (MRI) of the brain showed a well-defined left parietal mass, which demonstrated T2 mixed signal intensity (Fig.2A), T1 low signal intensity and mild peripheral nodular enhancement (Fig.2B). In addition, a repeat CT scan of the chest showed extensive ongoing mediastinal involvement, essentially unchanged in size from the previous CT scan.
Figure 1.
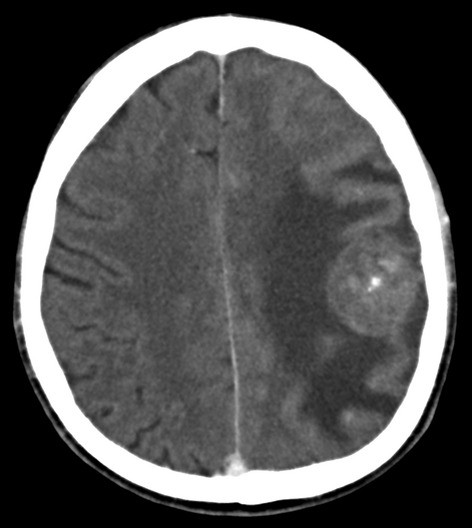
Contrast-enhanced axial CT brain demonstrating a heterogenously enhancing well-defined mass in the left parietal lobe with surrounding edema.
Figure 2.
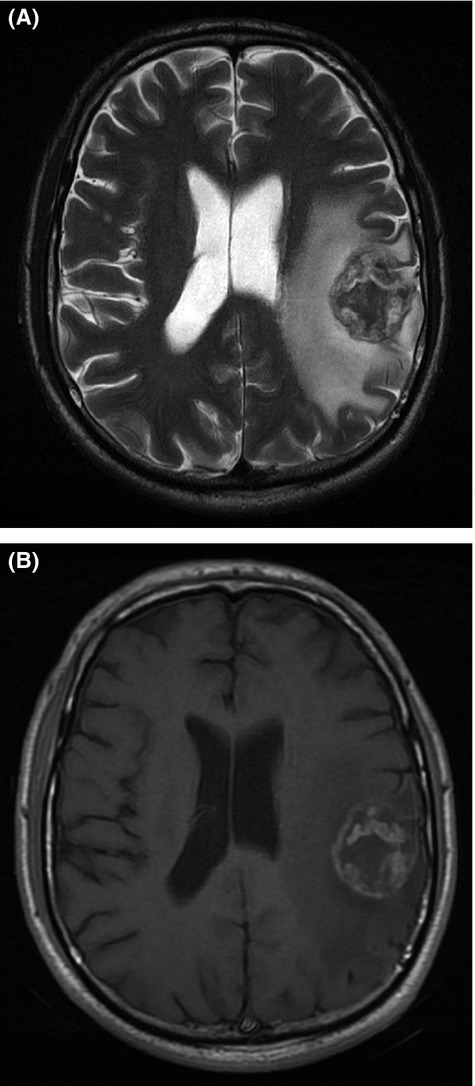
(A) T2W MRI demonstrating a mixed signal intensity mass in the left parietal lobe, with surrounding edema. (B) Contrast-enhanced axial T1W MRI demonstrating a peripheral and nodular enhancing mass in the left parietal lobe.
The patient was commenced on dexamethasone and anti-epileptic medication. The lesion was accessed through a left parietal craniotomy. The overlying dura appeared to be pale in color with some areas of increased vascularity. Dural vessels were cauterized and the underlying brain was also pale compared to normal surrounding brain parenchyma. The tumor appeared to be within 2 cm depth ‘almost surfacing’ and easily accessible. The specimen was taken piecemeal and there were no adverse events. The patient showed no signs of extension of neurological deficit, and his previous symptoms of right-sided upper limb sensory and motor deficit improved post surgery.
Microscopic examination of the brain biopsy of hematoxylin and eosin-stained sections revealed extensive coagulative tumor necrosis. There were several intermixed atypical Hodgkin cells which displayed pale eosinophilic cytoplasm, vesicular nuclei and prominent nucleoli. Several mummified tumor cells were noted (Fig.3). Anaplastic nuclear features were also evident in some areas. The tumor cells demonstrated membrane and paranuclear Golgi immunoreactivity for CD15 and CD30 (Figs. 4 & 5). The tumor lacked immunoexpression of CD20, LCA/CD45, ALK1, and EBV LMP1. Modified Ziehl-Neelsen and Periodic Acid-Schiff stains were negative for microorganisms.
Figure 3.
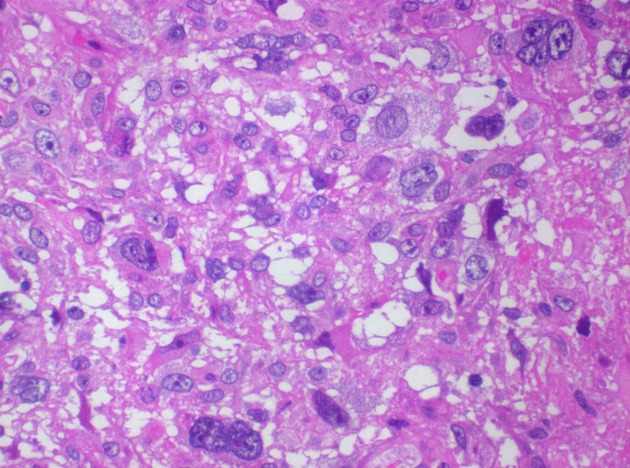
Hodgkin (Reed–Sternberg) cells with histiocyte-rich background (H&E stain, ×400 magnification).
Figure 4.
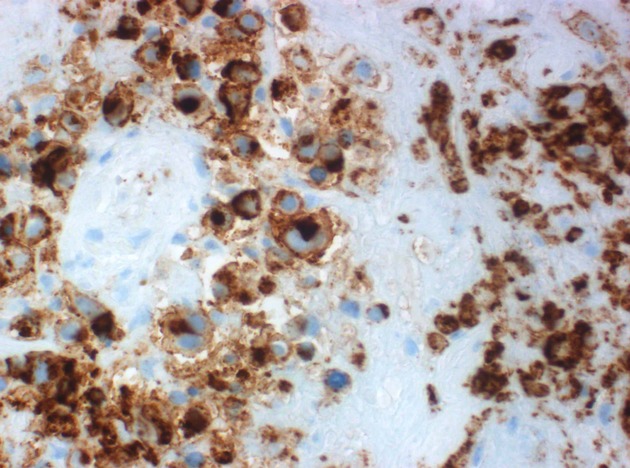
Hodgkin (Reed–Sternberg) cells displaying membrane and Golgi region CD15 immunoreactivity.
Figure 5.
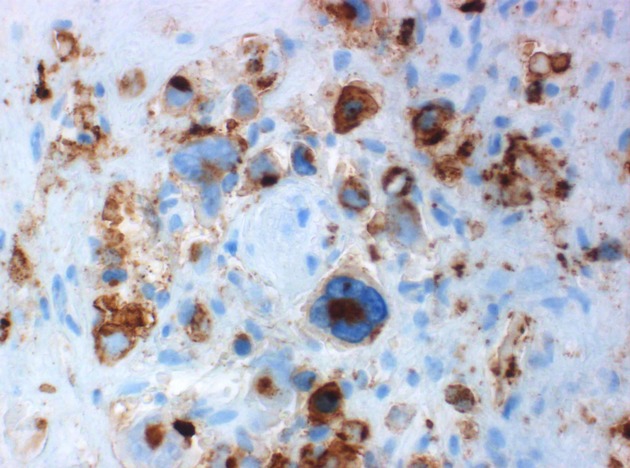
Membrane and Golgi region CD30 immunoreactivity.
These features were supportive of brain involvement by classical HL.
The preceding biopsy of the right cervical lymph node showed fibrotic bands and nodules with variable numbers of mummified, mononuclear, and classical Hodgkin/Reed–Sternberg cells which expressed CD15 and CD30. This was in keeping with nodular sclerosis, classical HL.
An assessment of relapsed classical HL, with CNS involvement as well as cervical and mediastinal involvement was made.
Cranial radiotherapy was planned post surgical recovery. Unfortunately, the patient developed neurogenic pulmonary edema on day 8 post brain biopsy, deteriorated rapidly, and died on day 10 post brain biopsy.
Discussion and Review of Published Literature
Classical HL is primarily a disease affecting lymph nodes, and neurological complications in this disease are rare. They can be divided into two main groups, those occurring as a result of the tumor – metastatic disease, and those occurring as an ancillary result of the disease – paraneoplastic phenomena, and treatment-related neurological complication. Paraneoplastic manifestations include cerebellar degeneration, acute inflammatory demyelinating polyradiculopathy, chorea, ataxia, stiff person syndrome, and myasthenia gravis [2].
CNS involvement in HL is uncommon, previously reported as occurring in 0.2–0.5% of patients with systemic HL [2–7], but more recently has been reported as occurring at a frequency of less than 0.02% [1]. The earlier higher frequency may be the result of some case reports identifying a number of cases as HL when they were possibly non-HL, a consequence of cases being reclassified as new criteria were adopted [6], or in some cases, inadequate diagnostic information existed [8]. Involvement of the CNS is considered so atypical that a space-occupying lesion in the brain of a patient with known HL should be thoroughly investigated to rule out a second disease process [9]. A possible increase in frequency of intracranial HL may be seen in the future as patients live longer [7].
CNS involvement occurs most frequently in the face of relapsing disease [4,5,7,8,10–13]. There is conflicting information in the literature regarding other possible risk factors, including suggestions of an increased risk with a family history of HL [14], immune compromise [15], infection with EBV [5,10,16], and male gender [7]. Histological subtypes most commonly described are mixed cellularity and nodular sclerosing, although there is concern as to whether the WHO-subclassification system for systemic HL should be applied to CNS HL [11].
Our patient initially presented with classical HL and tuberculosis, and this coexistence is well documented, resulting from two possible mechanisms, one being T-cell immune dysfunction related to HL, and the other, B-cell immune dysfunction resulting from therapy of HL [17]. His TB was diagnosed concurrently with the HL. Tuberculosis and HL can both involve the CNS although there is no literature describing an increased risk of tuberculous CNS involvement in HL. It remains to be investigated if breakdown of the blood brain barrier could increase the risk of cHL affecting the CNS in the face of CNS tuberculosis. There was, however, no evidence of CNS tuberculosis in our patient.
The most common presentation of intracranial involvement described in the literature is cranial nerve palsies, with other symptoms and signs including: headaches, paresis, papilledema, seizures and other neurological manifestations [2,12]. Our patient presented with focal seizures, headache, sensory and motor deficits of the right upper limb, due to a lesion involving the left parietal cortex. CNS sites most commonly involved, in decreasing frequency, are the brain parenchyma, leptomeninges and dura, corpus callosum and pituitary [2,12]. Our patient had involvement of the supratentorial brain parenchyma at the time of relapse. Intracranial involvement should be considered in any patient with known HL, who develops neurological complications, especially if there is dissemination of disease or relapse [12].
Three postulated mechanisms of intracranial involvement of HL exist, those being: direct extension through the bony skull or dura; meningeal involvement and metastasis; and hematogenous dissemination [2,4]. It has been suggested that the large size of the Reed–Sternberg cells may preclude their passage to the perivascular space of the CNS, and further filtering of the cells in the lungs, together with other, not well defined, intrinsic factors, prevent intracranial involvement, contributing to the rarity of intracranial involvement of HL [18].
CNS classical HL is atypical, and therefore meticulous staging is required, including a bone marrow examination, radiological imaging (Fluorodeoxyglucose (FDG) PET, MRI/CT Brain, CT scan of the neck, chest, abdomen, and pelvis), and an ophthalmological examination [3]. Investigations must include tissue biopsy, with intent to exclude non-HL, other primary brain tumors and intracranial infections [5,16,19].
Given the rarity of this presentation, no clinical trials have been conducted, nor conclusive treatment protocols established. Reports have described a variety of treatment modalities, including radiotherapy alone for isolated, initial CNS involvement [8], or whole-brain irradiation and combination chemotherapy [2]. Additional modalities described include intrathecal chemotherapy, especially in the face of meningeal involvement [17], stem cell transplantation [10], and surgery [10]. However, there is little evidence to support these modalities. There is currently insufficient data to recommend routine use of any specific treatment modality to prevent CNS involvement at the time of initial HL treatment [8], but currently, whole-brain irradiation and combination chemotherapy are advised [2,5,16,17]. CNS prophylaxis is not routinely recommended in HL [13], and more data are required to identify specific populations in whom this could be beneficial.
Median survival after diagnosis with intracranial HL is reportedly 46 months [2,15]. Prognosis, with treatment, although guarded, is not considered as pessimistically as it once was [4,10], and physicians should approach HL with CNS involvement with the intent to cure [12,13,19]. It is suggested that there is a better outcome in patients with disease limited to CNS initially, and in patients who relapse with CNS involvement only, compared to patients with evidence of multiple sites of relapse [11].
CNS involvement in HL is rare, and we report a patient with relapsed, refractory HL with intracranial involvement by HL. The need for a tissue diagnosis is essential in any patient with known HL who presents with a focal CNS lesion and new unexplained neurology. For now, the prognosis of this rare complication is poor and needs to be improved and optimized.
Conflict of Interest
None declared.
References
- 1.Re D, Fuchs M, Schober T, et al. CNS involvement of Hodgkin's lymphoma. J. Clin. Oncol. 2007;25:3182. doi: 10.1200/JCO.2007.12.5088. [DOI] [PubMed] [Google Scholar]
- 2.Grimm S, Chamberlain M. Hodgkin's lymphoma: a review of neurologic complications. Adv. Hematol. 2011;2011:624578. doi: 10.1155/2011/624578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson M, Kinney M, Scheithauer B, et al. Primary intracerebral Hodgkin's disease mimicking meningioma: case report. Neurosurgery. 2000;47:454–457. doi: 10.1097/00006123-200008000-00038. [DOI] [PubMed] [Google Scholar]
- 4.Apollonsky N, Edelman M, Johnson A, et al. Intracerebral presentation of Hodgkin disease mimicking meningioma in a young woman. J. Pediatr. Hematol. Oncol. 2008;30:369–372. doi: 10.1097/MPH.0b013e31816392b7. [DOI] [PubMed] [Google Scholar]
- 5.Galan L, Sanchez AC, Cantos B, et al. Central nervous system involvement in Hodgkin's lymphoma. Med. Oncol. 2011;28:505–508. doi: 10.1007/s12032-010-9692-z. [DOI] [PubMed] [Google Scholar]
- 6.Ashby MA, Barber PC, Holmes AE, et al. Primary intracranial Hodgkin's disease. Am. J. Surg. Pathol. 1988;12:294–299. [PubMed] [Google Scholar]
- 7.Cuttner J, Meyer R, Huang YP. Intracerebral involvement in Hodgkin's disease. Cancer. 1979;43:1497–1506. doi: 10.1002/1097-0142(197904)43:4<1497::aid-cncr2820430442>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Biagi J, MacKenzie R, Lim M, et al. Primary Hodgkin's disease of the CNS in an immunocompetent patient: a case study and review of the literature. Neuro. Oncol. 2000;2:239–243. doi: 10.1093/neuonc/2.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guermazi A, Brice P, de Kerviler E, et al. Extranodal Hodgkin disease: spectrum of disease. Radiographics. 2001;21:161–179. doi: 10.1148/radiographics.21.1.g01ja02161. [DOI] [PubMed] [Google Scholar]
- 10.Gerstner ER, Abrey LE, Schiff D, et al. CNS Hodgkin lymphoma. Blood. 2008;112:1658–1661. doi: 10.1182/blood-2008-04-151563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gessi M, Kuchelmeister K, Kellner U, et al. Unusual clinico-pathological features in primary Hodgkin's lymphomas of the central nervous system. Acta Neurochir. 2013;155:19–24. doi: 10.1007/s00701-012-1535-6. [DOI] [PubMed] [Google Scholar]
- 12.Akyuz C, Yalcin B, Ataham L, et al. Intracranial involvement in Hodgkin's disease. Pediatr. Hematol. Oncol. 2005;22:589–596. doi: 10.1080/08880010500198863. [DOI] [PubMed] [Google Scholar]
- 13.Sapozink M, Kaplan H. Intracranial Hodgkin's disease. Cancer. 1983;52:1301–1307. doi: 10.1002/1097-0142(19831001)52:7<1301::aid-cncr2820520728>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Ashigbi MY, Venkatraj U, Agarwal V, et al. Intracranial Hodgkin's disease in 2 patients with familial Hodgkin's disease. Med. Pediatr. Oncol. 1997;29:255–258. doi: 10.1002/(sici)1096-911x(199704)28:4<255::aid-mpo3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa BE, Brown JR, Nacimento A, et al. Hodgkin's lymphoma of the CNS masquerading as meningioma. J. Clin. Oncol. 2004;01:4228–4230. doi: 10.1200/JCO.2004.01.163. [DOI] [PubMed] [Google Scholar]
- 16.Corti M, Fioti MFV, Yampolsky C, et al. Central nervous system involvement in Hodgkin's lymphoma associated with Epstein-Barr virus in a patient with AIDS: case report and review of the literature. Braz. J. Infect. Dis. 2006;10:403–405. doi: 10.1590/s1413-86702006000600009. [DOI] [PubMed] [Google Scholar]
- 17.Shet AS, Saba N, Rausch D, et al. Intra-cranial lesions in a patient with Hodgkin lymphoma. Leuk. Lymphoma. 2004;45:419–422. doi: 10.1080/1042819031000139602. [DOI] [PubMed] [Google Scholar]
- 18.Morawa E, Ragam A, Sirota R, et al. Hodgkin's lymphoma involving the CNS. J. Clin. Oncol. 2007;25:1437–1438. doi: 10.1200/JCO.2006.10.1691. [DOI] [PubMed] [Google Scholar]
- 19.Gupta V, Ghosh J, Pant P, et al. Intracranial Hodgkin's disease. Indian J. Pediatr. 2006;73:370–371. doi: 10.1007/BF02825839. [DOI] [PubMed] [Google Scholar]


